Effects of Long-Term Endurance Exercise and Lithium Treatment on Neuroprotective Factors in Hippocampus of Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Subject and Method
2.2. Lithium Treatment
2.3. Exercise Protocol
2.4. Tissue and Blood Sampling
2.5. Analysis
2.5.1. H&E Staining
2.5.2. Blood Factor Analysis
2.5.3. Western Blotting
2.6. Statistical Analysis
3. Results
3.1. Body Composition
3.2. Toxicity Test
3.3. BDNF Production
3.4. GSK3β/Phospho-GSK3β Expression Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanson, C.D.; Clarke, C. Is expressed emotion related to estimates of abilitymade by older people with cognitive impairments and their partners? Aging Ment. Health 2013, 17, 535–543. [Google Scholar] [CrossRef]
- Alves, G.; Forsaa, E.B.; Pedersen, K.F.; Dreetz, G.M.; Larsen, J.P. Epidemiology of Parkinson’s disease. J. Neurol. 2008, 255, 18–32. [Google Scholar] [CrossRef]
- Wang, G.D.; Lai, D.J.; Burau, K.D.; Du, X.L. Potential gains in life expectancy from reducing heart disease, cancer, Alzheimer’s disease, kidney disease or HIV/AIDS as major causes of death in the USA. Public Health 2013, 127, 348–356. [Google Scholar] [CrossRef]
- Abbott, R.D.; Ross, G.W.; White, L.R.; Nelson, J.S.; Masaki, K.H.; Tanner, C.M.; Curb, J.D.; Blanchette, P.L.; Popper, J.S.; Petrovitch, H. Midlife adiposity and the future risk of Parkinson’s disease. Neurology 2002, 59, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Hassing, L.B.; Dahl, A.K.; Thorvaldsson, V.; Berg, S.; Gatz, M.; Pedersen, N.L.; Johansson, B. Overweight in midlife and risk of dementia: A 40-year follow-up study. Int. J. Obes. 2009, 33, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kåreholt, I.; Winblad, B.; Helkala, E.L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E. Cheaper, simpler, and better: Tips for treating seniors with Parkinson disease. Mayo Clin. Proc. 2011, 86, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Arida, R.M.; Scorza, F.A.; Scorza, C.A.; Cavalheiro, E.A. Is physical activity beneficial for recovery in temporal lobe epilepsy? Evidences from animal studies. Neurosci. Biobehav. Rev. 2009, 33, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Boyle, P.A.; Yu, L.; Shah, R.C.; Wilson, R.S.; Bennett, D.A. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012, 78, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Long, W.; Liu, G.; Zhang, X.; Yang, X. Effect of seabuckthorn (Hippophae rhamnoides ssp. sinensis) leaf extract on the swimming endurance and exhaustive exercise-induced oxidative stress of rats. J. Sci. Food Agric. 2012, 92, 736–742. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, S.D.; Bramham, C.R. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: New insights and implications for therapy. Curr. Opin. Drug Discov. Devel. 2006, 9, 580–586. [Google Scholar] [PubMed]
