What Accounts for Physical Activity during Pregnancy? A Study on the Sociodemographic Predictors of Self-Reported and Objectively Assessed Physical Activity during the 1st and 2nd Trimesters of Pregnancy
Abstract
1. Introduction
1.1. Physical Activity during Pregnancy: On Guidelines, Performance, and Determinants
1.2. Measurement Options for PA, Advantages, and Disadvantages
1.3. Study Aim
2. Materials and Methods
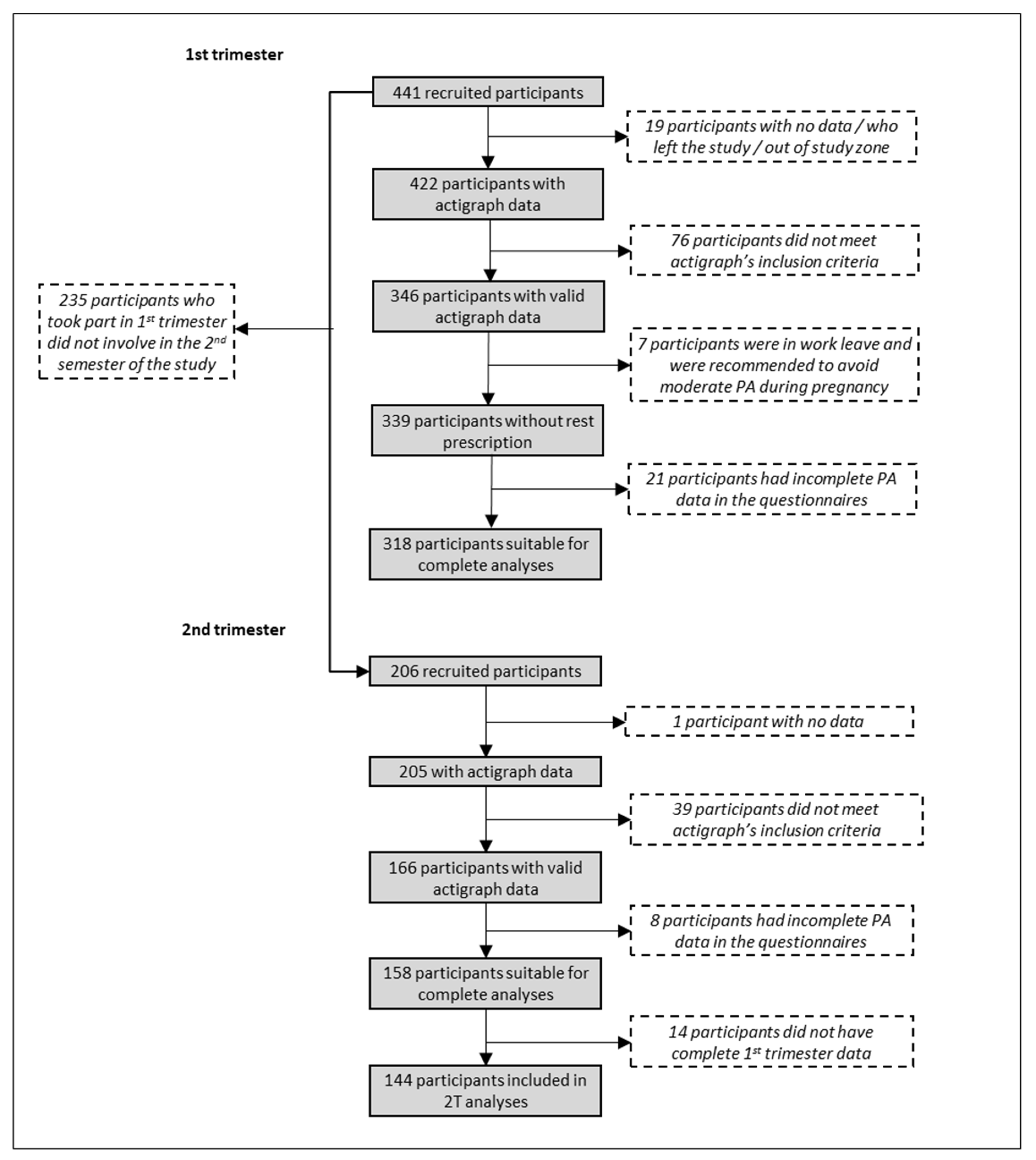
2.1. Study Sample
2.2. Study Instruments and Variables
2.2.1. Physical Activity
2.2.2. Covariates
2.3. Data Analysis
3. Results
3.1. PA Levels by Trimester and Fulfillment of International Recommendations
3.2. Comparison of PA Levels between the First and Second Trimesters of Pregnancy
