The Unrealised Potential for Predicting Pregnancy Complications in Women with Gestational Diabetes: A Systematic Review and Critical Appraisal
Abstract
1. Introduction
2. Materials and Methods
3. Results
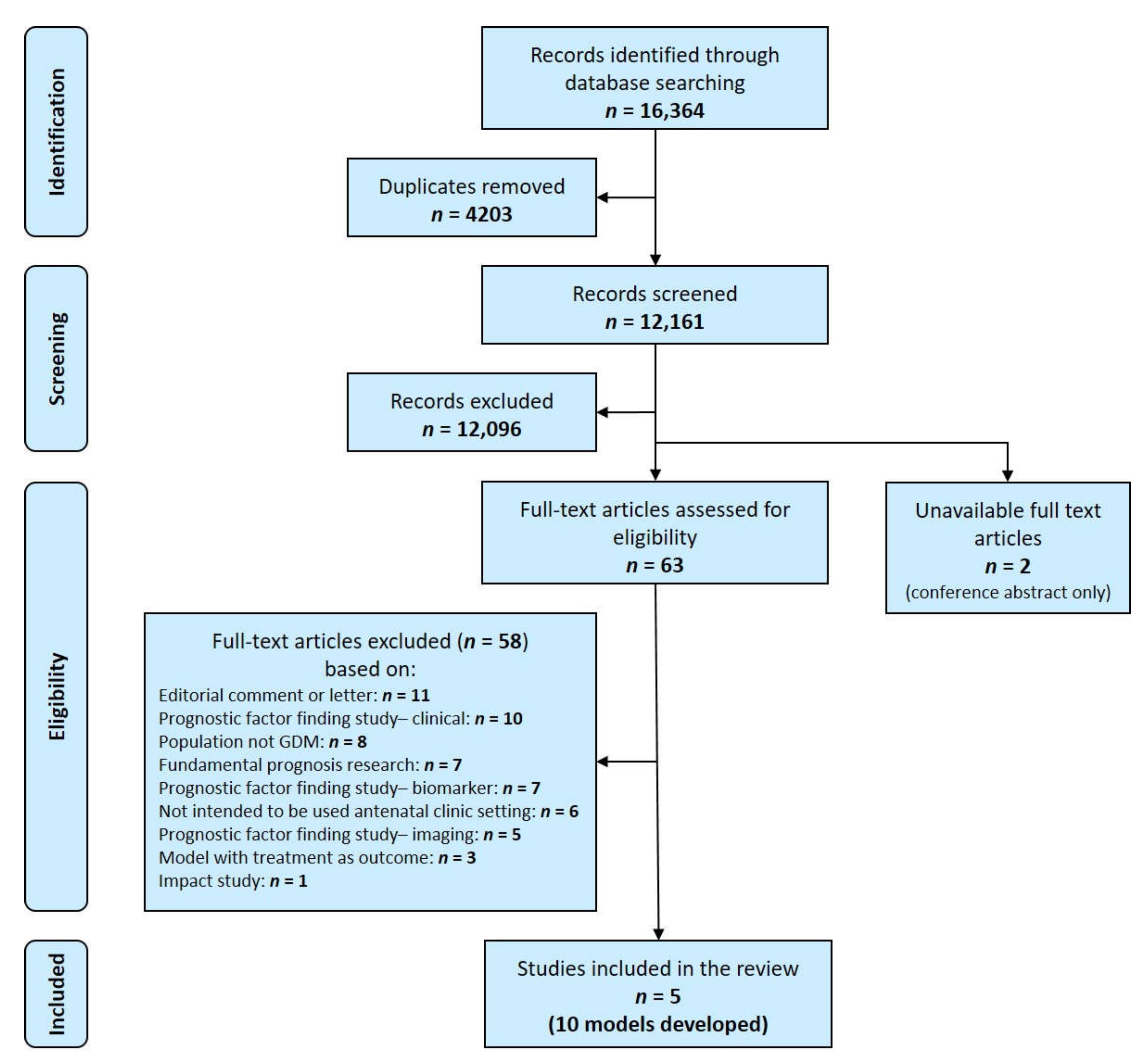
3.1. Study Selection
3.2. Study Characteristics
3.3. Characteristics of the Models
3.3.1. Source of Data and Participants
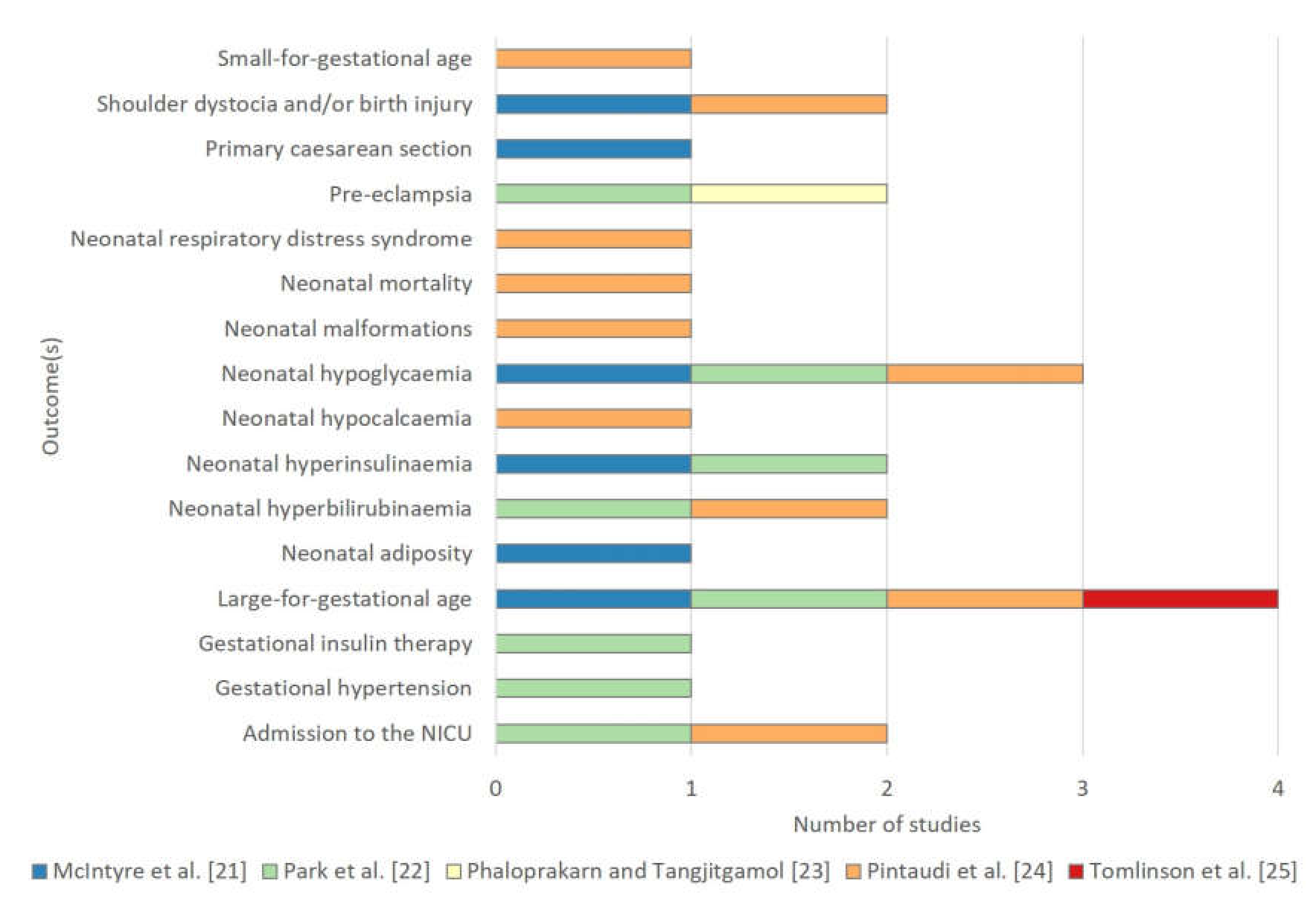
3.3.2. Outcome(s) to be Predicted
3.3.3. Candidate Predictors
3.3.4. Model Development
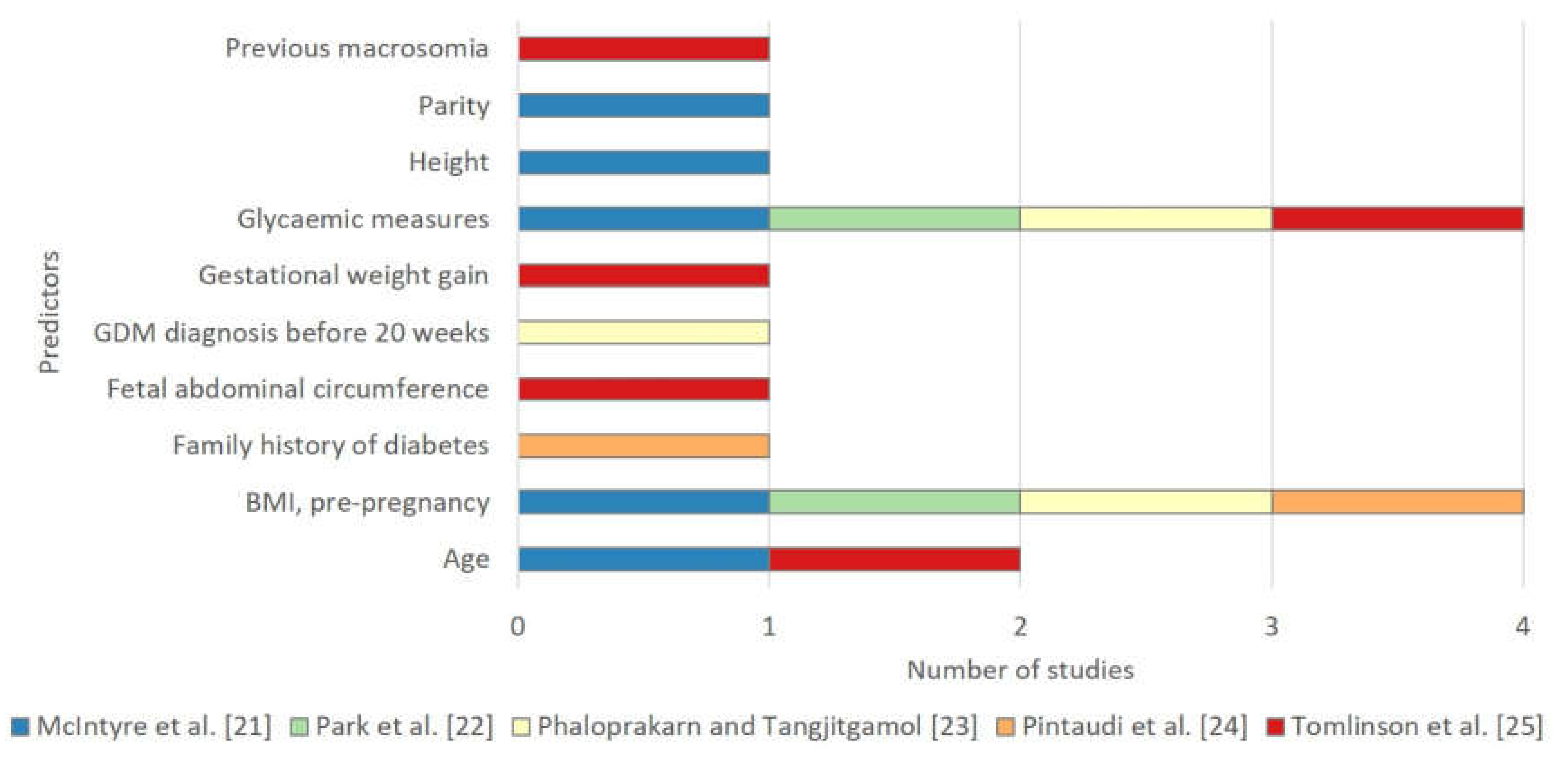
3.3.5. Predictors Selected in the Final Models
3.3.6. Model Evaluation
3.3.7. Model Presentation
3.4. Comparison of Predictive Performance
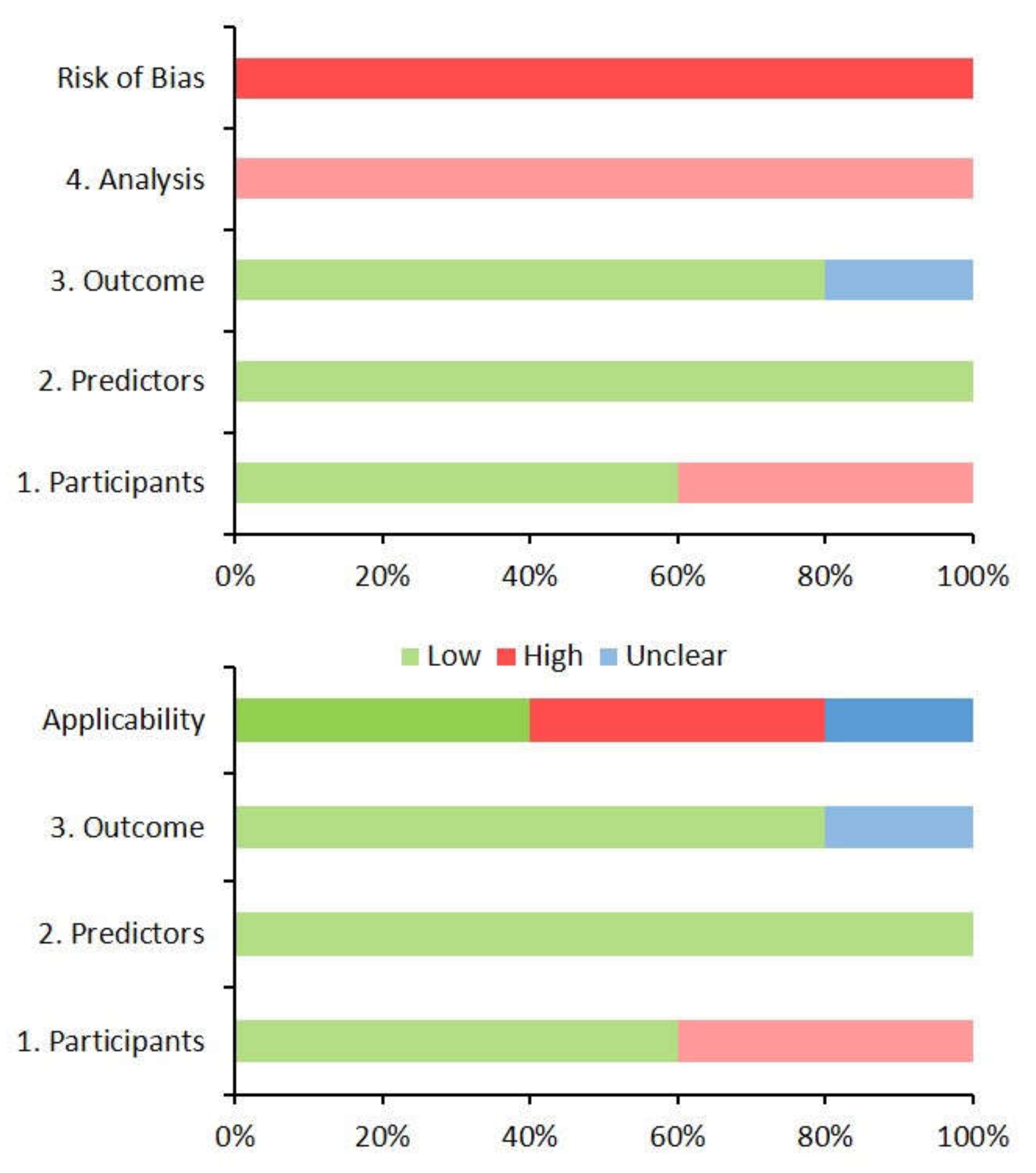
3.5. Risk of Bias and Concerns Regarding the Applicability of Models
4. Discussion
4.1. Models Identified
4.2. Characteristics of the Models
4.2.1. Outcome(s) to Be Predicted
4.2.2. Model Development
4.2.3. Predictors Selected in the Final Model
4.2.4. Model Evaluation
4.2.5. Model Presentation
4.3. Comparison of Predictive Performance
4.4. Risk of Bias and Concerns Regarding the Applicability of Models
4.4.1. Risk of Bias
4.4.2. Concerns Regarding Applicability
4.5. Strengths and Limitations of this Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the receiver operating characteristic curve |
| BMI | body mass index |
| c-statistic | concordance statistic |
| CHARMS | checklist for critical appraisal and data extraction for systematic reviews of prediction modelling studies |
| EPP | events per predictor |
| GDM | gestational diabetes |
| GWG | gestational weight gain |
| HAPO | hyperglycaemia and adverse pregnancy outcomes |
| LASSO | least absolute shrinkage and selection operator |
| LGA | large-for-gestational age |
| OGTT | oral glucose tolerance test |
| ROC | receiver operating characteristic |
| PROBAST | prediction model risk of bias overdiagnosis tool |
| RECPAM | recursive partitioning and amalgamation |
| TRIPOD | transparent reporting of a multivariable prediction model for individual prognosis or diagnosis |
References
- Scifres, C.; Feghali, M.; Althouse, A.D.; Caritis, S.; Catov, J. Adverse Outcomes and Potential Targets for Intervention in Gestational Diabetes and Obesity. Obstet. Gynecol. 2015, 126, 316–325. [Google Scholar] [CrossRef]
- Huet, J.; Beucher, G.; Rod, A.; Morello, R.; Dreyfus, M. Joint impact of gestational diabetes and obesity on perinatal outcomes. