Impact of Climate Variability and Abundance of Mosquitoes on Dengue Transmission in Central Vietnam
Abstract
:1. Introduction
2. Materials and Methods
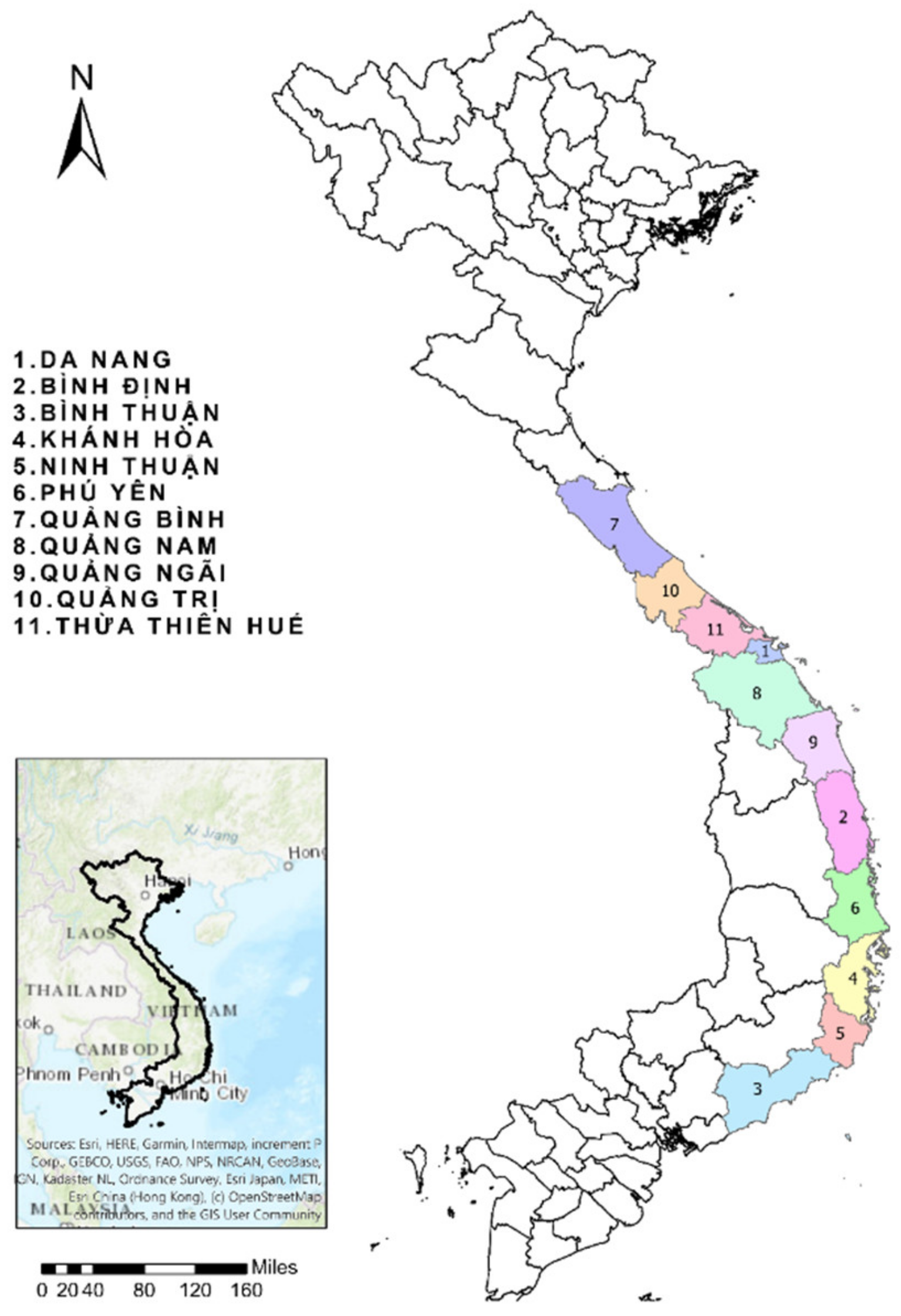
2.1. Study Area
2.2. Dengue Case Data
2.3. Mosquito Data
2.4. Climate Data
2.5. Statistical Analysis
3. Results
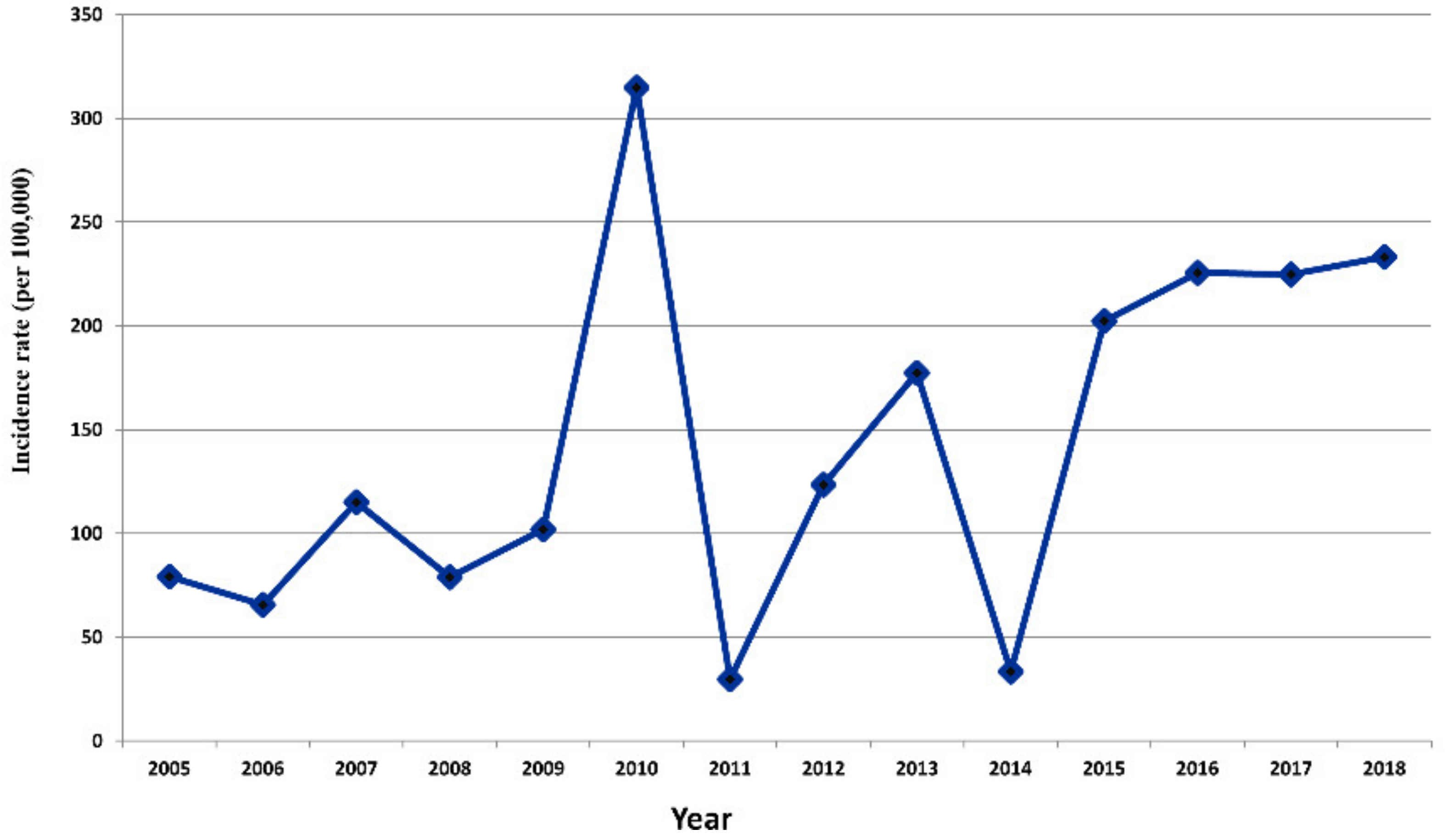
3.1. Dengue Transmission in Central Vietnam
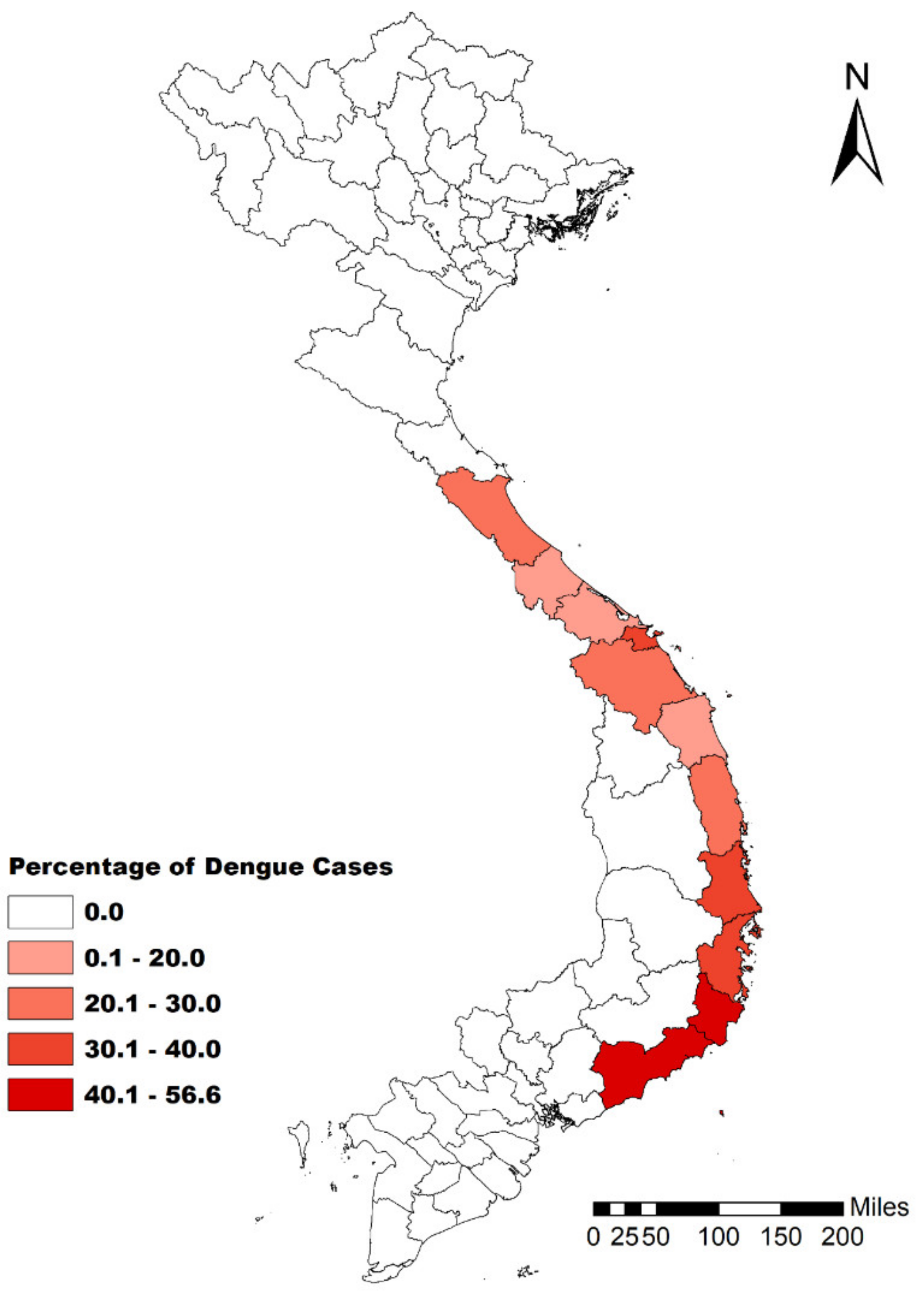
3.2. Spatiotemporal Patterns of Dengue Transmission in Central Vietnam
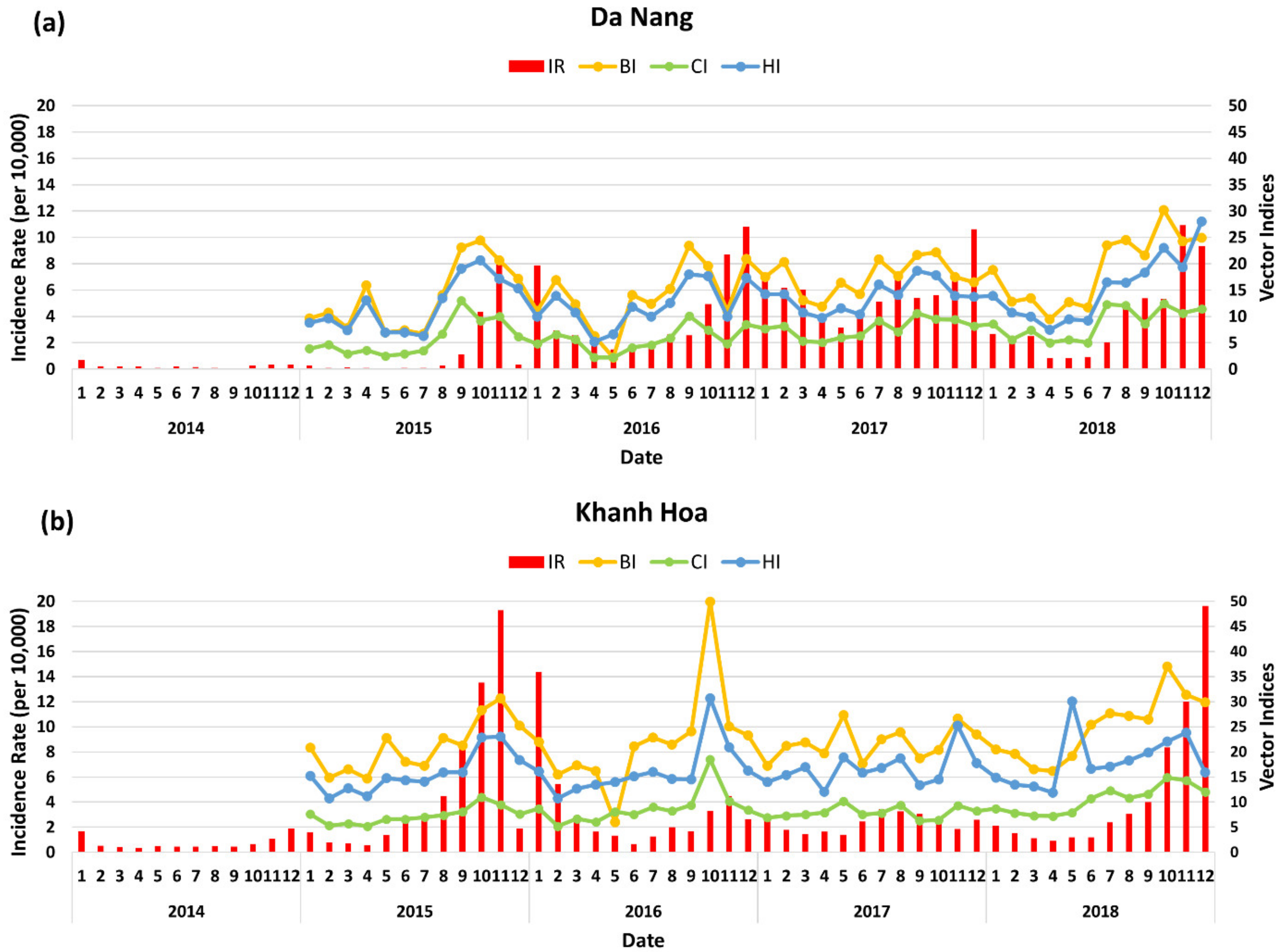
3.3. Dengue Transmission in Da Nang and Khah Hoa
3.4. Influence of Climate Variation and Vector Index on Dengue Transmission in Da Nang and Khanh Hoa
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Month | Da Nang | Khanh Hoa | ||||||
|---|---|---|---|---|---|---|---|---|
| DTM | NTM | MTM | Rainfall | DTM | NTM | MTM | Rainfall | |
| Jan | 23.15 | 16.44 | 20.17 | 16.69 | 24.57 | 17.27 | 20.87 | 5.99 |
| Feb | 25.83 | 17.39 | 21.6 | 5.98 | 26.37 | 18.06 | 22.15 | 4.15 |
| Mar | 28.44 | 20.8 | 24.71 | 11.23 | 29.53 | 20.13 | 24.97 | 4.33 |
| Apr | 31.08 | 22.01 | 26.45 | 6.66 | 31.46 | 22.14 | 26.81 | 1.51 |
| May | 31.47 | 23.34 | 27.57 | 20.95 | 31.6 | 22.71 | 27.14 | 6.58 |
| Jun | 31.57 | 24.16 | 28.21 | 23.82 | 31.06 | 22.84 | 27.04 | 0.66 |
| Jul | 29.73 | 23.54 | 27.15 | 14.34 | 30.9 | 22.72 | 26.59 | 4.48 |
| Aug | 30.76 | 23.1 | 26.66 | 36.36 | 31.18 | 22.26 | 26.31 | 3.08 |
| Sep | 29.17 | 22.37 | 25.74 | 39.45 | 29.58 | 21.45 | 25.48 | 24.99 |
| Oct | 26.22 | 20.59 | 23.7 | 114.96 | 27.85 | 19.9 | 23.78 | 94.93 |
| Nov | 25.71 | 19.32 | 22.62 | 268.54 | 26.19 | 19.54 | 23.3 | 71.63 |
| Dec | 24 | 14.93 | 20.51 | 39.34 | 24.47 | 17.25 | 20.9 | 9.45 |
References
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- World Health Organization. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever; WHO Regional Publication for South-Asia: New Delhi, India, 2011; pp. 159–168. [Google Scholar]