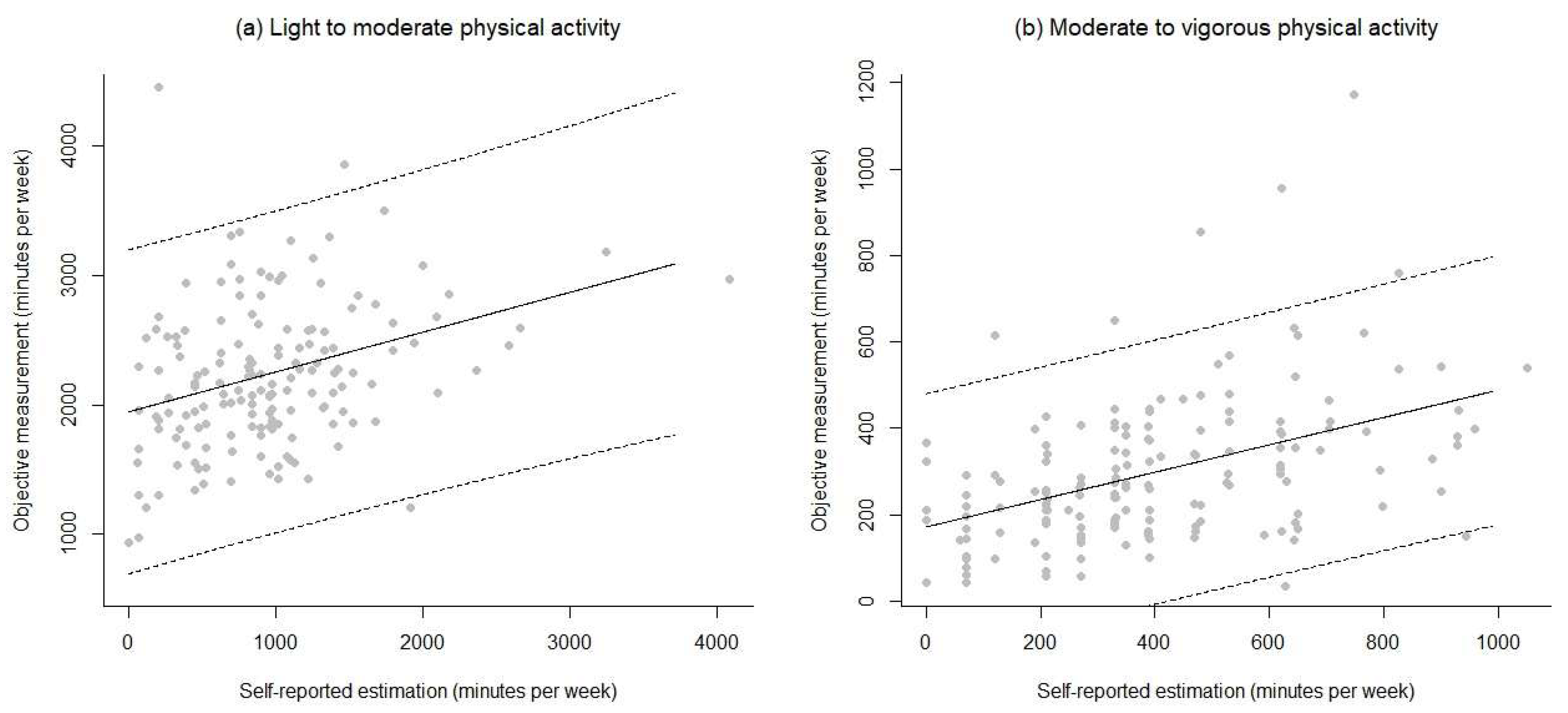
3.3. Associations and Agreement between Objective and Self-Reported Measures
3.4. Predictive Models of PA during Pregnancy
4. Discussion
4.1. PA Levels during Pregnancy and Agreement Between Self-Reported and Accelerometer Data
4.2. Determinants of PA during the 1st and 2nd Trimesters of Pregnancy
4.3. Strengths, Limitations, and Future Lines of Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pedersen, B.K.; Saltin, B. Exercise as medicine - Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sport. 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO. Available online: www.who.int (accessed on 16 December 2019).