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Yuen, L.; Wong, V.W.; Simmons, D. Ethnic Disparities in Gestational Diabetes. Curr. Diab. Rep. 2018, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Allard, C.; Battista, M.C.; Doyon, M.; Bouchard, L.; Ecker, J.L.; Perron, P.; Florez, J.C.; Thadhani, R.; Hivert, M.F. Heterogeneous Contribution of Insulin Sensitivity and Secretion Defects to Gestational Diabetes Mellitus. Diabetes Care 2016, 39, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia 2019, 62, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.S.; Abell, S.; Aroni, R.; Nankervis, A.; Boyle, J.; Teede, H. Ethnic differences in prevalence, risk factors, and perinatal outcomes of gestational diabetes mellitus: A comparison between immigrant ethnic Chinese women and Australian-born Caucasian women in Australia. J. Diabetes 2019, 11, 809–817. [Google Scholar] [CrossRef]
- Abell, S.K.; Teede, H.J. The IADPSG diagnostic criteria identify women with increased risk of adverse pregnancy outcomes in Victoria. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 564–568. [Google Scholar] [CrossRef]
- Behboudi-Gandevani, S.; Amiri, M.; Bidhendi Yarandi, R.; Ramezani Tehrani, F. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11. [Google Scholar] [CrossRef]
- Lavery, J.A.; Friedman, A.M.; Keyes, K.M.; Wright, J.D.; Ananth, C.V. Gestational diabetes in the United States: Temporal changes in prevalence rates between 1979 and 2010. BJOG 2017, 124, 804–813. [Google Scholar] [CrossRef]
- Moses, R.G.; Wong, V.C.; Lambert, K.; Morris, G.J.; San Gil, F. The prevalence of hyperglycaemia in pregnancy in Australia. Aust. N. Z. J. Obstet. Gynaecol. 2016, 56, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Lin, A.; Russell, H. Adopting the new World Health Organization diagnostic criteria for gestational diabetes: How the prevalence changes in a high-risk region in Australia. Diabetes Res. Clin. Pract. 2017, 129, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kleinrouweler, C.E.; Cheong-See, F.M.; Collins, G.S.; Kwee, A.; Thangaratinam, S.; Khan, K.S.; Mol, B.W.; Pajkrt, E.; Moons, K.G.; Schuit, E. Prognostic models in obstetrics: Available, but far from applicable. Am. J. Obstet. Gynecol. 2016, 214, 79–90 e36. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, J.C.; Altman, D.G. Commentary: Prognostic models: Clinically useful or quickly forgotten? BMJ 1995, 311, 1539–1541. [Google Scholar] [CrossRef]
- Damen, J.A.A.G.; Hooft, L. The increasing need for systematic reviews of prognosis studies: Strategies to facilitate review production and improve quality of primary research. Diagn. Progn. Res. 2019, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Cooray, S.D.; Boyle, J.A.; Soldatos, G.; Wijeyaratne, L.A.; Teede, H.J. Prognostic prediction models for pregnancy complications in women with gestational diabetes: A protocol for systematic review, critical appraisal and meta-analysis. Syst. Rev. 2019, 8, 270. [Google Scholar] [CrossRef]