- Hariri, A.R.; Goldberg, T.E.; Mattay, V.S.; Kolachana, B.S.; Callicott, J.H.; Egan, M.F.; Weinberger, D.R. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003, 23, 6690–6694. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Ferris, L.T.; Williams, J.S.; Shen, C.L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef]
- Griffin, É.W.; Mullally, S.; Foley, C.; Warmington, S.A.; O’Mara, S.M.; Kelly, A.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011, 104, 934–941. [Google Scholar] [CrossRef]
- Skriver, K.; Roig, M.; Lundbye-Jensen, J.; Pingel, J.; Helge, J.W.; Kiens, B.; Nielsen, J.B. Acute exercise improves motor memory: Exploring potential biomarkers. Neurobiol. Learn Mem. 2014, 116, 46–58. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chen, F.C.; Pan, C.Y.; Wang, C.H.; Huang, T.H.; Chen, T.C. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology 2014, 41, 121–131. [Google Scholar] [CrossRef]
- Boyko, M.; Nassar, A.; Kaplanski, J.; Zlotnik, A.; Sharon-Granit, Y.; Azab, A.N. Effects of acute lithium treatment on brain levels of inflammatory mediators in poststroke rats. Biomed. Res. Int. 2015, 2015, 916234. [Google Scholar] [CrossRef]
- Dhawan, D.; Singh, A.; Singh, B.; Bandhu, H.K.; Chand, B.; Singh, N. Effect of lithium augmentation on the trace elemental profile in diabetic rats. Biometals 1999, 12, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.N.; Andreazza, A.C.; Rosa, A.R.; Martins, M.R.; Valvassori, S.S.; Réus, G.Z.; Hatch, J.P.; Quevedo, J.; Kapczinski, F. Lithium increases nerve growth factor levels in the rat hippocampus in an animal model of mania. Behav. Pharmacol. 2006, 17, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Hajek, T.; Weiner, M.W. Neuroprotective effects of lithium in human brain? food for thought. Curr. Alzheimer Res. 2016, 13, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Khairova, R.; Pawar, R.; Salvadore, G.; Juruena, M.F.; de Sousa, R.T.; Soeiro-de-Souza, M.G.; Salvador, M.; Zarate, C.A.; Gattaz, W.F.; Machado-Vieira, R. Effects of lithium on oxidative stress parameters in healthy subjects. Mol. Med. Rep. 2012, 5, 680–682. [Google Scholar]
