Notifiable Respiratory Infectious Diseases in China: A Spatial–Temporal Epidemiology Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Resources
2.2. Time-Series Analysis
2.3. Seasonality Analysis
2.4. Spatial Autocorrelation Analysis
2.5. Software Tools
3. Results
3.1. Epidemiologic Trends
3.2. Seasonal Decomposition
3.3. Seasonal Scan Statistic
3.4. Global Spatial Autocorrelation
3.5. Local Spatial Autocorrelation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Chinese CDC | Chinese Center for Disease Control and Prevention |
| DALYs | Disability-Adjusted Life Years |
| HH | High–High |
| HL | High–Low |
| LH | Low–High |
| LISA | Local Indicator of Spatial Association |
| LL | Low–Low |
| MCV | Measles-Containing Vaccine |
| MuCV | Mumps-Containing Vaccine |
| NBS | National Bureau of Statistics |
| RIDs | Respiratory Infectious Diseases |
| SDGs | Sustainable Development Goals |
Appendix A
- : the number of geographical units (31 provincial units in this study).
- : the incidence of one certain type of RIDs in geographical unit i.
- : the incidence of one certain type of RIDs in geographical unit j.
- : the average incidence of one certain type of RIDs in all the geographical units.
- spatial-weighted n×n matrix which represents neighboring relations. = 1 if unit i is adjacent with unit j, and = 0 otherwise.
References
- WHO. World Health Statistics: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2016; ISBN 9789241565585. [Google Scholar]
- Vink, M.A.; Christoffel, M.; Bootsma, J.; Wallinga, J. Systematic reviews and meta- and pooled analyses serial intervals of respiratory infectious diseases: A systematic review and analysis. Am. J. Epidemiol. 2014, 180, 865–875. [Google Scholar] [CrossRef]
- Sheffield, E.R.S. The Global Impact of Respiratory Disease, 2nd ed.; European Respiratory Society: Sheffield, UK, 2017; ISBN 9781849840873. [Google Scholar]
- Wang, H.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Abraha, H.N.; Abu-Raddad, L.J.; Abu-Rmeileh, N.M.E.; et al. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1084–1150. [Google Scholar] [CrossRef]
- Region, E. Progress towards the SDGs: A Selection of Data from World Health Statistics 2018 SDG3: Ensure Healthy Lives and Promote Well-Being for all Ages; WHO: Geneva, Switzerland, 2018; pp. 4–7. [Google Scholar]