- Simmons, C.P.; Farrar, J.J.; Nguyen, V.V.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Murota, K.; Fujita, R.; Itokawa, K.; Kotaki, A.; Moi, M.L.; Ejiri, H.; Maekawa, Y.; Ogawa, K.; Tsuda, Y.; et al. Dengue virus infection in Aedes albopictus during the 2014 autochthonous dengue outbreak in Tokyo metropolis, Japan. Am. J. Trop. Med. Hyg. 2018, 98, 1460–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Halstead, S.B. Dengue antibody-dependent enhancement: Knowns and unknowns. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, M.G.; Alvarez, M.; Halstead, S.B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013, 158, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gaytan, S.I.; Diaz-Vasquez, F.J.; Duran-Arenas, L.G.; Lopez Cervantes, M.; Rothenberg, S.J. 20 Years spatial-temporal analysis of dengue fever and hemorrhagic fever in Mexico. Arch. Med. Res. 2017, 48, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Chowell, G.; Torre, C.A.; Munayco-Escate, C.; Suarez-Ognio, L.; Lopez-Cruz, R.; Hyman, J.M.; Castillo-Chavez, C. Spatial and temporal dynamics of dengue fever in Peru: 1994–2006. Epidemiol. Infect. 2008, 136, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.W.; Comrie, A.C.; Ernst, K. Climate and dengue transmission: Evidence and implications. Environ. Health Perspect. 2013, 121, 1264–1272. [Google Scholar] [CrossRef]
- Li, C.F.; Lim, T.W.; Han, L.L.; Fang, R. Rainfall, abundance of Aedes aegypti and dengue infection in Selangor, Malaysia. Southeast Asian J. Trop. Med. Public Health 1985, 16, 560–568. [Google Scholar]
- Paaijmans, K.P.; Wandago, M.O.; Githeko, A.K.; Takken, W. Unexpected high losses of Anopheles gambiae larvae due to rainfall. PLoS ONE 2007, 2, e1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedum, C.M.; Seidahmed, O.M.E.; Eltahir, E.A.B.; Markuzon, N. Statistical modeling of the effect of rainfall flushing on dengue transmission in Singapore. PLoS Negl. Trop. Dis. 2018, 12, e0006935. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, N.B.; Thomas, S.M.; Fischer, D.; Beierkuhnlein, C. Extrinsic incubation period of dengue: Knowledge, backlog, and applications of temperature dependence. PLoS Negl. Trop. Dis. 2013, 7, e2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, Z.; Lai, Z.; Zhou, T.; Jia, Z.; Gu, J.; Wu, K.; Chen, X.G. Temperature increase enhances Aedes albopictus competence to transmit dengue virus. Front. Microbiol. 2017, 8, 2337. [Google Scholar] [CrossRef] [Green Version]