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- de Oliveira, C.S.; dos Imakawa, T.S.; Moisés, E.C.D. Physical activity during pregnancy: Recommendations and assessment tools. Rev. Bras. Ginecol. e Obstet. 2017, 39, 424–432. [Google Scholar]
- Price, B.B.; Amini, S.B.; Kappeler, K. Exercise in pregnancy effect on fitness and obstetric outcomes. Aust. Fam. Physician 2014, 43, 541–542. [Google Scholar]
- Kramer, M.S. Aerobic Exercise for Women During Pregnancy. Birth 2003, 30, 278. [Google Scholar] [CrossRef]
- Davenport, M.H.; Meah, V.L.; Ruchat, S.M.; Davies, G.A.; Skow, R.J.; Barrowman, N.; Adamo, K.B.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; et al. Impact of prenatal exercise on neonatal and childhood outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.G.; Ricardo, L.I.; Evenson, K.R.; Hallal, P.C. Leisure-Time Physical Activity in Pregnancy and Maternal-Child Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Cohort Studies. Sport. Med. 2017, 47, 295–317. [Google Scholar] [CrossRef] [PubMed]
- ACOG Committee Opinion Physical Activity and Exercice During Pregnancy and the Postpartum Period. Obstet Gynecol. 2016, 388, 1969–1970.
- Dye, T.D.; Knox, K.L.; Artal, R.; Aubry, R.H.; Wojtowycz, M.A. Physical activity, obesity, and diabetes in pregnancy. Am. J. Epidemiol. 1997, 146, 961–965. [Google Scholar] [CrossRef]
- Artal, R. The role of exercise in reducing the risks of gestational diabetes mellitus in obese women. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 123–132. [Google Scholar] [CrossRef]
- Dipietro, L.; Evenson, K.R.; Bloodgood, B.; Sprow, K.; Troiano, R.P.; Piercy, K.L.; Vaux-Bjerke, A.; Powell, K.E. Benefits of Physical Activity during Pregnancy and Postpartum: An Umbrella Review. Med. Sci. Sports Exerc. 2019, 51, 1292–1302. [Google Scholar] [CrossRef]
- Wiebe, H.W.; Boulé, N.G.; Chari, R.; Davenport, M.H. The effect of supervised prenatal exercise on fetal growth. Obstet. Gynecol. 2015, 125, 1185–1194. [Google Scholar] [CrossRef]
- Aune, D.; Saugstad, O.D.; Henriksen, T.; Tonstad, S. Physical activity and the risk of preeclampsia: A systematic review and meta-analysis. Epidemiology 2014, 25, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Takami, M.; Tsuchida, A.; Takamori, A.; Aoki, S.; Ito, M.; Kigawa, M.; Kawakami, C.; Hirahara, F.; Hamazaki, K.; Inadera, H.; et al. Effects of physical activity during pregnancy on preterm delivery and mode of delivery: The Japan Environment and Children’s Study, birth cohort study. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.M.; Sobierajski, F.; Poitras, V.J.; Gray, C.E.; Yoo, C.; Skow, R.J.; Jaramillo Garcia, A.; Barrowman, N.; Meah, V.L.; et al. Impact of prenatal exercise on maternal harms, labour and delivery outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Walasik, I.; Kwiatkowska, K.; Szymusik, I. Physical Activity Patterns among 9000 Pregnant Women in Poland: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 1771. [Google Scholar] [CrossRef] [PubMed]
- de Oliveria Melo, A.S.; Silva, J.L.P.; Tavares, J.S.; Barros, V.O.; Leite, D.F.B.; Amorim, M.M.R. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: A randomized controlled trial. Obstet. Gynecol. 2012, 120, 302–310. [Google Scholar] [CrossRef]
- Wang, S.M.; Dezinno, P.; Maranets, I.; Berman, M.R.; Caldwell-Andrews, A.A.; Kain, Z.N. Low back pain during pregnancy: Prevalence, risk factors, and outcomes. Obstet. Gynecol. 2004, 104, 65–70. [Google Scholar] [CrossRef]
- Gobierno Vasco. Guía del embarazo, preconcepción, parto y puerperio saludable; Basque Government: Vitoria-Gasteiz, Spain, 2017; p. 48.
