Abstract
Following the recent electronic cigarette (e-cigarette) illness outbreak, the current review aimed to collect all related clinical cases for study and analysis and provide a critical synopsis of the proposed injury mechanism. Adhering to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines, e-cigarette-related clinical cases were identified via Google Scholar and PubMed databases. Additionally, references of published case reports and previous review papers were manually searched, revealing 159 publications presenting e-cigarette-related case reports and 19 reports by the Centers for Disease Control and Prevention. 238 individual cases were identified; 53% traumatic injuries due to e-cigarette explosion or self-combustion, 24% respiratory cases, and 12% poisonings. Additional cases pertained to oral, cardiovascular, immunologic, hematologic, allergic reactions, infant complications, and altered medication levels. Case reports were mainly published between 2016–2019 (78%). The oldest case, a lipoid pneumonia, was published in 2012. The current review showed that e-cigarette-related health effects extend beyond the acute lung injury syndrome, including traumatic, thermal injuries and acute intoxications. Physicians should be aware of the distinct clinical presentations and be trained to respond and treat effectively. Regulators and public health authorities should address the regulatory gap regarding electronic nicotine delivery systems (ENDS) and novel tobacco products.
1. Introduction
Introduced to the market in 2004 with the claim that the user inhales harmless vapor [1], the e-cigarette is marketed as a harm reduction product and has been proposed to be used as a smoking cessation tool, however there is a lack of clinical studies to support either of those effects [2].
Nearly two decades and four device generations later, there is growing scientific evidence that e-cigarette users are inhaling a mixture of irritative, toxic and carcinogenic compounds [3]. The device does not emit “side-stream smoke” as it is activated only by the user’s inspiratory effort; it does however produce secondhand aerosol (SHA) through the user’s exhalation [4]. SHA represents a documented source for passive exposure of bystanders as it contains micro-particulate matter (PM10 and PM2.5), volatile organic compounds and various other toxicants [5].
Following years of regulatory discussions, suggested policies and directives, e-cigarettes mainly targeting the youth continue to be underregulated especially outside the EU and UK; the easy online purchase and the customizable device and e-liquid mixture further complicate matters [6]. Regardless of their smoking status, adolescents tend to experiment with the nicotine containing «gadget» which predisposes them to addiction and smoking initiation [7]; e-cigarette users among high school students in the USA increased from 11.7% in 2017, to 20.8% in 2018 [8].
While the possible long-term health implications remain to be determined, studies showing immediate adverse health effects are mounting up. What has started as a small scale of laboratory-based studies on cells, tissues, animals, and humans [9], has evolved into a series of clinical case reports, including acute lung injury, poisoning, allergies, explosion accidents, and burn injuries [10].
The first case of e-cigarette-related lung injury was a case of lipoid pneumonia published in 2012 [11], furthermore from 2012 to 2015, 277 incidents of poisoning have been reported to National Poison Centers of 10 EU MS [12] and similarly increased poisoning incidents have been reported by US poison centers [13].
While in some countries, including the UK, the e-cigarette is used as a harm reduction product to aid smoking cessation, the severity of the 2019 acute lung injury outbreak in USA, mainly affecting adolescents and young adults was unanticipated. Having been attributed the term E-Vaping Acute Lung Injury (EVALI), earlier named Vaping Associated Lung Injury (VpALI) or Vaping Associated Pulmonary Injury (VAPI), and also an International Classification of Diseases (ICD) 10 coding [14], the syndrome continues to be under intense scientific scrutiny and diagnostic investigations, as the responsible causal factor and the underlying injury mechanism is not yet clear. On October 2019 the Centers for Disease Control and Prevention (CDC) issued a report which called for increased physician awareness, recommended the best approach of possible cases, and set the following criteria for EVALI diagnosis: “Vaping” or “dabbing” within 90 days prior to symptoms, pulmonary opacities on chest radiograph or ground-glass opacities on chest computerized tomography (CT) scan, negative infectious and immunologic panel, exclusion of alternative diagnoses [15,16]. CDC report issued in January 2020 showed that vitamin E acetate was identified as a possible causal factor for EVALI, however the contribution of other chemicals is yet to be determined [17].