- Li, C.; Sun, M.; Wang, Y.; Luo, L.; Yu, M.; Zhang, Y.; Wang, H.; Shi, P.; Chen, Z.; Wang, J.; et al. The centers for disease control and prevention system in China: Trends from 2002-2012. Am. J. Public Health 2016, 106, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yin, S.; Li, Y.; Wang, W.; Du, M.; Guo, W.; Xue, M.; Wu, J.; Liang, D.; Wang, R.; et al. Spatiotemporal epidemiology of, and factors associated with, the tuberculosis prevalence in northern China, 2010–2014. BMC Infect. Dis. 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Spatial-temporal epidemiological characteristics of tuberculosis in Shandong Province, China in 2011–2015. EC Pulmonol. Respir. Med. 2017, 5, 160–168. [Google Scholar]
- Wang, T.; Xue, F.; Chen, Y.; Ma, Y.; Liu, Y. The spatial epidemiology of tuberculosis in Linyi, China, 2005–2010. BMC Public Health 2012, 12, 0–7. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, L.; Pang, X.; Sun, M.; Ma, R.; Liu, D.; Wu, J. A 60-year review on the changing epidemiology of measles in capital Beijing, China, 1951–2011. BMC Public Health 2013, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.L.; Boulton, M.L.; Gillespie, B.W.; Zhang, Y.; Ding, Y.; Carlson, B.F.; Luo, X.; Montgomery, J.L.P.; Wang, X. Risk factors for measles among adults in Tianjin, China: Who should be controls in a case-control study? PLoS ONE 2017, 12, e0185465. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, L.; Zhou, M.; Chen, Z.; Li, F.; Wu, H.; Xiang, N.; Chen, E.; Tang, F.; Wang, D.; et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N. Engl. J. Med. 2014, 370, 520–532. [Google Scholar] [CrossRef]
- Gong, Z.; Lv, H.; Ding, H.; Han, J.; Sun, J.; Chai, C.; Cai, J.; Yu, Z.; Chen, E. Epidemiology of the avian influenza A (H7N9) outbreak in Zhejiang Province, China. BMC Infect. Dis. 2014, 14, 244. [Google Scholar] [CrossRef]
- Cui, A.; Zhu, Z.; Hu, Y.; Deng, X.; Sun, Z.; Zhang, Y.; Mao, N.; Xu, S.; Fang, X.; Gao, H.; et al. Mumps epidemiology and mumps virus genotypes circulating in mainland China during 2013–2015. PLoS ONE 2017, 12, e0169561. [Google Scholar] [CrossRef] [PubMed]
- Khor, C.; Sam, I.; Hooi, P.; Quek, K.; Chan, Y. Epidemiology and seasonality of respiratory viral infections in hospitalized children in Kuala Lumpur, Malaysia: A retrospective study of 27 years. BMC Pediatr. 2012, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.G.; Amstislavski, P.; Greene, A.; Haseeb, M.A. The landscape epidemiology of seasonal clustering of highly pathogenic avian influenza (H5N1) in domestic poultry in Africa, Europe and Asia. Transbound. Emerg. Dis. 2017, 64, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Batool, S.A.; Chaudhry, M.N. Seasonality and trend analysis