- Machado-Vieira, R.; Manji, H.K.; Zarate, C.A., Jr. The role of lithium in the treatment of bipolar disorder: Convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009, 11, 92–109. [Google Scholar] [CrossRef]
- Rahimi, H.R.; Dehpour, A.R.; Mehr, S.E.; Sharifzadeh, M.; Ghahremani, M.H.; Razmi, A.; Ostad, S.N. Lithium attenuates cannabinoid-induced dependence in the animal model: Involvement of phosphorylated ERK1/2 and GSK-3β signaling pathways. Acta Med. Iran 2014, 52, 656–663. [Google Scholar]
- Rahimi-Balaei, M.; Momeny, M.; Babaeikelishomi, R.; Ejtemaei-Mehr, S.; Tavangar, S.M.; Dehpour, A.R. The modulatory effect of lithium on doxorubicin-induced cardiotoxicity in rat. Eur. J. Pharmacol. 2010, 641, 193–198. [Google Scholar] [CrossRef]
- Lenox, R.H.; Hahn, C.G. Overview of the mechanism of action of lithium in the brain: Fifty-year update. J. Clin. Psychiatry 2000, 61, 5–15. [Google Scholar]
- Phiel, C.J.; Klein, P.S. Molecular targets of lithium action. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 789–813. [Google Scholar] [CrossRef]
- Leeds, P.R.; Yu, F.; Wang, Z.; Chiu, C.T.; Zhang, Y.; Leng, Y.; Linares, G.R.; Chuang, D.M. A new avenue for lithium: Intervention in traumatic brain injury. ACS Chem. Neurosci. 2014, 5, 422–433. [Google Scholar] [CrossRef]
- Hancock, C.R.; Han, D.H.; Chen, M.; Terada, S.; Yasuda, T.; Wright, D.C.; Holloszy, J.O. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl. Acad. Sci. USA 2008, 105, 7815–7820. [Google Scholar] [CrossRef] [PubMed]
- Hanak, A.S.; Chevillard, L.; El Balkhi, S.; Risède, P.; Peoc’h, K.; Mégarbane, B. Study of blood and brain lithium pharmacokinetics in the rat according to three different modalities of poisoning. Toxicol. Sci. 2015, 143, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.M., Jr.; Pritchard, H.D.; Braude, M.C.; D’Aguanno, W. Plasma and brain lithium levels after lithium carbonate and lithium chloride administration by different routes in rats. Proc. Soc. Exp. Biol. Med. 1971, 137, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Koltai, E.; Hart, N.; Taylor, A.W.; Goto, S.; Ngo, J.K.; Davies, K.J.; Radak, Z. Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R127–R134. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Y.; Hong, H.S.; Chen, L.L.; Lin, X.H.; Lin, J.H.; Lin, Z. Effects of exercise of different intensities on the angiogenesis, infarct healing, and function of the left ventricle in postmyocardial infarction rats. Coron. Artery Dis. 2011, 22, 497–506. [Google Scholar] [CrossRef]