- Sinclair, I.; St-Pierre, M.; Elgbeili, G.; Bernard, P.; Vaillancourt, C.; Gagnon, S.; Dancause, K.N. Psychosocial stress, sedentary behavior, and physical activity during pregnancy among canadian women: Relationships in a diverse cohort and a nationwide sample. Int. J. Environ. Res. Public Health 2019, 16, 5150. [Google Scholar] [CrossRef]
- Schlüssel, M.M.; De Souza, E.B.; Reichenheim, M.E.; Kac, G. Physical activity during pregnancy and maternal-child health outcomes: A systematic literature review. Cad. Saude Publica 2008, 24, 531–544. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Arena, R.; Riebe, D.; Thompson, P.D. ACSM’s Guidelines for Exercise Testing and Prescription, Ninth Edition; American College of Sports Medicine: Baltimore, MD, USA, 2014; ISBN 9781609136055. [Google Scholar]
- Evenson, K.R.; Barakat, R.; Brown, W.J.; Dargent-Molina, P.; Haruna, M.; Mikkelsen, E.M.; Mottola, M.F.; Owe, K.M.; Rousham, E.K.; Yeo, S.A. Guidelines for Physical Activity During Pregnancy: Comparisons From Around the World. Am. J. Lifestyle Med. 2014, 8, 102–121. [Google Scholar] [CrossRef]
- Pate, R.R.; Pratt, M.; Blair, S.N.; Haskell, W.L.; Macera, C.A.; Bouchard, C.; Buchner, D.; Ettinger, W.; Heath, G.W.; King, A.C.; et al. Public Health and Prevention and the American College of Sports Medicine. J. Am. Med. Assoc. 1995, 273, 402–407. [Google Scholar] [CrossRef]
- Physical Activity Guidelines Advisory Committee. Chapter 1. Physical Activity Behaviors: Steps, Bouts, and High Intensity Training. In Supplementary Material for the 2018 Physical Activity Guidelines Advisory Committee Scientific Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2018; pp. 1–26. [Google Scholar]
- Ekelund, U.; Tarp, J.; Steene-Johannessen, J.; Hansen, B.H.; Jefferis, B.; Fagerland, M.W.; Whincup, P.; Diaz, K.M.; Hooker, S.P.; Chernofsky, A.; et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ 2019, 366, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saint-Maurice, P.F.; Troiano, R.P.; Matthews, C.E.; Kraus, W.E. Moderate-to-Vigorous Physical Activity and All-Cause Mortality: Do Bouts Matter? J. Am. Heart Assoc. 2018, 7, 2003–2006. [Google Scholar] [CrossRef]
- Borodulin, K.M.; Evenson, K.R.; Wen, F.; Herring, A.H.; Benson, A.M. Physical activity patterns during pregnancy. Med. Sci. Sports Exerc. 2008, 40, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Downs, D.S.; Devlin, C.A.; Rhodes, R.E. The power of believing: Salient belief predictors of exercise behavior in normal weight, overweight, and obese pregnant women. J. Phys. Act. Heal. 2015, 12, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Evenson, K.R.; Savitz, D.A.; Huston, S.L. Leisure-time physical activity among pregnant women in the US. Paediatr. Perinat. Epidemiol. 2004, 18, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Rifas-Shiman, S.L.; Kleinman, K.P.; Rich-Edwards, J.W.; Peterson, K.E.; Gillman, M.W. Predictors of Change in Physical Activity During and After Pregnancy. Project Viva. Am. J. Prev. Med. 2007, 32, 312–319. [Google Scholar] [CrossRef]
- Nascimento, S.L.; Surita, F.G.; Godoy, A.C.; Kasawara, K.T.; Morais, S.S. Physical activity patterns and factors related to exercise during pregnancy: A cross sectional study. PLoS ONE 2015, 10, 1–14. [Google Scholar]