To the authors knowledge at the time of publication there are 7 reviews on e-cigarette-related health effects and case reports, each specifically focusing on one aspect of the e-cigarette-related injury: The respiratory effects [18,19], poisonings [20], burn injuries [21,22], radiologic appearance of lung injuries [23], and histologic findings [24]. The majority of those reviews were published before the 2019 e-cigarette-related injury outbreak. The most integrated review including case reports referring to various organ-systems was published in 2016 [10] prior to the recent outbreak and the most recent in September 2019 [19] presenting mainly respiratory cases, toxicology of e-liquids, as well as previous mainly laboratory based, in vitro, in vivo, and animal studies.
Considering the lack of an integrated up to date systematic literature review of clinical cases expanding across all medical disciplines, the aim of the current review was to collect all e-cigarette-related case reports for a comprehensive study and analysis, followed by a critical synopsis of the proposed injury theories, in an attempt to better understand the multifactorial process and possible mechanisms implicated in the etiology of the e-cigarette-related illness and injury.
2. Materials and Methods
Adhered to the PRISMA guidelines [25] the current study aimed to systematically review published case reports of e-cigarette-related illness and injury.
To identify published case reports we searched PubMed and Google Scholar databases without setting a time frame, in order not to miss the earliest of publications. The terms used were “e cig report”, “electronic cigarette report”, “e cig irritation”, “electronic cigarette irritation”, “e cig inflammation”, “e cig pneumonia”, “electronic cigarette pneumonia”, “e cig allergy”, “electronic cigarette allergy”, “nicotine intoxication”, “electronic cigarette respiratory effect”, “e cig cardiovascular”, “nicotine poisoning”, “e cig respiratory effect”, “nicotine salts”, “juul case report”, “pod case report”, “electronic cigarette health effect”, “electronic cigarette inflammation”, “electronic cigarette case”, “vape case report”, and “electronic cigarette review”. Cases identified from the references of case reports and previous review papers were manually searched in addition to articles found through specifically created alerts. Finally, all CDC reports regarding e-cigarette-related illness outbreak were searched.
2.1. Inclusion and Exclusion Criteria
Screening: The initial approach comprised of screening titles and abstracts, excluding papers presenting experimental and laboratory studies, animal, cellular and tissue studies and observational studies.
For inclusion we considered publications in the English language presenting e-cigarette-related case reports including papers, systematic literature reviews, conference abstracts, letters to the editors/correspondence and CDC reports, provided they were published in peer-reviewed journals.
Eligibility: Following screening, 2 of the authors separately studied the full manuscript of the selected papers. Each case report was evaluated for its clinical presentation (quality, information abundance, e-liquid composition) and documentation of e-cigarette causal implication. Cases considered non-eligible by both authors were excluded. Cases deemed of moderate to low quality were further evaluated by two additional researchers and those deemed of poor quality were excluded. Cases with comorbidities relevant to the diagnosis regardless of the e-cigarette use were excluded. We included however cases where comorbidities and e-cigarette use may have had an equal or additive contribution to the reported diagnosis.
2.2. Search Results
Up to 21 February 2020, our search resulted in 1914 papers regarding e-cigarettes in total, of which 215 presented case reports. Following the abovementioned inclusion criteria, our final dataset consisted of 159 papers (Figure 1). Additionally, 19 CDC reports were identified and will be presented separately.