- Ingui, B.J.; Rogers, M.A. Searching for clinical prediction rules in MEDLINE. J. Am. Med. Inform. Assoc. 2001, 8, 391–397. [Google Scholar] [CrossRef]
- Geersing, G.J.; Bouwmeester, W.; Zuithoff, P.; Spijker, R.; Leeflang, M.; Moons, K.G. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLoS ONE 2012, 7, e32844. [Google Scholar] [CrossRef]
- Moons, K.G.; de Groot, J.A.; Bouwmeester, W.; Vergouwe, Y.; Mallett, S.; Altman, D.G.; Reitsma, J.B.; Collins, G.S. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: The CHARMS checklist. PLoS Med. 2014, 11, e1001744. [Google Scholar] [CrossRef]
- Wolff, R.F.; Moons, K.G.M.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S.; Groupdagger, P. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Gibbons, K.S.; Lowe, J.; Oats, J.J.N. Development of a risk engine relating maternal glycemia and body mass index to pregnancy outcomes. Diabetes Res. Clin. Pract. 2018, 139, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, D.W.; Kwon, J.Y.; Park, Y.W.; Kim, Y.H.; Cho, H.Y. Development of a Screening Tool for Predicting Adverse Outcomes of Gestational Diabetes Mellitus: A Retrospective Cohort Study. Medicine (Baltimore) 2016, 95, e2204. [Google Scholar] [CrossRef]
- Phaloprakarn, C.; Tangjitgamol, S. Risk assessment for preeclampsia in women with gestational diabetes mellitus. J. Perinat. Med. 2009, 37, 617–621. [Google Scholar] [CrossRef]
- Pintaudi, B.; Fresa, R.; Dalfra, M.; Dodesini, A.R.; Vitacolonna, E.; Tumminia, A.; Sciacca, L.; Lencioni, C.; Marcone, T.; Lucisano, G.; et al. The risk stratification of adverse neonatal outcomes in women with gestational diabetes (STRONG) study. Acta Diabetol. 2018, 55, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, T.M.; Mostello, D.J.; Lim, K.H.; Pritchard, J.S.; Gross, G. Fetal overgrowth in pregnancies complicated by diabetes: Development of a clinical prediction index. Arch. Gynecol. Obstet. 2018, 298, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.; Hod, M.; McIntyre, H.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. New Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef]
- International Association of Diabetes Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Ensor, J.; Riley, R.D.; Moore, D.; Snell, K.I.; Bayliss, S.; Fitzmaurice, D. Systematic review of prognostic models for recurrent venous thromboembolism (VTE) post-treatment of first unprovoked VTE. BMJ Open 2016, 6, e011190. [Google Scholar] [CrossRef]
- Lamain-de Ruiter, M.; Kwee, A.; Naaktgeboren, C.A.; Franx, A.; Moons, K.G.M.; Koster, M.P.H. Prediction models for the risk of gestational diabetes: A systematic review. Diagn. Progn. Res. 2017, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.A.; Wong, T.; Ross, G.P.; Jalaludin, B.B.; Wong, V.W.; Smart, C.E.; Collins, C.E.; MacDonald-Wicks, L.; Flack, J.R. A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus. Diabetologia 2016, 59, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J. Diabetes and Pregnancy: Blood Sugar of Newborn Infants. Ph.D. Thesis, Danish Science Press, Copenhagen, Denmark, 1952. [Google Scholar]