of tuberculosis in Lahore, Pakistan from 2006 to 2013. J. Epidemiol. Glob. Health 2015, 5, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jiang, Y.; Guo, X.; Wu, Y.; Zhao, G. Influence of infectious disease seasonality on the performance of the outbreak detection algorithm in the China infectious disease automated-alert and response system. J. Int. Med. Res. 2018, 46, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huo, X.; Huai, Y.; Xiao, L.; Jiang, H.; Klena, J.; Greene, C.M.; Xing, X.; Huang, J.; Liu, S.; et al. Epidemiology, seasonality and treatment of hospitalized adults and adolescents with influenza in Jingzhou, China, 2010–2012. PLoS ONE 2016, 11, 2010–2012. [Google Scholar] [CrossRef][Green Version]
- Lee, H.S.; Thiem, V.D.; Anh, D.D.; Duong, T.N.; Lee, M.; Grace, D.; Nguyen-Viet, H. Geographical and temporal patterns of rabies post exposure prophylaxis (PEP) incidence in humans in the mekong river delta and southeast central coast regions in Vietnam from 2005 to 2015. PLoS ONE 2018, 13, e0194943. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, N.; Zhu, B.; Liu, J.; He, R. A descriptive analysis of the Spatio- temporal distribution of intestinal infectious diseases in China. BMC Infect. Dis. 2019, 19, 766. [Google Scholar] [CrossRef]
- Kim, E.H.; Bae, J.M. Seasonality of tuberculosis in the Republic of Korea, 2006–2016. Epidemiol. Health 2018, 40, e2018051. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Liu, Y.; Sun, D.; Ding, S.; Zhang, B.; Du, Z.; Xue, F. Detecting spatial-temporal clusters of HFMD from 2007 to 2011 in Shandong Province, China. PLoS ONE 2013, 8, e63447. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, J.; Fu, Y.; Zhang, B.; Mao, Y. Spatio-temporal epidemiology of viral hepatitis in China (2003-2015): Implications for prevention and control policies. Int. J. Environ. Res. Public Health 2018, 15, 661. [Google Scholar] [CrossRef] [PubMed]
- Ge, E.; Zhang, X.; Wang, X.; Wei, X. Spatial and temporal analysis of tuberculosis in Zhejiang Province, China, 2009–2012. Infect. Dis. Poverty 2016, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Fu, Y.; Liu, J.; Mao, Y. Notifiable sexually transmitted infections in China: Epidemiologic trends and spatial changing patterns. Sustainability 2017, 9, 1784. [Google Scholar] [CrossRef]