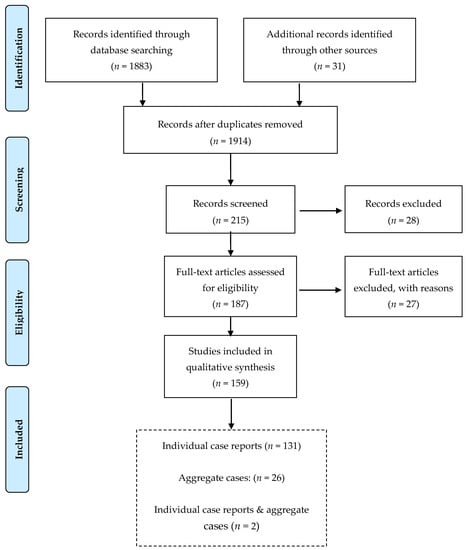
Figure 1.
Flow chart of the process to identify and screen published case reports of e-cigarette-related illness and injury.
2.3. Statistical Analysis
Descriptive analysis of the selected case reports was performed in Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, Texas, TX 77845, USA: StataCorp LP). Results are presented as percentages or frequencies.
3. Results
3.1. Classification of Cases
Selected case reports were classified in medical, poisonings and traumatic injuries. Medical cases were further categorized into respiratory, cardiovascular, allergic, autoimmune, and effect on medication metabolism among others; poisoning was further classified in accidental and suicidal and injuries in explosions and burns (Figure 2).
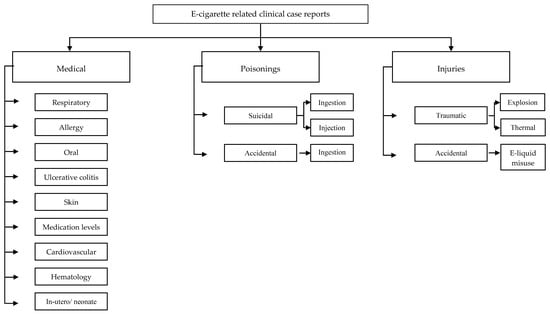
Figure 2.
Classification by type of injury of e-cigarette-related case reports.
We identified 133 publications presenting 238 individual cases including 91 papers, 25 letters, 7 conference abstracts, 6 correspondences, 1 brief report, 1 conference paper, 1 short communication, and 1 publication presented as “Massachusetts General, Interesting Case Series”. Majority of publications (63%) pertained to cases in the US while the remaining 37% were from the UK, Australia, Canada, China, Denmark, France, Germany, Ireland, Italy, Japan, Korea, Malaysia, the Netherlands, Poland, Portugal, Scotland, South Korea, Switzerland, and Turkey (Table 1). Case reports were mainly published between 2016–2019 (78%), although there was a scattered number in the years 2013-2015 and 2020. The oldest case report identified was a lipoid pneumonia published in 2012. Two of the abovementioned publications in addition to the individual patients also presented aggregate cases. Twenty-six further publications presenting only aggregate cases on e-cigarette adverse health effects were identified (Figure 1).

Table 1.
Type of injury by geographical location.
3.2. Respiratory
41 publications were identified, presenting 58 respiratory cases. Main findings are presented in Table 2 and Table 3. Most common diagnosis was EVALI (n = 15) [26,27,28,29,30,31,32,33,34,35,36] or EVALI with an additional finding (n = 1) [37]. Second most common diagnosis included either organizing pneumonia/ Bronchiolitis obliterans with organizing pneumonia (BOOP)/ respiratory bronchiolitis (n = 12) [38,39,40,41,42,43,44,45,46] or lipoid pneumonia (n = 9) [11,47,48,49,50,51]. In 4 cases vaping precipitated a pneumothorax [52,53,54] and in 2 exacerbated pre-existing asthma [55]. Other diagnoses included eosinophilic pneumonia (n = 4) [56,57,58,59], combination of organizing and lipoid pneumonia (n = 3) [40], hypersensitivity pneumonitis (n = 3) [46,60,61], diffuse alveolar hemorrhage (DAH) (n = 1) [62], acute respiratory distress syndrome (ARDS) (n = 1) [63], a combination of ARDS, organizing pneumonia and diffuse alveolar damage (DAD) (n = 1) [64], epiglottitis (n = 1) [65], and a possible EVALI on asthma grounds (n = 1) [46].