- Freinkel, N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980, 29, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.L., Jr.; Scholtens, D.M.; Kuang, A.; Linder, B.; Lawrence, J.M.; Lebenthal, Y.; McCance, D.; Hamilton, J.; Nodzenski, M.; Talbot, O.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 372–380. [Google Scholar] [CrossRef] [PubMed]
- West, N.A.; Crume, T.L.; Maligie, M.A.; Dabelea, D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia 2011, 54, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Crume, T.L.; Ogden, L.; West, N.A.; Vehik, K.S.; Scherzinger, A.; Daniels, S.; McDuffie, R.; Bischoff, K.; Hamman, R.F.; Norris, J.M.; et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: The Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia 2011, 54, 87–92. [Google Scholar] [CrossRef]
- Hammoud, N.M.; Visser, G.H.A.; van Rossem, L.; Biesma, D.H.; Wit, J.M.; de Valk, H.W. Long-term BMI and growth profiles in offspring of women with gestational diabetes. Diabetologia 2018, 61, 1037–1045. [Google Scholar] [CrossRef]
- Gillman, M.W. Interrupting Intergenerational Cycles of Maternal Obesity. Nestle Nutr. Inst. Workshop Ser. 2016, 85, 59–69. [Google Scholar] [CrossRef]
- Montori, V.M.; Permanyer-Miralda, G.; Ferreira-Gonzalez, I.; Busse, J.W.; Pacheco-Huergo, V.; Bryant, D.; Alonso, J.; Akl, E.A.; Domingo-Salvany, A.; Mills, E.; et al. Validity of composite end points in clinical trials. BMJ 2005, 330, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Snell, K.I.; Ensor, J.; Burke, D.L.; Harrell, F.E., Jr.; Moons, K.G.; Collins, G.S. Minimum sample size for developing a multivariable prediction model: PART II—Binary and time-to-event outcomes. Stat. Med. 2019, 38, 1276–1296. [Google Scholar] [CrossRef]
- Dawson, N.V.; Weiss, R. Dichotomizing continuous variables in statistical analysis: A practice to avoid. Med. Decis. Making 2012, 32, 225–226. [Google Scholar] [CrossRef] [PubMed]
- van Walraven, C.; Hart, R.G. Leave ’em alone—Why continuous variables should be analyzed as such. Neuroepidemiology 2008, 30, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Altman, D.G.; Sauerbrei, W. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat. Med. 2006, 25, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Wolff, R.F.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. Probast: A tool to assess risk of bias and applicability of prediction model studies: Explanation and elaboration. Ann. Intern. Med. 2019, 170, W1–W33. [Google Scholar] [CrossRef]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