- Parra-Amaya, M.; Puerta-Yepes, M.; Lizarralde-Bejarano, D.; Arboleda-Sánchez, S. Early detection for dengue using local indicator of spatial association (LISA) analysis. Diseases 2016, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Cai, S.; Zhang, H.; Lin, W.; Fan, Y.; Qiu, J.; Sun, L.; Chang, B.; Zhang, Z.; Nie, S. Spatial, temporal, and spatiotemporal analysis of malaria in Hubei Province, China from 2004–2011. Malar. J. 2015, 14, 145. [Google Scholar] [CrossRef]
- Cao, K.; Yang, K.; Wang, C.; Guo, J.; Tao, L.; Liu, Q.; Gehendra, M.; Zhang, Y.; Guo, X. Spatial-temporal epidemiology of tuberculosis in mainland China: An analysis based on Bayesian theory. Int. J. Environ. Res. Public Health 2016, 13, 469. [Google Scholar] [CrossRef]
- Guo, C.; Du, Y.; Shen, S.Q.; Lao, X.Q.; Qian, J.; Ou, C.Q. Spatiotemporal analysis of tuberculosis incidence and its associated factors in mainland China. Epidemiol. Infect. 2017, 145, 2510–2519. [Google Scholar] [CrossRef]
- Xiang, N.; Havers, F.; Chen, T.; Song, Y.; Tu, W.; Li, L.; Cao, Y.; Liu, B.; Zhou, L.; Meng, L.; et al. Use of national pneumonia surveillance to describe influenza A(H7N9) virus epidemiology, China, 2004-2013. Emerg. Infect. Dis. 2013, 19, 1784–1790. [Google Scholar] [CrossRef]
- Engelman, D.; Steer, A.C. The resurgence of scarlet fever in China Reappraising the cardiosafety of dihydroartemisinin-piperaquine. Lancet Infect. Dis. 2018, 18, 823–824. [Google Scholar]
- You, Y.; Davies, M.R.; Protani, M.; McIntyre, L.; Walker, M.J.; Zhang, J. Scarlet fever epidemic in China caused by streptococcus pyogenes serotype M12: Epidemiologic and molecular analysis. EBioMedicine 2018, 28, 128–135. [Google Scholar] [CrossRef]
- Ma, C.; Gregory, C.J.; Hao, L.; Wannemuehler, K.A.; Su, Q.; An, Z.; Quick, L.; Rodewald, L.; Ma, F.; Yan, R.; et al. Risk factors for measles infection in 0–7 month old children in China after the 2010 nationwide measles campaign: A multi-site case–control study, 2012–2013. Vaccine 2016, 34, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, S.; Wang, L.; Chin, D.P.; Wang, S.; Jiang, G.; Xia, H.; Zhou, Y.; Zhao, B.; Zhang, H.; et al. National Survey of Drug-Resistant Tuberculosis in China. N. Engl. J. Med. 2012, 366, 2070–2161. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Geater, A.; McNeil, E.; Deng, Q.; Dong, A.; Zhong, G. Spatial, temporal and spatio-temporal clusters of measles incidence at the county level in Guangxi, China during 2004–2014: Flexibly shaped scan statistics. BMC Infect. Dis. 2017, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Vencalek, O.; Kyncl, J. Analysis of the seasonal incidence of acute respiratory infections including influenza (ARI) in the Czech Republic—Possible contribution of the functional data boxplot in epidemiology. Biomed. Pap. 2017, 161, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gong, J.; Zhou, J.; Zhao, Y.; Tan, J.; Ibrahim, A.N.; Zhou, Y. A spatial, social and environmental study of tuberculosis in China using statistical and GIS technology. Int. J. Environ. Res. Public Health 2015, 12, 1425–1448. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Tian, B.C.; Kang, X.P.; Zhang, W.; Meng, X.P.; Zhang, J.B.; Lo, S.K. Public awareness of tuberculosis in China: A national survey of 69253 subjects. Int. J. Tuberc. Lung Dis. 2009, 13, 1493–1499. [Google Scholar]
- Zou, X.; Zhou, L.; Wu, H.; Chen, L.; Zhou, F.; Gong, C.; Ye, J.; Ling, L. The role of tuberculosis control institutes in delivering tuberculosis information to domestic migrants in China: A multi-level analysis of a nationwide cross-sectional survey. Int. J. Infect. Dis. 2019, 86, 94–101. [Google Scholar] [CrossRef]
- Ben Zakour, N.L.; Davies, M.R.; You, Y.; Chen, J.H.K.; Forde, B.M.; Stanton-Cook, M.; Yang, R.; Cui, Y.; Barnett, T.C.; Venturini, C.; et al. Transfer of scarlet fever-associated elements into the group A Streptococcus M1T1 clone. Sci. Rep. 2015, 5, 15877. [Google Scholar] [CrossRef]
- Liao, J.; Yu, S.; Yang, F.; Yang, M.; Hu, Y.; Zhang, J. Short-term effects of climatic variables on hand, foot, and mouth disease in Mainland China, 2008–2013: A multilevel spatial poisson regression model accounting for overdispersion. PLoS ONE 2016, 11, e0147054. [Google Scholar] [CrossRef]
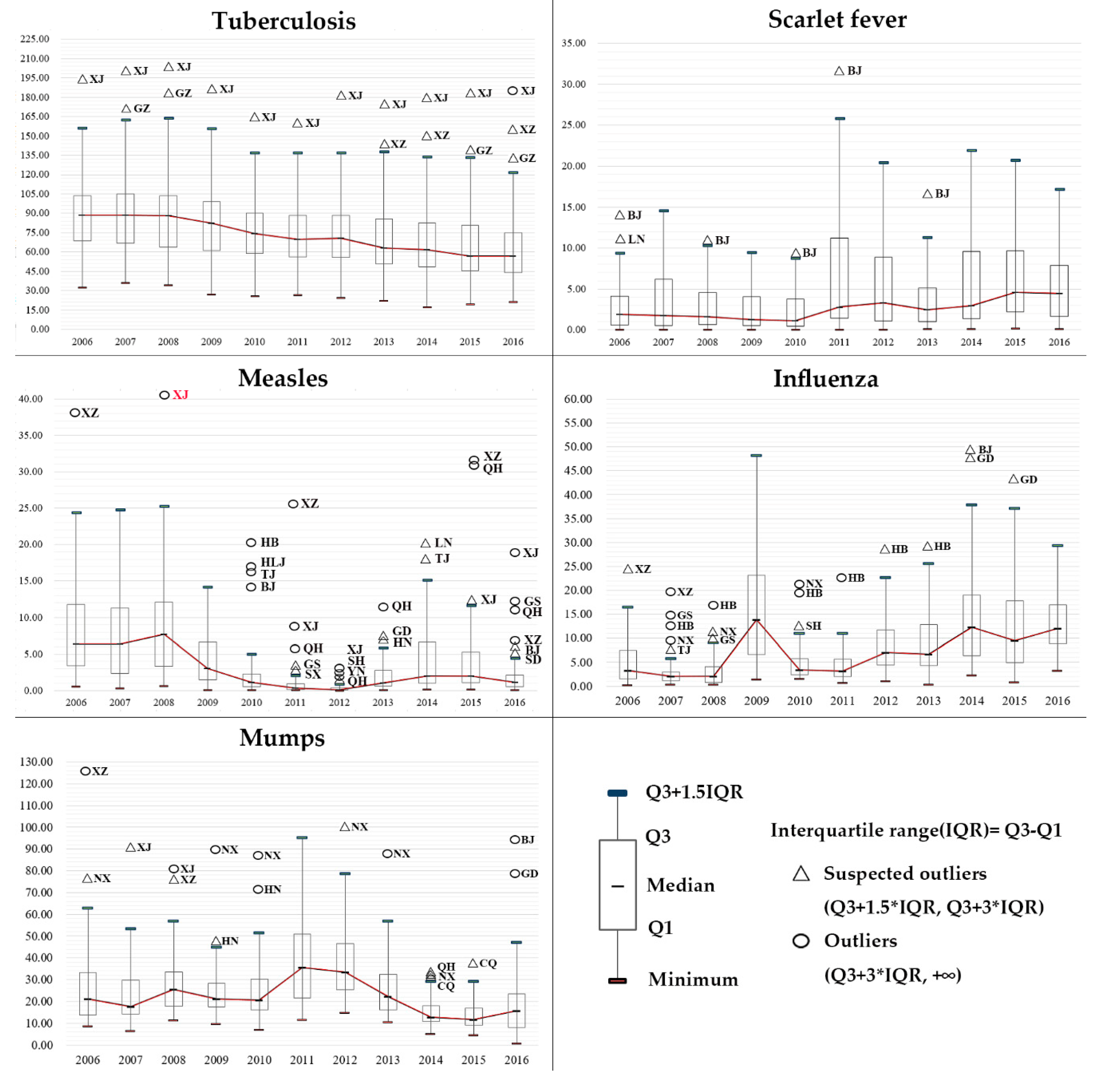
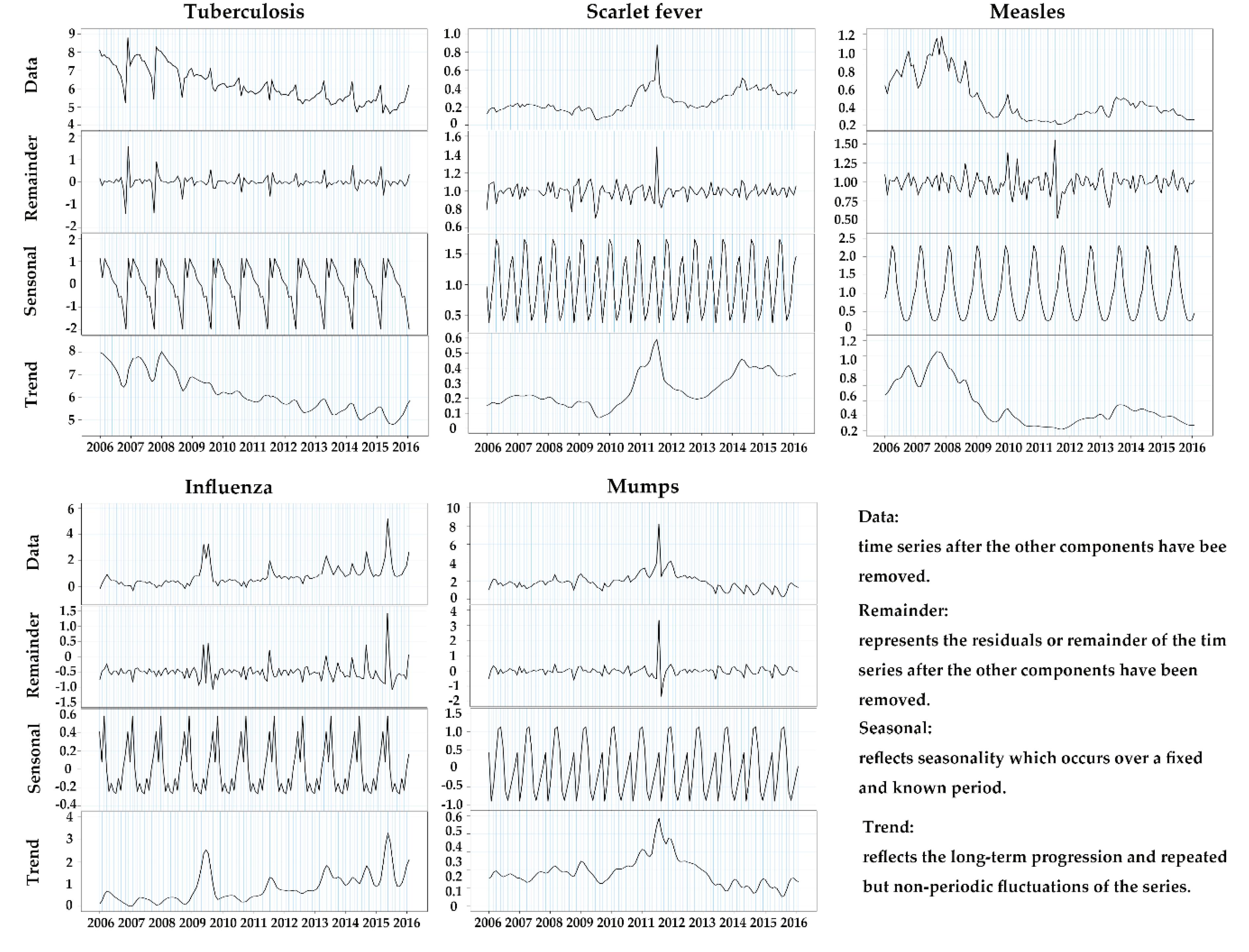
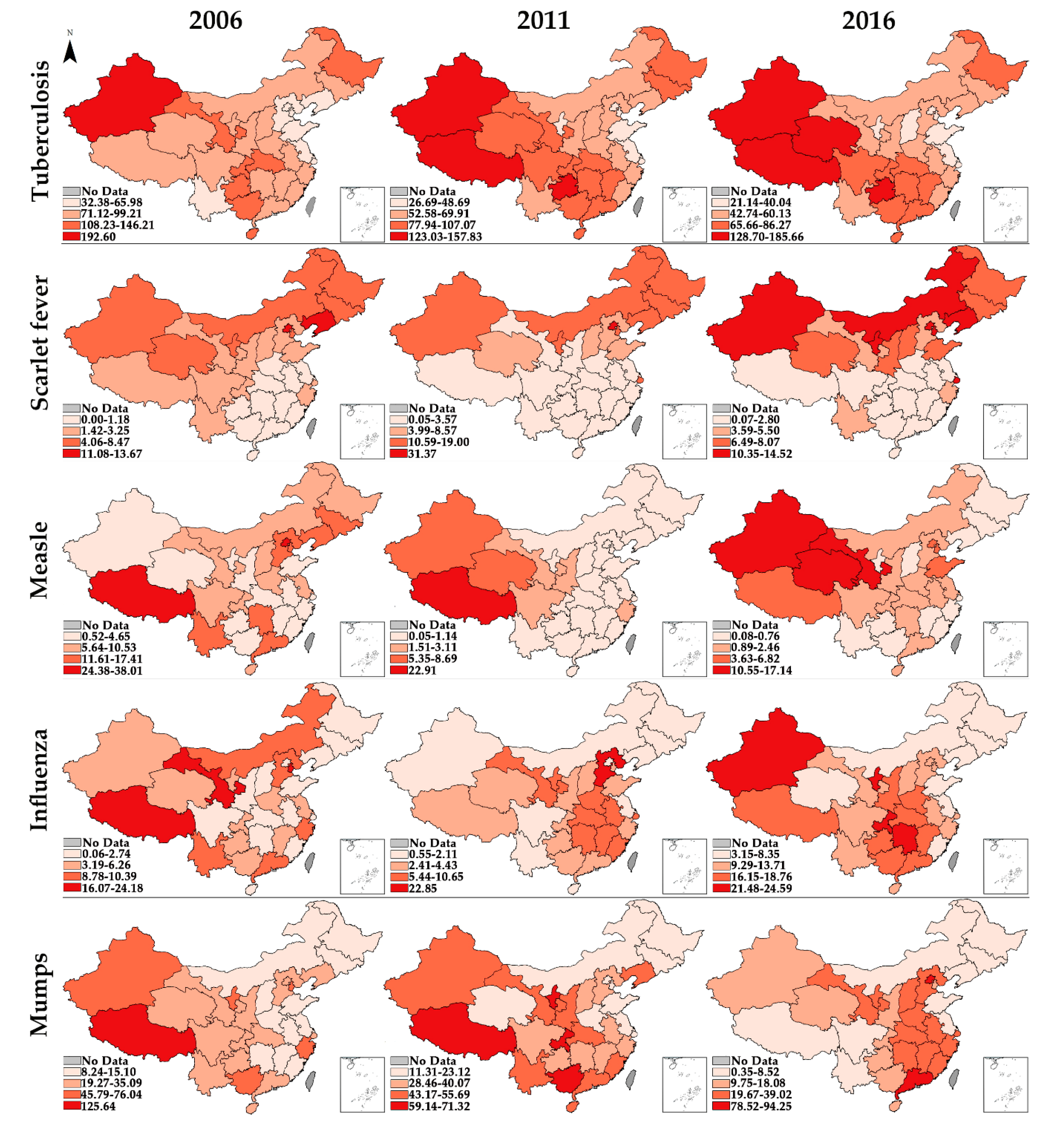
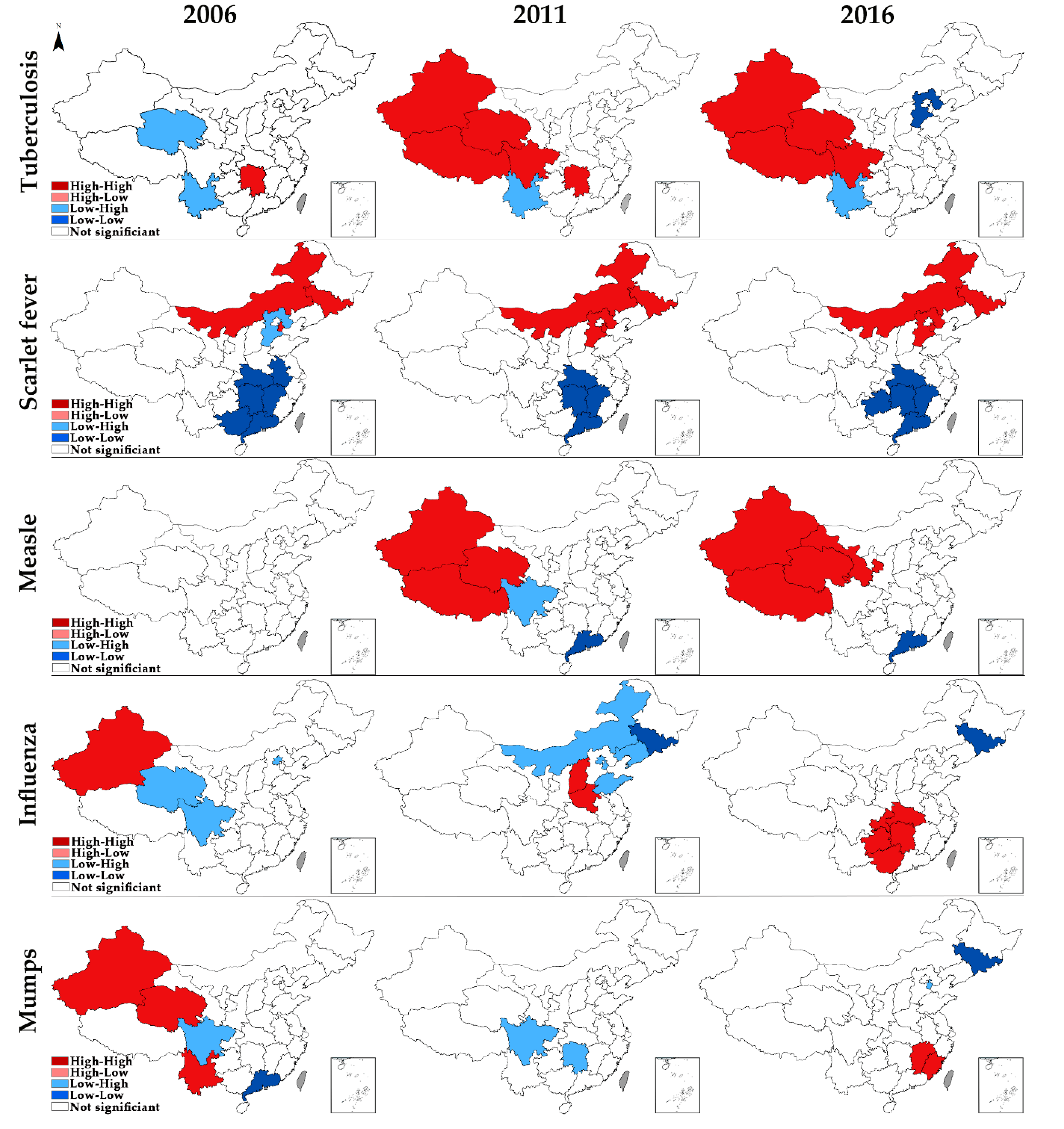
| Region | Tuberculosis | Scarlet Fever | Measles | Influenza | Mumps | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2016 | Growth | 2006 | 2016 | Growth | 2006 | 2016 | Growth | 2006 | 2016 | Growth | 2006 | 2016 | Growth | |
| Beijing | 51.29 | 31.01 | −4.91% *** | 13.67 | 12.08 | −1.23% | 24.38 | 5.75 | −13.45% * | 1.89 | 10.09 | 18.27% | 28.21 | 94.25 | 12.82% |
| Tianjin | 36.54 | 21.14 | −5.33% *** | 4.06 | 11.21 | 10.70% ** | 13.02 | 3.63 | −11.99% | 16.07 | 9.43 | −5.19% | 52.39 | 15.74 | −11.33% *** |
| Hebei | 62.54 | 45.34 | −3.16% *** | 2.64 | 5.21 | 7.04% ** | 11.61 | 2.46 | −14.37% | 10.39 | 9.97 | −0.41% * | 21.00 | 39.02 | 6.39% |
| Shanxi | 71.12 | 38.65 | −5.92% *** | 2.49 | 7.69 | 11.94% ** | 6.71 | 0.41 | −24.38% ** | 2.15 | 10.23 | 16.88% ** | 10.94 | 20.52 | 6.48% |