Table 2.
Respiratory cases: demographic, clinical, laboratory findings, and outcome.

Table 3.
Publications of e-cigarette case reports with cytologic and histologic findings.
Most patients were previously healthy (38/58). The majority were male (40/58) with median age 23 years old and interquartile range (IQR) 19–33 years old. The youngest person presented in the reports was 14 y.o. and the oldest person was 64 y.o. For the majority of cases it was not specified if they were dual users or if they used the e-cigarette for cessation (72%). While for 40% (23/58) of the cases it was not specified the substance used, 21 of cases used cannabis products solely, 6 used cannabis and nicotine in combination, 6 used cannabis and unknown liquid, while 2 used solely nicotine. Most common clinical symptom was dyspnea (48/58), cough (34/58), their combination (dyspnea and cough) (31/58) and fever (23/58). Sixty percent of patients (35/58) presented elevated white blood cell count (WBC), 7 patients had a normal WBC count while for 16 patients’ information was not provided. Information on infectious panels was available for 42 cases, all of which were negative. CT scan results were available for most cases (52/58), with most common finding being ground-glass opacities (GGO) (20/52) or GGO with consolidation (6/52). In total, GGO was mentioned in 37 cases. Ten cases required high flow nasal cannula (HFNC), 17 intubation/mechanical ventilation and 8 extracorporeal membrane oxygenation (ECMO) (Table 2). Bronchoscopy was performed in 43 cases; in 18 cases bronchoalveolar lavage (BAL) was positive for lipid-laden macrophages (LLMs), including Oil Red O Staining in 14 and 4 without Oil Red O Staining information. For 17 cases transbronchial biopsy was performed, 5 of whom were diagnosed with organizing pneumonia. For an additional 3 cases open lung biopsy was performed (Table 3). Majority was treated with corticosteroid administration (40/58) while 24 cases were also given antibiotics. Majority of patients recovered and were discharged home (n = 48), two patients were discharged but hospitalized again for asthma exacerbation, one left against medical advice however he was re-hospitalized, and 6 presented persisting abnormalities in lung function tests, long-term rehabilitation, psychiatric care. Additionally there was one fatality.
Additionally, 8 publications presenting respiratory aggregate cases were identified. A total of 104 cases with EVALI symptoms cared for in the authors’ institutions were presented in 6 papers [24,66,67,68,69,70], while in one publication [42] the focus was on Illinois and Wisconsin patients (53 cases) and in another publication [71] 60 EVALI patients admitted to 24 hospitals of Utah, Idaho, Wyoming and Nevada were presented.
3.3. CDC Reports on Respiratory Cases
Since the EVALI outbreak, CDC has published 19 reports in an effort to provide guidance to health care professionals and assist them identify patients with EVALI symptoms [15,72,73,74], to describe the patients’ characteristics [17,75,76,77,78,79,80,81,82,83] and to identify possible risk-factors associated with EVALI [6,84,85,86,87]. According to the most recent CDC report published on the 24th January 2020 [87], 2668 EVALI patients have been hospitalized in the USA and reported to the CDC until the 14th January 2020.
3.4. Traumatic Injury
42 publications presenting 126 cases of injury were identified. The majority of injuries were caused due to explosion (82/126), including 48 assembled devices [21,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114], 20 self-exploded batteries [95,97,102,111,112,115,116,117] and 14 without specifying [118]. Second most common injury was thermal burns (24/126) caused by 14 self-combusted batteries [21,111,115,119,120,121,122], 6 self-combusted assembled devices [115,120,123,124], 2 exploding devices [99,102], 1 in-pocket e-cigarette self-activation [125] and 1 case for which the ignition was induced by a motorcycle crash [111]. A combination of explosion and thermal burn caused by the explosion of the assembled device (7/126) [21,112,120,126,127] was the 3rd most common cause of injury. In 1 case their injury was caused by a flash burn [93] and in 12 it was not specified [128]. The type or generation of the device was not provided in the vast majority of papers.