- Collins, G.S.; de Groot, J.A.; Dutton, S.; Omar, O.; Shanyinde, M.; Tajar, A.; Voysey, M.; Wharton, R.; Yu, L.M.; Moons, K.G.; et al. External validation of multivariable prediction models: A systematic review of methodological conduct and reporting. BMC Med. Res. Methodol. 2014, 14, 40. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Allotey, J.; Fernandez-Felix, B.M.; Zamora, J.; Moss, N.; Bagary, M.; Kelso, A.; Khan, R.; van der Post, J.A.M.; Mol, B.W.; Pirie, A.M.; et al. Predicting seizures in pregnant women with epilepsy: Development and external validation of a prognostic model. PLoS Med. 2019, 16, e1002802. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Saville, B.R.; Lewis, R.J. Decision curve analysis. JAMA 2015, 313, 409–410. [Google Scholar] [CrossRef]
- Holmberg, L.; Vickers, A. Evaluation of prediction models for decision-making: Beyond calibration and discrimination. PLoS Med. 2013, 10, e1001491. [Google Scholar] [CrossRef] [PubMed]
- Localio, A.R.; Goodman, S. Beyond the usual prediction accuracy metrics: Reporting results for clinical decision making. Ann. Intern. Med. 2012, 157, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, L.J.; Snell, K.I.E.; Collins, G.S.; Riley, R.D. Guide to presenting clinical prediction models for use in clinical settings. BMJ 2019, 365, l737. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vergouwe, Y. Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur. Heart J. 2014, 35, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Janssen, K.J.; Kalkman, C.J.; Grobbee, D.E.; Bonsel, G.J.; Moons, K.G.; Vergouwe, Y. The risk of severe postoperative pain: Modification and validation of a clinical prediction rule. Anesth. Analg. 2008, 107, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Kappen, T.H.; Vergouwe, Y.; Van Klei, W.A.; Van Wolfswinkel, L.; Kalkman, C.J.; Moons, K.G.M. Adaptation of Clinical Prediction Models for Application in Local Settings. Med. Decis. Mak. 2012, 32, E1–E10. [Google Scholar] [CrossRef]
| Study | Type of Prediction Modelling Study | Model(s) |
|---|---|---|
| McIntyre et al. [21] | Development |
A. A risk engine relating maternal glycaemia and body mass index to pregnancy outcomes with a model developed for each of:
|
| Park et al. [22] | Development | B. Screening tool for predicting adverse outcomes a of GDM |
| Phaloprakarn and Tangjitgamol [23] | Development | C. A risk score based on clinical characteristics of GDM women for the development of preeclampsia |
| Pintaudi et al. [24] | Development | D. Subgroups at different risks of developing the composite adverse neonatal outcome b by RECPAM analysis |