| Neimenggu | 90.05 | 48.30 | −6.04% *** | 6.80 | 10.35 | 4.29% ** | 10.53 | 1.12 | −20.07% | 9.60 | 7.37 | −2.62% | 12.82 | 8.52 | −4.00% |
| Liaoning | 56.20 | 51.40 | −0.89% | 11.08 | 10.36 | −0.67% | 12.82 | 0.26 | −32.28% | 0.21 | 6.87 | 41.67% *** | 24.54 | 4.61 | −15.39% * |
| Jilin | 83.56 | 49.76 | −5.05% *** | 4.46 | 7.68 | 5.58% | 17.41 | 0.31 | −33.16% | 0.18 | 4.07 | 36.30% *** | 11.87 | 3.19 | −12.32% |
| Heilongjiang | 109.35 | 80.16 | −3.06% *** | 8.47 | 7.43 | −1.31% | 8.97 | 0.29 | −29.05% | 0.06 | 3.15 | 47.90% | 15.10 | 2.74 | −15.68% ** |
| Shanghai | 32.38 | 27.28 | −1.70% *** | 3.25 | 12.10 | 14.05% ** | 3.36 | 0.69 | −14.65% * | 0.43 | 9.81 | 36.62% ** | 21.33 | 19.67 | −0.81% ** |
| Jiangsu | 65.99 | 35.93 | −5.90% *** | 1.18 | 2.80 | 9.04% ** | 6.10 | 0.94 | −17.06% ** | 6.23 | 6.33 | 0.16% | 12.48 | 6.56 | −6.23% |
| Zhejiang | 88.74 | 48.78 | −5.81% *** | 1.90 | 4.46 | 8.91% ** | 3.21 | 0.59 | −15.57% | 8.78 | 12.77 | 3.82% * | 46.94 | 26.13 | −5.69% *** |
| Anhui | 85.63 | 56.77 | −4.03% *** | 0.30 | 1.33 | 16.10% ** | 2.45 | 1.17 | −7.13% | 2.34 | 12.69 | 18.43% *** | 14.31 | 23.76 | 5.20% |
| Fujian | 85.87 | 42.74 | −6.74% *** | 0.36 | 1.59 | 16.04% *** | 4.65 | 0.63 | −18.12% ** | 1.74 | 7.72 | 16.09% ** | 14.52 | 27.15 | 6.46% |
| Jiangxi | 98.49 | 71.99 | −3.09% *** | 0.05 | 0.10 | 7.98% ** | 3.68 | 0.50 | −18.10% *** | 4.73 | 16.75 | 13.48% *** | 8.66 | 20.83 | 9.18% |
| Shandong | 42.24 | 30.78 | −3.12% *** | 1.43 | 7.22 | 17.56% *** | 3.50 | 4.47 | 2.46% | 0.18 | 6.50 | 43.44% *** | 8.24 | 7.34 | −1.14% |
| Henan | 95.98 | 60.13 | −4.57% *** | 0.66 | 1.56 | 9.04% ** | 7.78 | 1.19 | −17.12% ** | 3.19 | 16.80 | 18.08% *** | 9.13 | 23.47 | 9.90% * |
| Hubei | 108.23 | 74.70 | −3.64% *** | 0.40 | 1.73 | 15.69% *** | 4.02 | 1.21 | −11.31% ** | 2.74 | 17.89 | 20.63% | 19.27 | 19.96 | 0.35% |
| Hunan | 91.99 | 75.50 | −1.96% *** | 0.17 | 1.15 | 20.90% *** | 11.99 | 1.16 | −20.83% ** | 1.41 | 23.41 | 32.41% | 13.35 | 23.56 | 5.85% |
| Guangdong | 95.22 | 71.82 | −2.78% *** | 0.37 | 2.40 | 20.42% *** | 15.10 | 1.17 | −22.57% ** | 8.78 | 16.24 | 6.35% ** | 20.45 | 78.52 | 14.40% |
| Guangxi | 127.23 | 86.27 | −3.81% *** | 0.46 | 1.09 | 8.93% ** | 1.90 | 0.08 | −27.15% ** | 4.29 | 18.76 | 15.90% | 45.79 | 18.08 | −8.87% |
| Hainan | 140.54 | 84.18 | −5.00% *** | 0.00 | 0.07 | − | 9.23 | 1.76 | −15.27% | 0.58 | 13.71 | 37.21% *** | 21.11 | 11.59 | −5.82% |
| Chongqing | 124.67 | 73.34 | −5.17% *** | 1.42 | 1.60 | 1.21% * | 5.64 | 1.13 | −14.86% | 4.15 | 24.41 | 19.39% | 23.30 | 10.54 | −7.63% |