The vast majority of cases were in the USA (99/126), male (120/126) with a median age of 28 years old. Most common affected body areas were the thighs (85/126) followed by hands (49/126). Twenty-three cases sustained facial injuries, with three including eye injuries. In total 55 cases sustained injuries in multiple body areas. Information on total burn surface area (TBSA) was given for the majority of cases (101/126), with median TBSA 4% (IQR: 2–6%). Skin grafting was performed in 40 patients in total, including 25 who required both skin grafting and excision, 1 finger amputation, and 2 foreign body removals, while 18 patients required minor surgical procedures. The majority of cases (105/126) were discharged with no further complications while for 6 cases there was no discharge information provided. For the rest 15 cases complications included amputation (1/15), back pain (2/15), post-traumatic stress disorder (PTSD) (1/15), pain and scarring (1/15), scarring (2/15), photophobia (1/15), eye (2/15), teeth (1/15), and neurologic (3/15) complications, as well as discharge to burn center (1/15).
Additionally, 10 publications presenting aggregate cases with traumatic injury were identified: 5 publications [129,130,131,132,133] presented a total of 86 cases from the authors’ institutions; 3 publications [90,134,135] presented a total of 311 cases from the USA (however the total number of cases could be overestimated as those publications could have some mutual cases presented). Finally, 2 additional publications [136,137] presented USA burn centers cases estimated as 2035 in 2015–2017 and 1007 in 2016.
3.5. Poisoning
Twenty-five papers presenting 28 cases of nicotine poisonings were identified. Interestingly, publications were not limited to the USA but were scattered around the world (6 from the USA, 3 from Korea, 2 from each of the UK, Italy and Germany and 1 from each of Poland, Denmark, Canada, China, France, Japan, the Netherlands, South Korea, Switzerland, and Turkey).
Poisonings were caused by accidental (9/28) [138,139,140,141,142,143,144,145,146] or intentional ingestion (14/28) [147,148,149,150,151,152,153,154,155,156,157,158] of e-liquid, intravenous injection of e-liquid (4/28) [151,159,160,161], or both ingestion and injection (1/28) [162].
Accidental ingestion was observed only to young children with a median age of 2 years old (IQR: 0.85–4) and mainly to females (6/8). Information on sex was not available for 1 case. Nicotine concentration was available for 3 cases, with nicotine ingested being 8.2 mg, 50 mg, and 60 mg. Two children required admission to the intensive care unit (ICU) and intubation, while most children did not require an invasive treatment (5/9). The most severe outcome was death (2/9), followed by hearing complications (1/9), while the majority of cases were discharged home with no complications (6/9).
Suicidal attempts (i.e., ingestion and injection of e-liquid) were mainly by adults with a median age of 27 years old (IQR: 22–36). Majority of cases were male (13/19). Total nicotine intake was available for 8 cases, ranging from 2100 mg to 128.8 mg. ICU admission was required in 8 cases, including 3 intubations. Information on treatment was available for 9 cases with most common being the administration of activated charcoal (5/9). Eight cases were discharged without complications, 4 cases were reportedly improved without further information, 1 case remained semi-comatose without awareness (Cerebral Performance Category 4), and 6 were fatal (3 deaths at hospital and 3 at the scene).
Additionally, 10 papers presenting aggregate data on poisonings were identified. Between 2010–2013 the USA Poison Centers received 1700 calls regarding exposures to electronic cigarettes [13], between 2010–2014 the calls were 2405 [163] whereas between 2010–2018 the calls were 17,358 [164]. In Texas, Poison Centers received 225 calls between 2009–2014 [165], while in Utah 52 cases were reported to have been poisoned by a synthetic cannabinoid in 2017–2018 [166]. Between 2012–2015, 277 calls were made to 10 European Countries’ Poison Centers [12], while in the UK, between 2008-2016, 278 calls were made to the National Poisons Information Service (NPIS) regarding children under 16 years old [167]. Between 2012–2018, 148 cases of acute exposure to e-cigarettes were reported to Czech Toxicological Information Centre [69]. Finally, 2 publications presented an estimate on the poisonings of under 5 year-olds between 2013–2017 (4745 cases) [168] and in 2018 (885 cases) [169].