- Margolis, B.; Ziberstein, A.; Franks, C.; Felder, S.; Kremer, S.; Ullrich, A.; Rhee, S.G.; Skorecki, K.; Schlessinger, J. Effect of phospholipase C-gamma overexpression on PDGF induced second messengers and mitogenesis. Science 1990, 248, 607–610. [Google Scholar] [CrossRef]
- Lowry, E.C.; Blumber, J.M.; Rhea, R.L.; Ranson, J.P. Serum levels of orally administered penicillin. US Armed Forces Med. J. 1951, 2, 265–270. [Google Scholar]
- Chiu, C.T.; Chuang, D.M. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol. Ther. 2010, 128, 281–304. [Google Scholar] [CrossRef]
- Huang, H.C.; Klein, P.S. Multiple roles for glycogen synthase kinase-3 as a drug target in Alzheimer’s disease. Curr. Drug Targets. 2006, 7, 1389–1397. [Google Scholar] [CrossRef]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen synthase kinase-3(GSK3): Inflammation, diseases, and therapeutics. Neurochem. Res. 2007, 32, 577–595. [Google Scholar] [CrossRef]
- Li, X.; Jope, R.S. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology 2010, 35, 2143–2154. [Google Scholar] [CrossRef]
- Meijer, L.; Flajolet, M.; Greengard, P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol. Sci. 2004, 25, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Michalek, S.M.; Jope, R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010, 31, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.K.; Chuang, D.M. Lithium neuroprotection: Molecular mechanisms and clinical implications. Expert Rev. Mol. Med. 2004, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.K.; Wiest, C.; Chuang, D.M. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci. Biobehav. Rev. 2007, 31, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.H.; Wendland, J.R.; Chuang, D.M. Lithium inhibits Smad3/4 transactivation via increased CREB activity induced by enhanced PKA and AKT signaling. Mol. Cell Neurosci. 2008, 37, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Shi, T.; Chuang, D.M.; Qian, Y. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils. Brain Res. 2007, 1184, 270–276. [Google Scholar] [CrossRef]
- Tao, X.; West, A.E.; Chen, W.G.; Corfas, G.; Greenberg, M.E. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron 2002, 33, 383–395. [Google Scholar] [CrossRef]
- Yasuda, S.; Liang, M.H.; Marinova, Z.; Yahyavi, A.; Chuang, D.M. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol. Psychiatry 2009, 14, 51–59. [Google Scholar] [CrossRef]
- Chuang, D.M.; Wang, Z.; Chiu, C.T. GSK-3 as a target for lithium-induced neuroprotection against excitotoxicity in neuronal cultures and animal models of ischemic stroke. Front. Mol. Neurosci. 2011, 4, 15. [Google Scholar] [CrossRef]