| Tomlinson et al. [25] | Development | E. The fetal overgrowth index |
| Model | Source of Data | Study Setting | Study Dates | Sample Size | |
|---|---|---|---|---|---|
| A risk engine relating maternal glycaemia and body mass index to pregnancy outcomes [21] | Prospective cohort (post hoc analysis) | Single centre The antenatal clinic, Mater Misericordiae Mothers’ Hospital, Australia | Jul 2000– Apr 2006 | n = 1248 | |
| Screening tool for predicting adverse outcomes of GDM [22] | Retrospective cohort | Single centre Admitted patients, Severance Hospital, South Korea | Mar 2001–Apr 2013 | n = 802 (306 in GDM group) | |
| A risk score based on clinical characteristics of GDM women for the development of preeclampsia [23] | Retrospective cohort | Single centre The antenatal clinic, Vajira Hospital, Thailand | Jan 2003– Feb 2008 | n = 813 | |
| Subgroups at different risks of developing the composite adverse neonatal outcome [24] | Retrospective cohort | Multi-centre Specialised GDM clinics, Various hospitals, Italy | Jan 2012–May 2015 | n = 2736 | |
| The fetal overgrowth index [25] | Retrospective cohort | Single centre Collaborative diabetes in pregnancy program, South Shore Hospital, USA | Mar 2010–May 2012 | n = 275 |
| Models | Inclusion Criteria | Exclusion Criteria | Nulliparous | Ethnicity | Diagnostic Criteria Used For GDM | Treatment Status |
|---|---|---|---|---|---|---|
| A risk engine relating maternal glycaemia and body mass index to pregnancy outcomes [21] | Pregnant women enrolled in HAPO study a | Multiple pregnancy, stillbirth, congenital anomaly, non-Caucasian mother, birth < 33 weeks gestation, missing data for key independent variables, age ≤ 18 years, uncertainty of gestational age, inability to complete oral glucose-tolerance test within 32 weeks gestation, assisted conception, previous diabetes requiring pharmacologic treatment or infection with hepatitis B or C virus | 54.5% | 100% Caucasian | NA (Participants blinded to results of OGTT) | No GDM treatment (patients and clinicians blinded to OGTT result) |
| Screening tool for predicting adverse outcomes of GDM [22] | Two groups: 1) Women with GDM, 2) pregnant women with false-positive glucose challenge tests | Multiple pregnancy, pre-gestational diabetes, diagnosis with GDM at <24 weeks gestation, anomalous foetuses, chronic hypertension | NR | NR | Universal screening with ACOG approach b | Treatment not reported |
| A risk score based on clinical characteristics of GDM women for the development of preeclampsia [23] | Women with GDM | Multiple pregnancy, risk factors for pre-eclampsia, smoking | 40.0% | 92% Thai, 8% South East Asian | Universal screening with ACOG approach b | GDM treatment as per standardised institutional protocol |