- Alto, B.W.; Juliano, S.A. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): Implications for range expansion. J. Med. Entomol. 2001, 38, 646–656. [Google Scholar] [CrossRef] [Green Version]
- Patz, J.A.; Epstein, P.R.; Burke, T.A.; Balbus, J.M. Global climate change and emerging infectious diseases. JAMA 1996, 275, 217–223. [Google Scholar] [CrossRef]
- Anyamba, A.; Chretien, J.P.; Britch, S.C.; Soebiyanto, R.P.; Small, J.L.; Jepsen, R.; Forshey, B.M.; Sanchez, J.L.; Smith, R.D.; Harris, R.; et al. Global disease outbreaks associated with the 2015–2016 El Nino event. Sci. Rep. 2019, 9, 1930. [Google Scholar] [CrossRef] [Green Version]
- Chaves, L.F.; Satake, A.; Hashizume, M.; Minakawa, N. Indian Ocean dipole and rainfall drive a Moran effect in East Africa malaria transmission. J. Infect. Dis. 2012, 205, 1885–1891. [Google Scholar] [CrossRef] [Green Version]
- Sajid, N.H.; Yamada, T. Possible impacts of Indian Ocean Dipole modeevents on global climate. Clim. Res. 2003, 25, 151–169. [Google Scholar] [CrossRef]
- Saji, N.H.; Goswami, B.N.; Vinayachandran, P.N.; Yamagata, T. A dipole mode in the tropical Indian Ocean. Nature 1999, 401, 360–363. [Google Scholar] [CrossRef]
- Johansson, M.A.; Cummings, D.A.; Glass, G.E. Multiyear climate variability and dengue—El Nino southern oscillation, weather, and dengue incidence in Puerto Rico, Mexico, and Thailand: A longitudinal data analysis. PLoS Med. 2009, 6, e1000168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, D.O.; Troyo, A.; Beier, J.C. El Nino Southern Oscillation and vegetation dynamics as predictors of dengue fever cases in Costa Rica. Environ. Res. Lett. 2009, 4, 140111–140118. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.W.; Chaves, L.F.; Chen, P.J. Effects of local and regional climatic fluctuations on dengue outbreaks in southern Taiwan. PLoS ONE 2017, 12, e0178698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu-Helmersson, J.; Brannstrom, A.; Sewe, M.O.; Semenza, J.C.; Rocklov, J. Estimating past, present, and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front. Public Health 2019, 7, 148. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, L.; Vanlerberghe, V.; Alfonso, L.; Marquetti Mdel, C.; Guzman, M.G.; Bisset, J.; van der Stuyft, P. Aedes aegypti larval indices and risk for dengue epidemics. Emerg. Infect. Dis. 2006, 12, 800–806. [Google Scholar] [CrossRef] [Green Version]
- Chadee, D.D. Dengue cases and Aedes aegypti indices in Trinidad, West Indies. Acta Trop. 2009, 112, 174–180. [Google Scholar] [CrossRef]
- Sulaiman, S.; Pawanchee, Z.A.; Arifin, Z.; Wahab, A. Relationship between Breteau and House indices and cases of dengue/dengue hemorrhagic fever in Kuala Lumpur, Malaysia. J. Am. Mosq. Control Assoc. 1996, 12, 494–496. [Google Scholar]
- Arboleda, S.; Jaramillo, O.N.; Peterson, A.T. Spatial and temporal dynamics of Aedes aegypti larval sites in Bello, Colombia. J. Vector Ecol. 2012, 37, 37–48. [Google Scholar] [CrossRef]
- Sivagnaname, N.; Gunasekaran, K. Need for an efficient adult trap for the surveillance of dengue vectors. Indian J. Med. Res. 2012, 136, 739–749. [Google Scholar]
- Ooi, E.E.; Goh, K.T.; Gubler, D.J. Dengue prevention and 35 years of vector control in Singapore. Emerg. Infect. Dis. 2006, 12, 887–893. [Google Scholar] [CrossRef]
- Rawlins, S.C.; Martinez, R.; Wiltshire, S.; Legall, G. A comparison of surveillance systems for the dengue vector Aedes aegypti in Port of Spain, Trinidad. J. Am. Mosq. Control Assoc. 1998, 14, 131–136. [Google Scholar] [PubMed]
- Cuong, H.Q.; Vu, N.T.; Cazelles, B.; Boni, M.F.; Thai, K.T.; Rabaa, M.A.; Quang, L.C.; Simmons, C.P.; Huu, T.N.; Anders, K.L. Spatiotemporal dynamics of dengue epidemics, southern Vietnam. Emerg. Infect. Dis. 2013, 19, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Anders, K.L.; Nguyet, N.M.; Chau, N.V.; Hung, N.T.; Thuy, T.T.; Lien le, B.; Farrar, J.; Wills, B.; Hien, T.T.; Simmons, C.P. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 2011, 84, 127–134. [Google Scholar] [CrossRef]