- Rousham, E.K.; Clarke, P.E.; Gross, H. Significant changes in physical activity among pregnant women in the UK as assessed by accelerometry and self-reported activity. Eur. J. Clin. Nutr. 2006, 60, 393–400. [Google Scholar] [CrossRef]
- Petersen, A.M.; Leet, T.L.; Brownson, R.C. Correlates of physical activity among pregnant women in the United States. Med. Sci. Sports Exerc. 2005, 37, 1748–1753. [Google Scholar] [CrossRef]
- Perales, M.; Santos-Lozano, A.; Ruiz, J.R.; Lucia, A.; Barakat, R. Benefits of aerobic or resistance training during pregnancy on maternal health and perinatal outcomes: A systematic review. Early Hum. Dev. 2016, 94, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Baena-García, L.; Ocón-Hernández, O.; Acosta-Manzano, P.; Coll-Risco, I.; Borges-Cosic, M.; Romero-Gallardo, L.; de la Flor-Alemany, M.; Aparicio, V.A. Association of sedentary time and physical activity during pregnancy with maternal and neonatal birth outcomes. The GESTAFIT Project. Scand. J. Med. Sci. Sport. 2019, 29, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, J.; Terzic-Supic, Z.; Bjegovic-Mikanovic, V.; Piperac, P.; Dugalic, S.; Gojnic-Dugalic, M. Factors associated with the leisure-time physical activity (LTPA) during the first trimester of the pregnancy: The cross-sectional study among pregnant women in Serbia. Int. J. Environ. Res. Public Health 2020, 17, 1366. [Google Scholar] [CrossRef] [PubMed]
- Sytsma, T.T.; Zimmerman, K.P.; Manning, J.B.; Jenkins, S.M.; Nelson, N.C.; Clark, M.M.; Boldt, K.; Borowski, K.S. Perceived Barriers to Exercise in the First Trimester of Pregnancy. J. Perinat. Educ. 2018, 27, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Fell, D.B.; Joseph, K.S.; Armson, B.A.; Dodds, L. The impact of pregnancy on physical activity level. Matern. Child Health J. 2009, 13, 597–603. [Google Scholar] [CrossRef]
- Ning, Y.; Williams, M.A.; Dempsey, J.C.; Sorensen, T.K.; Frederick, I.O.; Luthy, D.A. Correlates of recreational physical activity in early pregnancy. J. Matern. Neonatal Med. 2003, 13, 385–393. [Google Scholar] [CrossRef]
- Rutkowska, E.; Łepecka-Klusek, C. The role of physical activity in preparing women for pregnancy and delivery in Poland. Health Care Women Int. 2002, 23, 919–923. [Google Scholar] [CrossRef]
- Mudd, L.M.; Nechuta, S.; Pivarnik, J.M.; Paneth, N. Factors associated with women’s perceptions of physical activity safety during pregnancy. Prev. Med. (Baltim) 2009, 49, 194–199. [Google Scholar] [CrossRef]
- Zhang, J.; Savitz, D.A. Exercise during pregnancy among US women. Ann. Epidemiol. 1996, 6, 53–59. [Google Scholar] [CrossRef]
- Gaston, A.; Cramp, A. Exercise during pregnancy: A review of patterns and determinants. J. Sci. Med. Sport 2011, 14, 299–305. [Google Scholar] [CrossRef]
- Watson, P.E.; McDonald, B.W. Activity levels in pregnant New Zealand women: Relationship with socioeconomic factors, well-being, anthropometric measures, and birth outcome. Appl. Physiol. Nutr. Metab. 2007, 32, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Chasan-Taber, L.; Schmidt, M.D.; Pekow, P.; Sternfeld, B.; Manson, J.A.; Markenson, G. Correlates of physical activity in pregnancy among latina women. Matern. Child Health J. 2007, 11, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Horns, P.N.; Ratcliffe, L.P.; Leggett, J.C.; Swanson, M.S. Pregnancy outcomes among active and sedentary primiparous women. J. Obstet. Gynecol. Neonatal Nurs. 1996, 25, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Vuillemin, A. Limits to the measurement of habitual physical activity by questionnaires: Commentary. Br. J. Sports Med. 2003, 37, 206. [Google Scholar]