| Subgroups at different risks of developing the composite adverse neonatal outcome [24] | Women with GDM | Multiple pregnancy | 45.3% | 44.8% Caucasian | Risk factor-based screening with IADPSG approach c | GDM treatment as per standardised institutional protocol |
| The fetal overgrowth index [25] | Women with pre-gestational diabetes or GDM | Multiple pregnancy, birth <20 weeks gestation | 50.5% | 82% White | Universal screening with ACOG approach b | GDM treatment as per standardised institutional protocol |
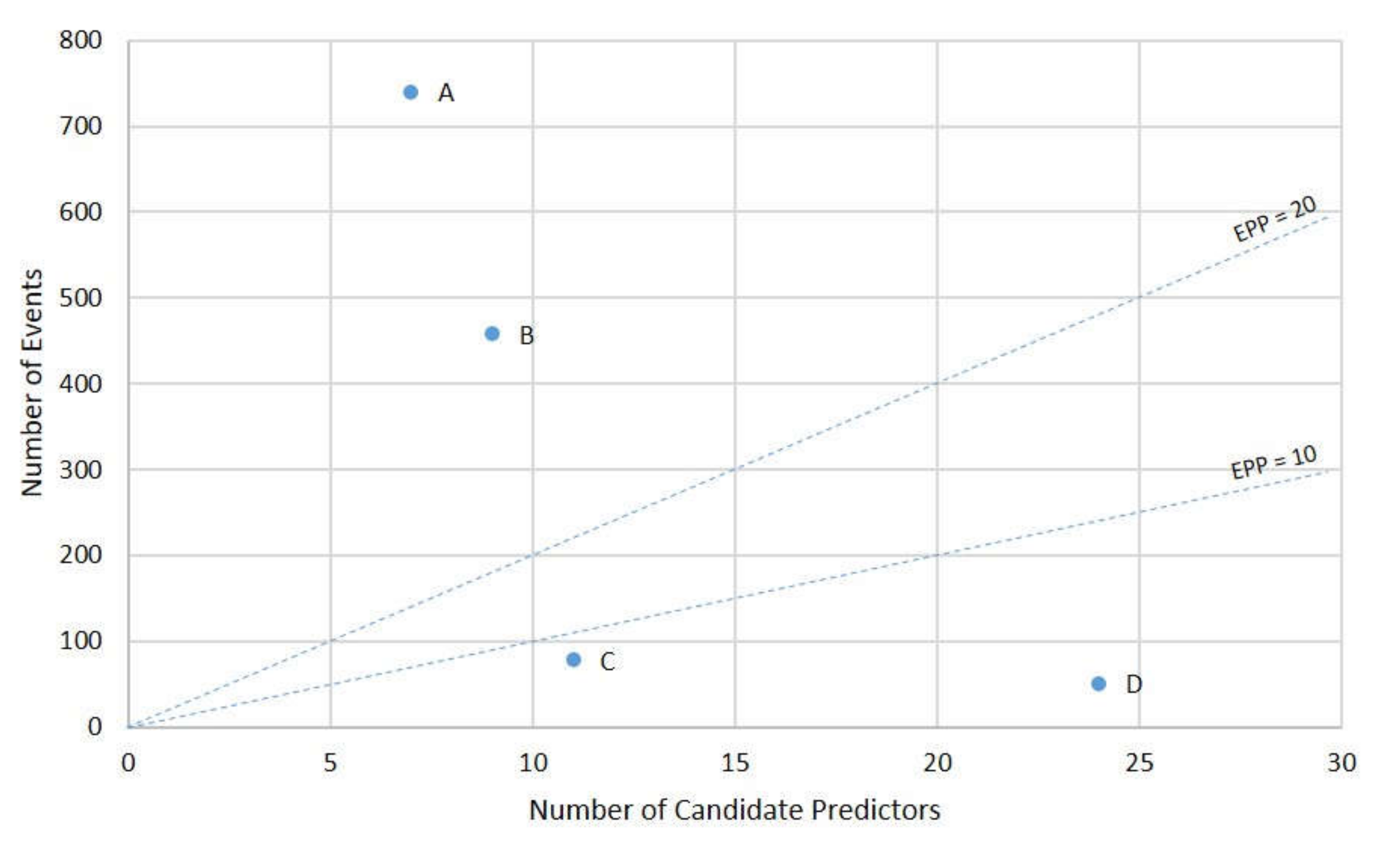
| Model | Outcome(s) to Be Predicted | Candidate Predictors | Events per Predictor | |||
|---|---|---|---|---|---|---|
| Type | Event | Number of Events | Number | Type | ||
| A risk engine relating maternal glycaemia and body mass index to pregnancy outcomes [21] | Single | (1) primary caesarean delivery | 241 | NR | NR | ─ |
| (2) birth injury (including shoulder dystocia) | 29 | ─ | ||||
| (3) LGA | 175 | ─ | ||||
| (4) neonatal adiposity | 100 | ─ | ||||
| (5) neonatal hyperinsulinemia | 76 | ─ | ||||
| (6) neonatal hypoglycaemia | 73 | ─ | ||||
| Screening tool for predicting adverse outcomes of GDM [22] | Composite | “adverse outcomes of GDM”: neonatal hypoglycaemia, hyperbilirubinemia, and hyperinsulinemia; admission to the NICU; LGA; gestational insulin therapy; preeclampsia; gestational hypertension | 458 | 9 | demographics, patient history, physical examination, investigations | 51 a |
| A risk score based on clinical characteristics of GDM women for the development of preeclampsia [23] | Single | preeclampsia | 78 | 11 | demographics, patient history, physical examination, investigations, disease characteristics | 7 |
| Subgroups at different risks of developing the composite adverse neonatal outcome [24] | Composite | “neonatal adverse outcome”: fetal growth large or small for gestational age, mortality (neonatal deaths and stillbirths), malformations, shoulder dystocia, NICU need, hypoglycaemia, hypocalcaemia, hyperbilirubinemia, and respiratory distress syndrome | 740 | 7 | demographics, patient history, physical examination, investigations | 106 |
| The fetal overgrowth index [25] | Single | fetal overgrowth: birthweight ≥ 90th gestational-related optimal weight centile | 51 | 24 | demographics, patient history, physical examination, investigations | 2 |
| Model | Selected Predictors | Presentation Format | Evaluation | Performance | |
|---|---|---|---|---|---|
| Calibration | Discrimination (c-statistic a [95% CI]) | ||||