3.6. Allergy
Four publications presenting 5 cases of allergic contact dermatitis to nickel were identified [170,171,172,173]. Majority of patients were female (3/5), in their late 30s or early 50s. For all cases the dermatitis was treated with the avoidance of the e-cig.
3.7. Effect on Medication Metabolism and Plasma Levels
We identified 2 publications presenting 2 cases for which the use of the electronic cigarette increased their clozapine levels [174,175] and 1 publication presenting a patient with epilepsy for whom it increased their seizure frequency [176]. Two of the patients were 16 years old and 23 years old females and there was one 52-year-old male.
3.8. Ulcerative Colitis
We identified 2 publications presenting 2 cases for whom the e-cig was associated with their ulcerative colitis. The first was a 2013 publication [177] from the USA presenting the case of a 35-year-old male for whom their ulcerative colitis improved after the initiation of an electronic cigarette. The second was a 2014 publication [178] from France, presenting a 49-year-old female smoker for whom their ulcerative colitis reappeared after switching from combustible to electronic cigarette.
3.9. Misuse of E-liquid
Accidental misuse of e-liquid was presented in 2 publications [179,180]. Two case reports (a 50-year-old and a 32-year-old female) were presented, who have mistaken the e-liquid bottle for eye-drops. Both had immediately realized their mistake and washed out the liquid before attending the emergency department (ED). Treatment was described for one of the cases for whom their misuse resulted in corneal burn, including eye irrigation, analgesics, anti-inflammatory and antibiotic eye-drops.
3.10. Injury Caused by Falling with E-cigarette in Mouth
A case report of a male in the USA who fell while he had his e-cigarette in his mouth [181] was presented in a letter published in 2018. The age of the patient was not published. The reason of the fall was speculated to be due to loss of consciousness resulting in ICU admission, tracheostomy, intraoperative examination, esophagogastroduodenoscopy and feeding tube placement, which was needed even at 6-month follow-up.
3.11. Additional Diagnoses and Health Effects Attributed to Electronic Cigarette Use
Thirteen publications were identified; 4 oral cases including a lingua villosa nigra [182], a lichenoid eruption [183], a necrotic ulcer [184], and an acute uvulitis [185], 2 cases with skin-grafts compromise [186,187], 2 cases of coronary events [188,189] in 16 and 24 year-old males, 1 case of neonatal necrotizing enterocolitis due to in-utero exposure [190], and 1 case of polycythemia [191]. Finally, in one case the e-cigarette use was attributed an anti-inflammatory [192] and in 2 cases an antibacterial [193,194] effect.
Detailed information for each case report included in the current review is presented in the Supplementary Materials Table S1: “E-cigarette related case reports by type of injury”.
4. Discussion
The shift towards novel noncombustible products has significantly altered the diagnostic algorithm by introducing to clinical practice new risk factors for adverse health effects.
The current review is the first to show the potential of e-cigarette use to lead not only to acute and severe lung injury syndromes, but also to acute poisonings, traumatic injuries and to interfere with medication bioavailability, in addition to providing a new vehicle for the inhalational abuse of several psychoactive medications and recreational drugs that may easily be added in the e-liquid.
Three major categories of cases were reported: medical, poisonings and injuries. Majority of respiratory cases were associated with Tetrahydrocannabinol (THC) use, whereas, cases of acute poisonings were mainly associated with nicotine use.
4.1. Respiratory Injuries
Majority of reported cases referred to previously healthy adolescents and young adults, a finding of great significance as studies have shown that adolescents addicted to nicotine are predisposed to addiction to other substance/substances as well [195]. Users experienced a range of acute and severe lung injury syndromes, that led to hospitalizations, need for mechanical ventilation, use of ECMO and ultimately loss of life.