- Sylvia, L.G.; Bernstein, E.E.; Hubbard, J.L.; Keating, L.; Anderson, E.J. Practical guide to measuring physical activity. J. Acad. Nutr. Diet. 2014, 114, 199–208. [Google Scholar] [CrossRef]
- Conway, M.R.; Marshall, M.R.; Schlaff, R.A.; Pfeiffer, K.A.; Pivarnik, J.M. Physical Activity Device Reliability and Validity during Pregnancy and Postpartum. Med. Sci. Sports Exerc. 2018, 50, 617–623. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Delisle Nyström, C.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sport. Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef]
- Skender, S.; Ose, J.; Chang-Claude, J.; Paskow, M.; Brühmann, B.; Siegel, E.M.; Steindorf, K.; Ulrich, C.M. Accelerometry and physical activity questionnaires - A systematic review. BMC Public Health 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Colley, R.C.; Butler, G.; Garriguet, D.; Prince, S.A.; Roberts, K.C. Comparison of self-reported and accelerometer-measured physical activity among Canadian youth. Heal. Reports 2019, 30, 3–12. [Google Scholar]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Connor Gorber, S.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5. [Google Scholar] [CrossRef]
- Metcalf, K.M.; Baquero, B.I.; Coronado Garcia, M.L.; Francis, S.L.; Janz, K.F.; Laroche, H.H.; Sewell, D.K. Calibration of the global physical activity questionnaire to Accelerometry measured physical activity and sedentary behavior. BMC Public Health 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sport. Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Guxens, M.; Ballester, F.; Espada, M.; Fernández, M.F.; Grimalt, J.O.; Ibarluzea, J.; Olea, N.; Rebagliato, M.; Tardón, A.; Torrent, M.; et al. Cohort profile: The INMA-INfancia y Medio Ambiente-(environment and childhood) project. Int. J. Epidemiol. 2012, 41, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Falissard, B. psy: Various Procedures Used in Psychometry. In R Package Version 1.1; 2012; Available online: https://rdrr.io/cran/psy/ (accessed on 16 December 2019).
- Shrout, P.E.; Fleiss, J.L. Intraclass correlation: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.C.; Hinlkey, D. V Bootstrap Methods and Their Applications; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Devore, J.L.; Berk, N.K. Modern Mathematical Statistics with Applications; Springer: New York, NY, USA, 2012. [Google Scholar]
- Dunnet, C.W. A Multiple Comparison Procedure for Comparing Several Treatments with a Control. J. Am. Stat. Assoc. 1955, 50, 1096–1121. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing, Version 3.6.1.; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Marquet, O.; Miralles-Guasch, C. Walking short distances. The socioeconomic drivers for the use of proximity in everyday mobility in Barcelona. Transp. Res. Part A Policy Pract. 2014, 70, 210–222. [Google Scholar] [CrossRef]
- Hesketh, K.R.; Evenson, K.R.; Stroo, M.; Clancy, S.M.; Østbye, T.; Benjamin-Neelon, S.E. Physical activity and sedentary behavior during pregnancy and postpartum, measured using hip and wrist-worn accelerometers. Prev. Med. Rep. 2018, 10, 337–345. [Google Scholar] [CrossRef]

| Variable | N | N* | Mean | SE Mean | SD | Min. | Max. | Q1 | Median | Q3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Trimester | ||||||||||