- Wartel, T.A.; Prayitno, A.; Hadinegoro, S.R.; Capeding, M.R.; Thisyakorn, U.; Tran, N.H.; Moureau, A.; Bouckenooghe, A.; Nealon, J.; Taurel, A.F. Three decades of dengue surveillance in five highly endemic South East Asian countries. Asia Pac. J. Public Health 2017, 29, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Cuong, H.Q.; Hien, N.T.; Duong, T.N.; Phong, T.V.; Cam, N.N.; Farrar, J.; Nam, V.S.; Thai, K.T.; Horby, P. Quantifying the emergence of dengue in Hanoi, Vietnam: 1998–2009. PLoS Negl. Trop. Dis. 2011, 5, e1322. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Nguyen-Viet, H.; Nam, V.S.; Lee, M.; Won, S.; Duc, P.P.; Grace, D. Seasonal patterns of dengue fever and associated climate factors in 4 provinces in Vietnam from 1994 to 2013. BMC Infect. Dis. 2017, 17, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, H.H.; Okumura, J.; Hashizume, M.; Tran, D.N.; Yamamoto, T. Regional differences in the growing incidence of dengue fever in Vietnam explained by weather variability. Trop. Med. Health 2014, 42, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Quyen, D.L.; Thanh Le, N.; Van Anh, C.T.; Nguyen, N.B.; Hoang, D.V.; Montgomery, J.L.; Kutcher, S.C.; Hoang Le, N.; Hien, N.T.; Hue Kien, D.T.; et al. Epidemiological, serological, and virological features of dengue in Nha Trang City, Vietnam. Am. J. Trop. Med. Hyg. 2018, 98, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Mai, V.Q.; Mai, T.T.X.; Tam, N.L.M.; Nghia, L.T.; Komada, K.; Murakami, H. Prevalence and risk factors of dengue infection in Khanh Hoa Province, Vietnam: A stratified cluster sampling survey. J. Epidemiol. 2018, 28, 488–497. [Google Scholar] [CrossRef] [Green Version]
- Thao, N.D.; Takagi, H.; Esteban, M. Coastal Disasters and Climate Change in Vietnam; Elsevier: Amsterdam, Netherlands, 2014; pp. 175–198. [Google Scholar]
- Vietnam Ministry of Health. Guidance on Monitoring, Prevention, and Control of Dengue; Vietnam Ministry of Health: Hanoi, Vietnam, 2014.