- Gould, T.D.; Chen, G.; Manji, H.K. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology 2004, 29, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kaidanovich-Beilin, O.; Milman, A.; Weizman, A.; Pick, C.G.; Eldar-Finkelman, H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol. Psychiatry 2004, 55, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tso, P.; Woods, S.C. Receptor CD36 links a risk-associated allele to obesity and metabolic disorders. J. Biol. Chem. 2018, 293, 13349–13350. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Miller, D.L.; Roecklein, K.A. The aging hippocampus: Interactions between exercise, depression, and BDNF. Neuroscientist 2012, 18, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Leem, Y.H.; Kato, M.; Chang, H. Regular exercise and creatine supplementation prevent chronic mild stress-induced decrease in hippocampal neurogenesis via Wnt/GSK3β/β-catenin pathway. J. Exerc. Nutrition Biochem. 2018, 30, 1–6. [Google Scholar] [CrossRef]
- Wang, L.R.; Kim, S.H.; Baek, S.S. Effects of treadmill exercise on the anxiety-like behavior through modulation of GSK3β/β-catenin signaling in the maternal separation rat pup. J. Exerc. Rehabil. 2019, 26, 206–212. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, W.W.; Ma, P.; Ma, Z.X.; Hao, M.; Adelusi, T.I.; Du, L.; Yin, X.X.; Lu, Q. Swimming training alleviated insulin resistance through Wnt3a/β-catenin signaling in type 2 diabetic rats. Iran. J. Basic Med. Sci. 2017, 20, 1220–1226. [Google Scholar]
- Pena, G.S.; Paez, H.G.; Johnson, T.K.; Halle, J.L.; Carzoli, J.P.; Visavadiya, N.P.; Zourdos, M.C.; Whitehurst, M.A.; Khamoui, A.V. Hippocampal growth factor and myokine cathepsin B expression following aerobic and resistance training in 3xTg-AD mice. Int. J. Chronic Dis. 2020, 2020, 5919501. [Google Scholar] [CrossRef]
- Lista, I.; Sorrentino, G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol. Neurobiol. 2010, 30, 493–503. [Google Scholar] [CrossRef]
- Tang, S.W.; Chu, E.; Hui, T.; Helmeste, D.; Law, C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci. Lett. 2008, 431, 62–65. [Google Scholar] [CrossRef]
- Neeper, S.A.; Gómez-Pinilla, F.; Choi, J.; Cotman, C. Exercise and brain neurotrophins. Nature 1995, 373, 109. [Google Scholar] [CrossRef] [PubMed]