| SLMPA | 314 | 25 | 875.9 | 14.2 | 252.3 | 0 | 1110 | 190 | 330 | 525 |
| OLMPA | 339 | 0 | 2239.5 | 34.1 | 604.1 | 0 | 3710 | 390 | 840 | 1200 |
| SMVPA | 315 | 24 | 348.7 | 8.2 | 151.6 | 28. | 871.5 | 168 | 253.7 | 374.5 |
| OMVPA | 339 | 0 | 278.7 | 31.9 | 588 | 962.5 | 4214 | 1804.6 | 2202 | 2622 |
| 2nd Trimester | ||||||||||
| SLMPA | 158 | 195 | 975.5 | 49.8 | 626.2 | 0 | 4080 | 528.8 | 900 | 1287.5 |
| OLMPA | 166 | 187 | 2242.9 | 50.4 | 649.3 | 938 | 6501.3 | 1830.4 | 2167.8 | 2575.3 |
| SMVPA | 158 | 195 | 425.9 | 20.5 | 258 | 0 | 1110 | 210 | 390 | 620 |
| OMVPA | 166 | 187 | 295.2 | 13.1 | 169.1 | 35 | 1172 | 180.5 | 266 | 384.4 |
| Pair of Variables | N | Mean | SD | SE | Mean Difference | SD* | SE* | 95% C.I. | t-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1TSLMPA | 140 | 871.8 | 566 | 47.8 | −97.6 | 526.3 | 44.5 | (−185.6, −9.7) | −2.20 | 0.030 |
| 2TSLMPA | 140 | 969.5 | 629.1 | 53.2 | ||||||
| 1TOLMPA | 151 | 2226.2 | 558 | 45.4 | 6.9 | 135.1 | 11 | (−14.8, 28.6) | 0.63 | 0.530 |
| 2TOLMPA | 151 | 2238 | 555.5 | 45.2 | ||||||
| 1TSMVPA | 140 | 393.8 | 261.7 | 22.1 | −31.6 | 204.2 | 17.3 | (−65.8, 2.5) | −1.83 | 0.069 |
| 2TSMVPA | 140 | 425.4 | 263.1 | 22.2 | ||||||
| 1TOMVPA | 151 | 304.9 | 155.9 | 12.7 | −11.8 | 393.9 | 32.1 | (−75.2, 51.5) | −0.37 | 0.713 |
| 2TOMVPA | 151 | 298 | 164.7 | 13.4 |
| 1st Trimester of Pregnancy | 2nd Trimester of Pregnancy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coef. | 95% C.I. Coef. | p−Value | Contribution to R 2 | Coef. | 95% C.I. Coef. | p−vValue | Contribution to R 2 | ||
| Response variable SLMPA | Response variable SLMPA | ||||||||
| Constant | 462 | (137, 786) | 0.005 | Constant | 308 | (−37, 653) | 0.080 | ||
| Number of children | 209.70 | (120.5, 298.80) | <0.001 | 8.69% | TV Habit | 17,72 | (5.75, 29.69) | 0.004 | 1.72% |
| Preference for exercise | 101.20 | (42.9, 159.40) | 0.001 | 3.12% | Preference for exercise | 142,20 | (43.50, 240.90) | 0.005 | 8.34% |
| Car ownership | −105.50 | (−207.7, −3.30) | 0.043 | 2.44% | |||||
| Work Situation: Employed and Active | REF | 5.64% | Work Situation: Employed and Active | REF | 19.20% | ||||
| Unemployed | 229 | (−23, 482) | 0.075 | Unemployed | −300 | (−799, 199) | 0.236 | ||
| Student | 796 | (26, 1566) | 0.043 | - | |||||
| Domestic work only | 634 | (318, 950) | <0.001 | Domestic work only | 1605 | (1052, 2157) | <0.001 | ||
| Employed on maternal leave | 107 | (−180, 395) | 0.463 | Employed on maternal leave | 119 | (−298, 536) | 0.573 | ||
| Total explained variance | 19.89% | Total explained variance | 29.26% | ||||||
| Response variable OLMPA | Response variable OLMPA | ||||||||
| Constant | 2524 | (2097, 2951) | <0.001 | Constant | 1788 | (1587, 1989) | <0.001 | ||
| Number of children | 252.40 | (166.6, 338.10) | <0.001 | 13.84% | Number of children | 295.10 | (175.30, 414.80) | <0.001 | 13.81% |
| TV habit | −9.70 | (−16.18, −3.22) | 0.003 | 1.55% | |||||
| Education level | −170 | (−267.8, −72.20) | 0.001 | 3.08% | |||||
| Total explained variance | 18.47% | Total explained variance | 13.81% | ||||||
| Response variable SMVPA | Response variable SMVPA | ||||||||
| Constant | 147.10 | (28.80, 265.40) | 0.015 | Constant | 216.70 | (58.90, 374.40) | 0.007 | ||
| Preference for exercise | 107.70 | (83.90, 131.40) | <0.001 | 19.88% | Preference for exercise | 103.50 | (61.90, 145.20) | <0.001 | 15.17% |