- Chuang, T.W.; Henebry, G.M.; Kimball, J.S.; Vanroekel-Patton, D.L.; Hildreth, M.B.; Wimberly, M.C. Satellite microwave remote sensing for environmental modeling of mosquito population dynamics. Remote Sens. Environ. 2012, 125, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Rogers, D.J.; Randolph, S.E.; Snow, R.W.; Hay, S.I. Satellite imagery in the study and forecast of malaria. Nature 2002, 415, 710–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, T.W.; Wimberly, M.C. Remote sensing of climatic anomalies and West Nile virus incidence in the northern Great Plains of the United States. PLoS ONE 2012, 7, e46882. [Google Scholar] [CrossRef] [PubMed]
- Wolter, K.; Timlin, M.S. Monitoring ENSO in COADS with a seasonally adjusted principal component index. In Proceedings of the 17th Climate Diagnostics Workshop, Norman, OK, USA, 18–23 October 1992; pp. 52–57. [Google Scholar]
- Chang, A.Y.; Parrales, M.E.; Jimenez, J.; Sobieszczyk, M.E.; Hammer, S.M.; Copenhaver, D.J.; Kulkarni, R.P. Combining Google Earth and GIS mapping technologies in a dengue surveillance system for developing countries. Int. J. Health Geogr. 2009, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisen, L.; Eisen, R.J. Using geographic information systems and decision support systems for the prediction, prevention, and control of vector-borne diseases. Annu. Rev. Entomol. 2011, 56, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Rotela, C.; Fouque, F.; Lamfri, M.; Sabatier, P.; Introini, V.; Zaidenberg, M.; Scavuzzo, C. Space-time analysis of the dengue spreading dynamics in the 2004 tartagal outbreak, Northern Argentina. Acta Trop. 2007, 103, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Siqueira-Junior, J.B.; Maciel, I.J.; Barcellos, C.; Souza, W.V.; Carvalho, M.S.; Nascimento, N.E.; Oliveira, R.M.; Morais-Neto, O.; Martelli, C.M. Spatial point analysis based on dengue surveys at household level in central Brazil. BMC Public Health 2008, 8, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheong, Y.L.; Burkart, K.; Leitao, P.J.; Lakes, T. Assessing weather effects on dengue disease in Malaysia. Int. J. Environ. Res. Public Health 2013, 10, 6319–6334. [Google Scholar] [CrossRef]
- Honorio, N.A.; Nogueira, R.M.; Codeco, C.T.; Carvalho, M.S.; Cruz, O.G.; Magalhaes Mde, A.; de Araujo, J.M.; de Araujo, E.S.; Gomes, M.Q.; Pinheiro, L.S.; et al. Spatial evaluation and modeling of Dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2009, 3, e545. [Google Scholar] [CrossRef]
- Astrom, C.; Rocklov, J.; Hales, S.; Beguin, A.; Louis, V.; Sauerborn, R. Potential distribution of dengue fever under scenarios of climate change and economic development. Ecohealth 2012, 9, 448–454. [Google Scholar] [CrossRef]
- Lindén, A.; Mäntyniemi, S. Using the negative binomial distribution to model overdispersion in ecological count data. Ecology 2011, 92, 1414–1421. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Iformation-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Hammond, S.N.; Balmaseda, A.; Perez, L.; Tellez, Y.; Saborio, S.I.; Mercado, J.C.; Videa, E.; Rodriguez, Y.; Perez, M.A.; Cuadra, R.; et al. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am. J. Trop. Med. Hyg. 2005, 73, 1063–1070. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lei, H.Y.; Nguyen, T.L.; Lin, Y.S.; Huang, K.J.; Le, B.L.; Lin, C.F.; Yeh, T.M.; Do, Q.H.; Vu, T.Q.; et al. Dengue hemorrhagic fever in infants: A study of clinical and cytokine profiles. J. Infect. Dis. 2004, 189, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Kittigul, L.; Pitakarnjanakul, P.; Sujirarat, D.; Siripanichgon, K. The differences of clinical manifestations and laboratory findings in children and adults with dengue virus infection. J. Clin. Virol. 2007, 39, 76–81. [Google Scholar] [CrossRef]