| A risk engine relating maternal glycaemia and body mass index to pregnancy outcomes b [21] | Fasting, one hour, two hour OGTT results c, age, height, BMI at time of OGTT, parity | Regression coefficients without baseline components | Internal validation (apparent) | NR | Primary caesarean delivery: 0.694 (0.661–0.727) Birth injury: 0.699 (0.568–0.830) LGA: 0.654 (0.624–0.684) Adiposity 0.608 (0.570–0.645) Hyperinsulinaemia 0.601 (0.545–0.657) Hypoglycaemia 0.574 (0.517–0.632) |
| Screening tool for predicting adverse outcomes of GDM [22] | BMI at time of diagnosis, fasting glucose from OGTT d | Regression coefficients without baseline components | Internal validation (apparent) | GOF (p = 0.27) | 0.642 (NR) |
| A risk score based on clinical characteristics of GDM women for the development of preeclampsia [23] | First trimester BMI ≥ 27 kg/m2, GDM diagnosed within 20 weeks of gestation, poor glycaemic control e | Simplified scoring system | Internal validation (apparent) | GOF (p = 0.792) | 0.911 (0.877–0.946) |
| Subgroups at different risks of developing the composite adverse neonatal outcome [24] | Pre-pregnancy BMI, family history of diabetes | Decision tree consisting of four patient subgroup classes | NR | NR | NR |
| The fetal overgrowth index [25] | High fasting glucose f, enlarged abdominal circumference g, excessive weight gain h, history of macrosomia i, Age ≤ 30 | Simplified scoring system | Internal validation (bootstrapping) | NR | 0.89 (0.888–0.891) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooray, S.D.; Wijeyaratne, L.A.; Soldatos, G.; Allotey, J.; Boyle, J.A.; Teede, H.J. The Unrealised Potential for Predicting Pregnancy Complications in Women with Gestational Diabetes: A Systematic Review and Critical Appraisal. Int. J. Environ. Res. Public Health 2020, 17, 3048. https://doi.org/10.3390/ijerph17093048
Cooray SD, Wijeyaratne LA, Soldatos G, Allotey J, Boyle JA, Teede HJ. The Unrealised Potential for Predicting Pregnancy Complications in Women with Gestational Diabetes: A Systematic Review and Critical Appraisal. International Journal of Environmental Research and Public Health. 2020; 17(9):3048. https://doi.org/10.3390/ijerph17093048
Chicago/Turabian StyleCooray, Shamil D., Lihini A. Wijeyaratne, Georgia Soldatos, John Allotey, Jacqueline A. Boyle, and Helena J. Teede. 2020. "The Unrealised Potential for Predicting Pregnancy Complications in Women with Gestational Diabetes: A Systematic Review and Critical Appraisal" International Journal of Environmental Research and Public Health 17, no. 9: 3048. https://doi.org/10.3390/ijerph17093048
APA StyleCooray, S. D., Wijeyaratne, L. A., Soldatos, G., Allotey, J., Boyle, J. A., & Teede, H. J. (2020). The Unrealised Potential for Predicting Pregnancy Complications in Women with Gestational Diabetes: A Systematic Review and Critical Appraisal. International Journal of Environmental Research and Public Health, 17(9), 3048. https://doi.org/10.3390/ijerph17093048