- Russo-Neustadt, A.; Beard, R.C.; Cotman, C.W. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology 1999, 21, 679–682. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Pilc, A.; Majerczak, J.; Grandys, M.; Zapart-Bukowska, J.; Duda, K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharmacol. 2008, 59, 119–132. [Google Scholar] [PubMed]
- Adlard, P.A.; Cotman, C.W. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience 2004, 124, 985–992. [Google Scholar] [CrossRef]
- Pizzorusso, T.; Ratto, G.M.; Putignano, E.; Maffei, L. Brain-derived neurotrophic factor causes cAMP response element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. J. Neurosci. 2000, 20, 2809–2816. [Google Scholar] [CrossRef]
- Rodgers, E.E.; Theibert, A.B. Functions of PI 3-kinase in development of the nervous system. Int. J. Dev. Neurosci. 2002, 20, 187–197. [Google Scholar] [CrossRef]
- Chen, M.J.; Russo-Neustadt, A.A. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res. Mol. Brain Res. 2005, 135, 181–193. [Google Scholar] [CrossRef]
- Duman, R.S.; Voleti, B. Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci. 2012, 35, 47–56. [Google Scholar] [CrossRef]
- Graham, L.C.; Grabowska, W.A.; Chun, Y.; Risacher, S.L.; Philip, V.M.; Saykin, A.J.; Alzheimer’s Disease Neuroimaging Initiative (ADNI); Sukoff Rizzo, S.J.; Howell, G.R. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol. Aging 2019, 80, 154–172. [Google Scholar] [CrossRef]
- Park, H.S.; Park, S.S.; Kim, C.J.; Shin, M.S.; Kim, T.W. Exercise Alleviates Cognitive Functions by Enhancing Hippocampal Insulin Signaling and Neuroplasticity in High-Fat Diet-Induced Obesity. Nutrients 2019, 11, 1603. [Google Scholar] [CrossRef]
- Bichet, D.G. Lithium, cyclic AMP signaling, A-kinase anchoring proteins, and aquaporin-2. J. Am. Soc. Nephrol. 2006, 7, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Robben, J.H.; Knoers, N.V.; Deen, P.M. Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am. J. Physiol. Renal Physiol. 2006, 291, F257–F270. [Google Scholar] [CrossRef] [PubMed]
- Trepiccione, F.; Christensen, B.M. Lithium-induced nephrogenic diabetes insipidus: New clinical and experimental findings. J. Nephrol. 2010, 23, S43–S48. [Google Scholar] [PubMed]
- Presne, C.; Fakhouri, F.; Noël, L.H.; Stengel, B.; Even, C.; Kreis, H.; Mignon, F.; Grünfeld, J.P. Lithium-induced nephropathy: Rate of progression and prognostic factors. Kidney Int. 2003, 64, 585–592. [Google Scholar] [CrossRef]
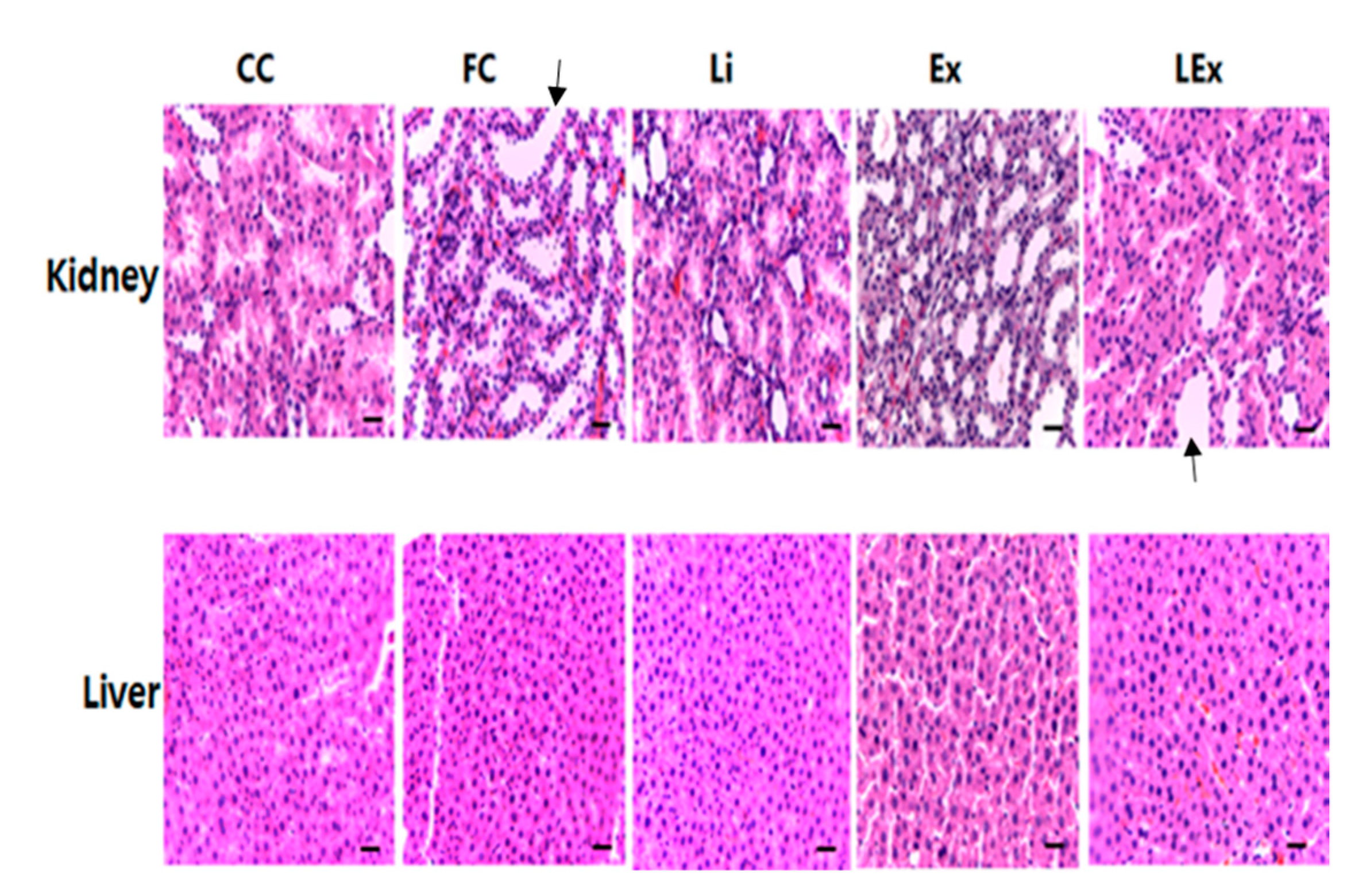
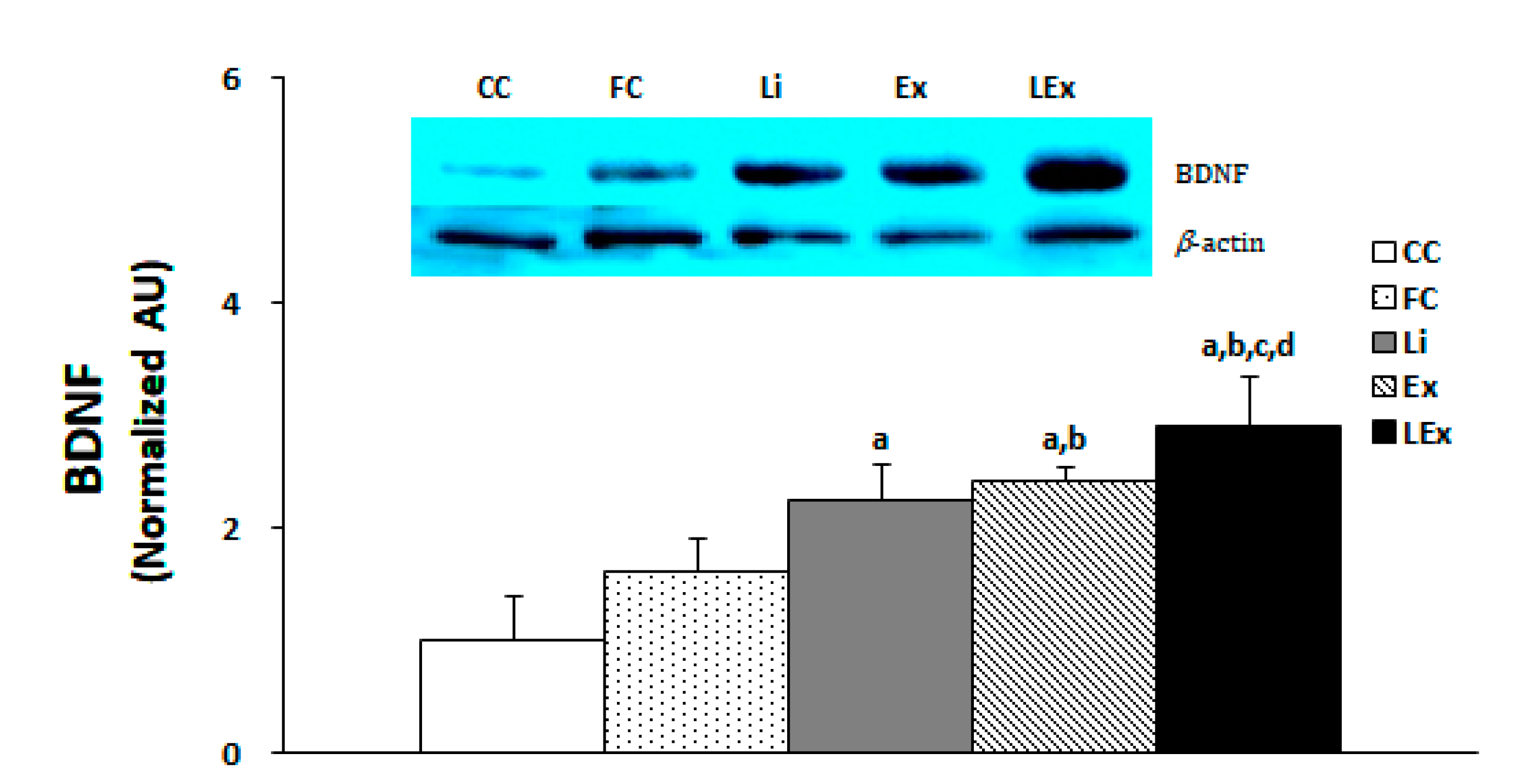

| Group | 1 Week | 3 Week | 6 Week | 9 Week | 12 Week |
|---|---|---|---|---|---|