- Higa, Y.; Yen, N.T.; Kawada, H.; Son, T.H.; Hoa, N.T.; Takagi, M. Geographic distribution of Aedes aegypti and Aedes albopictus collected from used tires in Vietnam. J. Am. Mosq. Control Assoc. 2010, 26, 1–9. [Google Scholar] [CrossRef]
- Kawada, H.; Higa, Y.; Nguyen, Y.T.; Tran, S.H.; Nguyen, H.T.; Takagi, M. Nationwide investigation of the pyrethroid susceptibility of mosquito larvae collected from used tires in Vietnam. PLoS Negl. Trop. Dis. 2009, 3, e391. [Google Scholar] [CrossRef] [Green Version]
- Minh An, D.T.; Rocklov, J. Epidemiology of dengue fever in Hanoi from 2002 to 2010 and its meteorological determinants. Glob. Health Action 2014, 7, 23074. [Google Scholar] [CrossRef]
- Huy, B.V.; Hoa, L.N.M.; Thuy, D.T.; Van Kinh, N.; Ngan, T.T.D.; Duyet, L.V.; Hung, N.T.; Minh, N.N.Q.; Truong, N.T.; Chau, N.V.V. Epidemiological and clinical features of dengue infection in adults in the 2017 outbreak in Vietnam. Biomed. Res. Int. 2019, 2019, 3085827. [Google Scholar] [CrossRef] [Green Version]
- Atique, S.; Abdul, S.S.; Hsu, C.Y.; Chuang, T.W. Meteorological influences on dengue transmission in Pakistan. Asian Pac. J. Trop. Med. 2016, 9, 954–961. [Google Scholar] [CrossRef]
- Xu, Z.; Bambrick, H.; Yakob, L.; Devine, G.; Lu, J.; Frentiu, F.D.; Yang, W.; Williams, G.; Hu, W. Spatiotemporal patterns and climatic drivers of severe dengue in Thailand. Sci. Total Environ. 2019, 656, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Pham, N.T.T.; Nguyen, C.T.; Vu, D.T.; Nakamura, K. Mapping of dengue vulnerability in the Mekong Delta region of Viet Nam using a water-associated disease index and remote sensing approach. APN Sci. Bull. 2018, 8, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.C.; Chaves, L.F.; Tsai, K.H.; Chuang, T.W. Increased adult Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) abundance in a dengue transmission hotspot, compared to a coldspot, within Kaohsiung City, Taiwan. Insects 2018, 9, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, F.S.; Tseng, Y.T.; Hsu, P.S.; Chen, C.D.; Lian Ie, B.; Chao, D.Y. Re-assess vector indices threshold as an early warning tool for predicting dengue epidemic in a dengue non-endemic country. PLoS Negl. Trop. Dis. 2015, 9, e0004043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, H.V.; Doan, H.T.; Phan, T.T.; Minh, N.N. Ecological factors associated with dengue fever in a Central Highlands province, Vietnam. BMC Infect. Dis. 2011, 11, 172. [Google Scholar] [CrossRef] [PubMed]

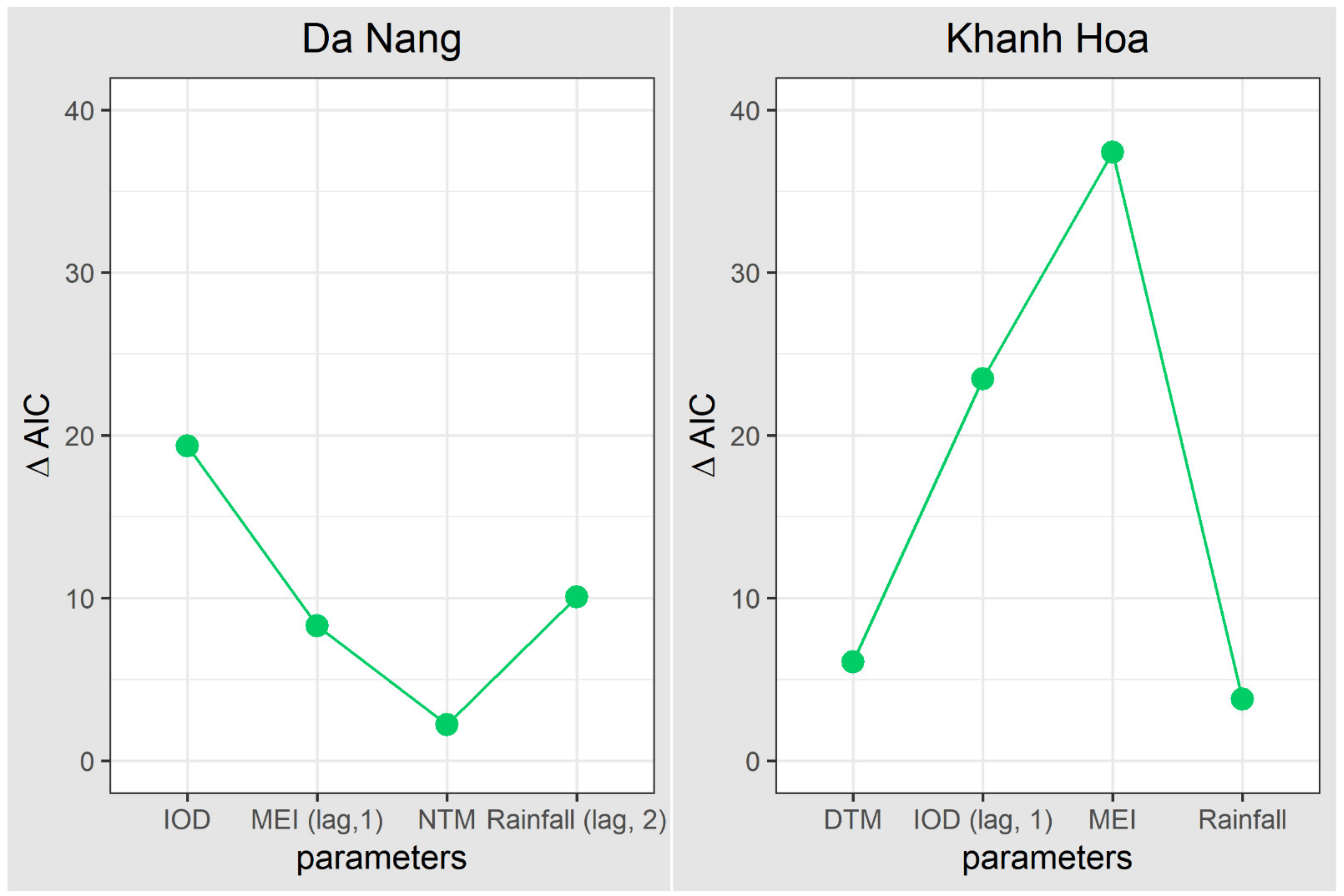

| Variable | Area | Median (IQR) | Mean (SD) | Min | Max |
|---|---|---|---|---|---|
| DTM | Da Nang | 28.44 (4.7) | 28.09 (3.2) | 21.3 | 33.36 |
| Khanh Hoa | 29.26 (4.5) | 28.73 (2.8) | 22.89 | 33.08 | |
| NTM | Da Nang | 21.52 (4.6) | 20.67 (3.1) | 13.08 | 25.44 |