| Sichuan | 99.21 | 65.66 | −4.04% *** | 1.73 | 2.10 | 1.96% ** | 9.95 | 0.99 | −20.61% * | 2.50 | 10.69 | 15.63% | 23.73 | 4.90 | −14.59% ** |
| Guizhou | 146.21 | 130.66 | −1.12% ** | 0.81 | 2.05 | 9.74% *** | 1.01 | 0.09 | −21.50% * | 4.73 | 17.54 | 14.00% | 32.67 | 9.75 | −11.39% ** |
| Yunnan | 60.42 | 55.47 | −0.85% *** | 1.75 | 3.59 | 7.46% ** | 14.47 | 0.34 | −31.28% ** | 10.08 | 12.02 | 1.77% | 35.09 | 5.63 | −16.71% * |
| Xizang | 91.17 | 154.37 | 5.41% *** | 2.89 | 2.44 | −1.67% | 38.01 | 6.82 | −15.78% | 24.18 | 17.19 | −3.35% | 125.64 | 0.35 | −44.53% ** |
| Shaanxi | 87.12 | 56.30 | −4.27% *** | 2.43 | 8.07 | 12.76% ** | 2.70 | 0.89 | −10.49% ** | 0.95 | 16.15 | 32.73% ** | 21.08 | 15.83 | −2.83% |
| Gansu | 108.23 | 58.13 | −6.03% *** | 2.86 | 5.50 | 6.76% ** | 6.39 | 11.14 | 5.72% | 16.24 | 9.29 | −5.43% | 34.06 | 32.73 | −0.40% |
| Qinghai | 75.21 | 128.70 | 5.52% *** | 6.10 | 6.49 | 0.63% | 3.44 | 10.55 | 11.85% ** | 3.26 | 8.35 | 9.86% ** | 32.12 | 13.15 | −8.55% ** |
| Ningxia | 76.86 | 40.04 | −6.31% *** | 4.16 | 14.52 | 13.31% *** | 0.52 | 0.76 | 3.87% | 5.29 | 21.48 | 15.05% | 76.04 | 21.68 | −11.79% |
| Xinjiang | 192.60 | 185.66 | −0.37% | 4.70 | 12.69 | 10.45% ** | 1.49 | 17.14 | 27.69% | 6.26 | 24.59 | 14.67% | 47.93 | 10.64 | −13.97% ** |
| SUM | 86.23 | 61.38 | −3.34% *** | 2.11 | 4.35 | 7.50% ** | 7.62 | 1.82 | −13.34% ** | 4.40 | 22.51 | 17.73% ** | 20.76 | 12.84 | −4.69% |
| Infections | Time Frame(Months) | Number of Cases | Expected Cases | Observed/Expected | Relative Risk | Log-Likelihood Ratio | p-Value |
|---|---|---|---|---|---|---|---|
| Tuberculosis | January to May | 6,709,673 | 6,047,427.81 | 1.11 | 1.20 | 61,857.01 | 0.001 |
| Scarlet fever | May to June | 128,684 | 77,291.75 | 1.66 | 1.92 | 17,795.86 | 0.001 |
| Measles | February to June | 450,685 | 264,491.11 | 1.70 | 3.35 | 109,997.33 | 0.001 |
| Influenza | November to April | 893,826 | 711,198.12 | 1.26 | 1.67 | 46,159.38 | 0.001 |
| Mumps | April to July | 1,559,324 | 1,108,119.59 | 1.41 | 1.77 | 131,010.74 | 0.001 |
| Year | Tuberculosis | Scarlet Fever | Measles | Influenza | Mumps | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moran’s I | Z−Value | p−Value | Moran’s I | Z−Value | p−Value | Moran’s I | Z−Value | p−Value | Moran’s I | Z−Value | p−Value | Moran’s I | Z−Value | p−Value | |
| 2006 | 0.108 | 1.2335 | 0.1109 | 0.3783 | 3.8401 | 0.0018 | 0.034 | 0.6079 | 0.25 | −0.0647 | −0.3374 | 0.3898 | 0.172 | 2.0203 | 0.0327 |