| CC | 39.9 ± 4.5 | 48.7 ± 1.8 | 52.4 ± 1.8 | 56.9 ± 3.8 | 57.5 ± 4.2 |
| FC | 53.0 ± 3.3 a | 64.0 ± 0.8 a | 68.5 ± 2.3 a | 73.0 ± 0.8a | 72.0 ± 0.9 a |
| Li | 50.1 ± 2.6 a | 63.0 ± 1.9 a | 65.3 ± 4.6 a | 82.4 ± 2.1 a | 77.3 ± 0.5 a |
| Ex | 49.3 ± 4.4 a | 57.3 ± 0.9 a | 69.8 ± 2.3 a | 67.3 ± 2.4 a | 70.9 ± 1.3 a |
| Lex | 50.0 ± 4.4a | 67.2 ± 4.3a | 76.0 ± 1.9 a | 75.9 ± 0.2 a | 72.9 ± 0.3 a |
| Group | 0 Week | 8 Week | 12 Week |
|---|---|---|---|
| CC | 301.0 ± 3.0 | 403.0 ± 13.0 | 462.0 ± 9.5 |
| FC | 300.0 ± 2.7 | 500.4 ± 1.2 a | 590.2 ± 20.0 a |
| Li | 299.8 ± 2.0 | 503.8 ± 1.5 a | 536.5 ± 8.4 a,b |
| Ex | 302.1 ± 1.5 | 505.0 ± 6.4 a | 555.8 ± 16.0 a,b |
| Lex | 300.8 ± 0.9 | 502.0 ± 3.5 a | 527.3 ± 24.2 a,b |
| Group | Retroperitoneal | Epididymal | Mesenteric | Visceral |
|---|---|---|---|---|
| CC | 16.39 ± 1.63 | 15.88 ± 0.14 | 10.97 ± 1.12 | 43.24 ± 5.02 |
| FC | 28.46 ± 0.72 a | 23.12 ± 0.66 a | 18.64 ± 1.12 a | 70.23 ± 1.02 a |
| Li | 23.00 ± 2.00 a,b | 20.15 ± 1.63 a | 14.65 ± 0.97 a,b | 56.04 ± 3.01 a,b |
| Ex | 22.05 ± 2.61 a,b | 15.42 ± 0.86 b,c | 14.13 ± 1.23 a,b | 51.61 ± 4.35 a,b |
| Lex | 24.75 ± 1.50 a,b | 16.64 ± 0.80 b | 15.89 ± 1.33 a | 59.49 ± 4.10 a,b |
| CC | FC | Li | Ex | Lex | |
|---|---|---|---|---|---|
| ALT | 2.58 ± 3.0 | 2.53 ± 0.43 | 2.11 ± 0.21 | 2.92 ± 0.42 | 2.15 ± 0.39 |
| AST | 0.35 ± 0.02 | 0.39 ± 0.02 | 0.36 ± 0.01 | 0.37 ± 0.02 | 0.35 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Cheon, W.; Kim, K. Effects of Long-Term Endurance Exercise and Lithium Treatment on Neuroprotective Factors in Hippocampus of Obese Rats. Int. J. Environ. Res. Public Health 2020, 17, 3317. https://doi.org/10.3390/ijerph17093317
Park J, Cheon W, Kim K. Effects of Long-Term Endurance Exercise and Lithium Treatment on Neuroprotective Factors in Hippocampus of Obese Rats. International Journal of Environmental Research and Public Health. 2020; 17(9):3317. https://doi.org/10.3390/ijerph17093317
Chicago/Turabian StylePark, Jusik, Wookwang Cheon, and Kijin Kim. 2020. "Effects of Long-Term Endurance Exercise and Lithium Treatment on Neuroprotective Factors in Hippocampus of Obese Rats" International Journal of Environmental Research and Public Health 17, no. 9: 3317. https://doi.org/10.3390/ijerph17093317
APA StylePark, J., Cheon, W., & Kim, K. (2020). Effects of Long-Term Endurance Exercise and Lithium Treatment on Neuroprotective Factors in Hippocampus of Obese Rats. International Journal of Environmental Research and Public Health, 17(9), 3317. https://doi.org/10.3390/ijerph17093317