| Khanh Hoa | 20.3 (4.7) | 20.52 (2.2) | 15.95 | 23.77 | |
| MTM | Da Nang | 25.3 (4.2) | 24.59 (2.9) | 18.45 | 29.33 |
| Khanh Hoa | 25.24 (3.3) | 24.61 (2.4) | 18.9 | 28.55 | |
| Rainfall | Da Nang | 16.21 (48.3) | 49.86 (102.2) | 0 | 563.65 |
| Khanh Hoa | 4.09 (12.9) | 19.32 (43.4) | 0 | 239.32 |
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Province/Variable | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value |
| Da Nang | ||||||||||
| NTM | 1.293 | 0.06 | 1.443 | 0.076 | 1.001 | 0.0065 | 1.001 | 0.172 | ||
| Rainfall (lag, 2) | 2.414 | 0.0028 | 2.409 | 0.0033 | 2.265 | 0.0094 | 2.442 | <0.001 | ||
| IOD | 2.654 | 0.0018 | 2.908 | <0.001 | 2.377 | 0.022 | 1.489 | 0.018 | ||
| MEI (lag, 1) | 3.123 | 0.0043 | 2.929 | 0.0194 | 3.349 | <0.001 | 2.425 | 0.068 | ||
| AIC | 405.873 | 408.094 | 415.94 | 425.219 | 414.171 | |||||
| ∆AIC | 2.221 | 10.067 | 19.346 | 8.298 | ||||||
| Khanh Hoa | ||||||||||
| DTM | 1 | 0.0031 | 1.429 | 0.004 | 1.988 | 0.002 | 1 | 0.0119 | ||
| Rainfall | 1.715 | 0.033 | 1.888 | 0.018 | 1 | 0.0028 | 1.033 | 0.277 | ||
| IOD (lag, 1) | 2.821 | <0.001 | 2.702 | <0.001 | 2.93 | <0.001 | 3.011 | <0.001 | ||
| MEI | 3.187 | <0.001 | 3.503 | <0.001 | 3.313 | <0.001 | 3.063 | <0.001 | ||
| AIC | 333.38 | 339.45 | 337.19 | 356.86 | 370.78 | |||||
| ∆AIC | 6.07 | 3.81 | 23.48 | 37.4 | ||||||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Province/Variable | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value |
| Da Nang | ||||||||
| IOD (lag, 1) | 2.676 | 0.0152 | 2.817 | 0.0298 | 2.205 | 0.001 | ||
| MEI | 3.455 | 0.0071 | 3.416 | 0.0243 | 3.178 | <0.001 | ||
| CI (lag, 3) | 2.968 | 0.0094 | 1.955 | 0.0094 | 3.107 | <0.001 | ||
| AIC | 294.2421 | 300.8429 | 309.1359 | 411.4024 | ||||
| ∆AIC | 6.6008 | 14.8938 | 117.1603 | |||||
| Khanh Hoa | ||||||||
| IOD (lag, 1) | 2.203 | <0.001 | 1.901 | <0.001 | 2.877 | <0.001 | ||
| MEI | 3.188 | <0.001 | 3.218 | <0.001 | 3.682 | <0.001 | ||
| CI (lag, 1) | 1 | <0.001 | 2.865 | <0.001 | 1 | 0.001 | ||
| AIC | 245.8264 | 258.581 | 273.5994 | 343.5945 | ||||
| ∆AIC | 12.7546 | 27.773 | 97.7681 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.T.; Le, H.X.; Nguyen, D.T.; Ho, H.Q.; Chuang, T.-W. Impact of Climate Variability and Abundance of Mosquitoes on Dengue Transmission in Central Vietnam. Int. J. Environ. Res. Public Health 2020, 17, 2453. https://doi.org/10.3390/ijerph17072453
Nguyen LT, Le HX, Nguyen DT, Ho HQ, Chuang T-W. Impact of Climate Variability and Abundance of Mosquitoes on Dengue Transmission in Central Vietnam. International Journal of Environmental Research and Public Health. 2020; 17(7):2453. https://doi.org/10.3390/ijerph17072453
Chicago/Turabian StyleNguyen, Luong Thi, Huy Xuan Le, Dong Thanh Nguyen, Ha Quang Ho, and Ting-Wu Chuang. 2020. "Impact of Climate Variability and Abundance of Mosquitoes on Dengue Transmission in Central Vietnam" International Journal of Environmental Research and Public Health 17, no. 7: 2453. https://doi.org/10.3390/ijerph17072453
APA StyleNguyen, L. T., Le, H. X., Nguyen, D. T., Ho, H. Q., & Chuang, T.-W. (2020). Impact of Climate Variability and Abundance of Mosquitoes on Dengue Transmission in Central Vietnam. International Journal of Environmental Research and Public Health, 17(7), 2453. https://doi.org/10.3390/ijerph17072453