| Car ownership | −44.00 | (−84.30, −3.70) | 0.032 | 1.18% | |||||
| Number of children | −84.40 | (−143.90, −24.80) | 0.006 | 2.54% | |||||
| Work Situation | 4.60% | ||||||||
| Employed and Active | REF | ||||||||
| Unemployed | 57.40 | (−125.70, 240.50) | 0.536 | ||||||
| Domestic work only | 168 | (−78, 413) | 0.179 | ||||||
| Employed on maternal leave | 220.60 | (46.80, 394.50) | 0.013 | ||||||
| Total explained variance | 21.06% | Total explained variance | 22.31% | ||||||
| Response variable OMVPA | Response variable OMVPA | ||||||||
| Constant | 149.70 | (57.8, 241.60) | 0.001 | Constant | 99.70 | (−23.20, 222.60) | 0.111 | ||
| Preference for walking | 57.79 | (40.12, 75.47) | <0.001 | 11.72% | Preference for walking | 48.10 | (15.40, 80.80) | 0.004 | 4.64% |
| Car ownership | −29.10 | (−55.1, −3.20) | 0.028 | 1.06% | Civil status | 5.64% | |||
| Work Situation: Employed and Active | REF | 2.61% | Married (REF) | REF | |||||
| Unemployed | −1.20 | (−65.1, 62.80) | 0.971 | Living with a partner | 57 | (−4.6, 118.5) | 0.069 | ||
| Student | 44.30 | (−118.6, 207.20) | 0.593 | Divorced or separated | −17 | (−337, 303) | 0.916 | ||
| Domestic work only | −55.40 | (−131.4, 20.50) | 0.152 | Single | 118.5 | (29.5, 207.4) | 0.009 | ||
| Employed on maternal leave | 95 | (22.80, 167.30) | 0.01 | ||||||
| Total explained variance | 15.39% | Total explained variance | 10.28% | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendinueta, A.; Esnal, H.; Arrieta, H.; Arrue, M.; Urbieta, N.; Ubillos, I.; Whitworth, K.W.; Delclòs-Alió, X.; Vich, G.; Ibarluzea, J. What Accounts for Physical Activity during Pregnancy? A Study on the Sociodemographic Predictors of Self-Reported and Objectively Assessed Physical Activity during the 1st and 2nd Trimesters of Pregnancy. Int. J. Environ. Res. Public Health 2020, 17, 2517. https://doi.org/10.3390/ijerph17072517
Mendinueta A, Esnal H, Arrieta H, Arrue M, Urbieta N, Ubillos I, Whitworth KW, Delclòs-Alió X, Vich G, Ibarluzea J. What Accounts for Physical Activity during Pregnancy? A Study on the Sociodemographic Predictors of Self-Reported and Objectively Assessed Physical Activity during the 1st and 2nd Trimesters of Pregnancy. International Journal of Environmental Research and Public Health. 2020; 17(7):2517. https://doi.org/10.3390/ijerph17072517
Chicago/Turabian StyleMendinueta, Ana, Haritz Esnal, Haritz Arrieta, Miren Arrue, Nerea Urbieta, Itziar Ubillos, Kristina W. Whitworth, Xavier Delclòs-Alió, Guillem Vich, and Jesus Ibarluzea. 2020. "What Accounts for Physical Activity during Pregnancy? A Study on the Sociodemographic Predictors of Self-Reported and Objectively Assessed Physical Activity during the 1st and 2nd Trimesters of Pregnancy" International Journal of Environmental Research and Public Health 17, no. 7: 2517. https://doi.org/10.3390/ijerph17072517
APA StyleMendinueta, A., Esnal, H., Arrieta, H., Arrue, M., Urbieta, N., Ubillos, I., Whitworth, K. W., Delclòs-Alió, X., Vich, G., & Ibarluzea, J. (2020). What Accounts for Physical Activity during Pregnancy? A Study on the Sociodemographic Predictors of Self-Reported and Objectively Assessed Physical Activity during the 1st and 2nd Trimesters of Pregnancy. International Journal of Environmental Research and Public Health, 17(7), 2517. https://doi.org/10.3390/ijerph17072517