| 2007 | 0.1334 | 1.4341 | 0.0815 | 0.4563 | 4.4060 | 0.0002 | −0.2396 | −1.8489 | 0.0220 | −0.0636 | −0.3443 | 0.4021 | 0.3355 | 3.5332 | 0.0016 |
| 2008 | 0.1001 | 1.1422 | 0.1260 | 0.4767 | 4.5124 | 0.0002 | −0.0434 | −0.2322 | 0.4298 | −0.0354 | −0.0731 | 0.4922 | 0.2268 | 2.3393 | 0.0182 |
| 2009 | 0.1162 | 1.2921 | 0.0968 | 0.4454 | 4.2746 | 0.0002 | 0.1162 | 1.2789 | 0.1055 | 0.2025 | 2.0695 | 0.0274 | 0.0090 | 0.3644 | 0.3368 |
| 2010 | 0.1266 | 1.3756 | 0.0852 | 0.4858 | 4.6689 | 0.0002 | 0.4776 | 4.8974 | 0.0011 | −0.0502 | −0.2139 | 0.4518 | −0.0082 | 0.1913 | 0.3925 |
| 2011 | 0.2266 | 2.2525 | 0.0161 | 0.3984 | 3.9892 | 0.0008 | 0.2995 | 4.7804 | 0.0032 | −0.0621 | −0.3611 | 0.3735 | 0.0663 | 0.8351 | 0.1985 |
| 2012 | 0.2844 | 2.8054 | 0.0053 | 0.4254 | 4.0517 | 0.0003 | −0.0777 | −0.4547 | 0.3509 | 0.0439 | 0.6526 | 0.2422 | 0.1488 | 1.6101 | 0.0597 |
| 2013 | 0.3171 | 3.0903 | 0.0031 | 0.3028 | 3.1607 | 0.0004 | −0.0840 | −0.5553 | 0.2982 | 0.0349 | 0.5764 | 0.2686 | 0.1776 | 1.9877 | 0.0309 |
| 2014 | 0.2732 | 2.7102 | 0.0073 | 0.4329 | 4.0828 | 0.0004 | 0.3177 | 3.1262 | 0.0053 | 0.0123 | 0.3777 | 0.3255 | 0.1060 | 1.2057 | 0.1149 |
| 2015 | 0.3114 | 3.0845 | 0.0034 | 0.3723 | 3.2113 | 0.0039 | 0.3643 | 4.1316 | 0.0066 | −0.0721 | −0.4012 | 0.3584 | 0.1231 | 1.3948 | 0.0859 |
| 2016 | 0.3497 | 3.4609 | 0.0017 | 0.3761 | 3.5555 | 0.0006 | 0.5228 | 5.5626 | 0.0004 | 0.1991 | 2.0050 | 0.0295 | −0.0454 | −0.1709 | 0.4639 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; He, R.; Zhu, B.; Liu, J.; Zhang, N. Notifiable Respiratory Infectious Diseases in China: A Spatial–Temporal Epidemiology Analysis. Int. J. Environ. Res. Public Health 2020, 17, 2301. https://doi.org/10.3390/ijerph17072301
Mao Y, He R, Zhu B, Liu J, Zhang N. Notifiable Respiratory Infectious Diseases in China: A Spatial–Temporal Epidemiology Analysis. International Journal of Environmental Research and Public Health. 2020; 17(7):2301. https://doi.org/10.3390/ijerph17072301
Chicago/Turabian StyleMao, Ying, Rongxin He, Bin Zhu, Jinlin Liu, and Ning Zhang. 2020. "Notifiable Respiratory Infectious Diseases in China: A Spatial–Temporal Epidemiology Analysis" International Journal of Environmental Research and Public Health 17, no. 7: 2301. https://doi.org/10.3390/ijerph17072301
APA StyleMao, Y., He, R., Zhu, B., Liu, J., & Zhang, N. (2020). Notifiable Respiratory Infectious Diseases in China: A Spatial–Temporal Epidemiology Analysis. International Journal of Environmental Research and Public Health, 17(7), 2301. https://doi.org/10.3390/ijerph17072301