In the majority of cases, the injury involved distal airway and parenchymal areas and the histologic findings of acute lung injury patterns, mainly DAD and organizing pneumonia, were consistent with an underlying inflammatory pathophysiology [39]. The inflammatory pathway response, possibly triggered by the inhalational exposure to e-cigarette aerosols, was expressed as various types of pneumonitis (lipoid, organizing, eosinophilic, hypersensitivity, interstitial) often complicated by ARDS [196].
No single causal factor has yet been identified for EVALI, however it is worth noting that most cases in the current review and over 80% of those reported to the CDC [87], have used cannabinoids. Vaping cannabinoid oils has been associated with lipoid pneumonia [29] and LLMs were detected in almost half (19/35) of the bronchoscopy specimens included in the current review.
Although there was not a consistent method of LLM staining, measurement and reporting, and their role in diagnosis of EVALI has been questioned by researchers [197], majority of lipoid pneumonia cases in this review complied with the diagnostic criteria [198], suggesting a possibly new risk factor for exogenous lipoid pneumonia [199].
The 6th report issued by CDC [84] was dedicated to discussing the lipoid pneumonia cases in association with vaping; diagnosis criteria used, included: Lipid-containing e-liquid use (such as marijuana oils), consistent imaging (CT scan/radiography), exclusion of differential diagnoses and presence of LLMs in BAL cytology preferably using oil Red-O or Sudan stain.
Travis S. et al. [22], suggest that even non-oil e-liquid ingredients may potentially trigger the endogenous phospho-lipidosis mechanism and lead to an amiodarone like lung toxicity, therefore representing a risk factor for endogenous lipoid pneumonia as well. Furthermore, vaping has been causally associated with acute eosinophilic pneumonia similarly to smoking [200,201].
The current review also highlighted the adverse effect of vaping on asthma, which similarly to that of smoking, is translated in asthma exacerbation with potentially more frequent, severe, or difficult to control asthma attacks [54,202].
Pneumothorax cases, although pertaining to predisposed individuals (blebs and bullae on CT scan, compatible body type), indicate that vaping should be also considered as a risk factor for pneumothorax and emphysema. Cannabis vaping in particular, could have induce barotrauma, spontaneous pneumothorax and bullous emphysema similarly to cannabis smoking; the deep inhalation practiced by marihuana users has been proposed to possibly lead to more negative alveolar pressure and alveolar-capillary membrane injury [203]. Furthermore, the anticoagulant activity known to be exerted by cannabis could explain the occurrence of hemoptysis and diagnosis of DAH [204].
The etiology of organizing pneumonia includes infectious agents, medications, chemicals, and radiation, suggesting that exposure to an inhalational trigger originating from the e-liquid constituents and, or their degradation products, possibly diacetyl, could be the cause [205]. Diacetyl has been previously suggested as the cause of BOOP in diacetyl plant workers [206] and workers in microwave popcorn industry [207], while rats exposed in diacetyl inhalation developed airway epithelium changes, consistent with BOOP [208]; diagnosis requires evidence of exposure, exclusion of infection or other illness, compatible lung function tests, chest CT scan and ultimately lung biopsy, criteria that have been met by the case reports included in the current review.
Carl A. Vas et al. [209], in a study funded by British American Tobacco (BAT), showed that the use of acetoine in e-liquids leads to a continuous diacetyl formation even during storage time, a process dependent on the influence of light, nicotine concentration, increased e-liquid pH and levels of propylene glycol (PG)/vegetable glycerin (VG); therefore the authors suggest that acetoine addition to e-liquids should be avoided. Vitamin E acetate has been suggested as another possible toxicant especially for EVALI [86]. Vitamin E acetate was present in the majority of the bronchoalveolar lavage (BAL) samples collected from EVALI patients, who used THC, while it was not identified in the healthy controls; e-cig liquids containing THC, usually also contain various concentrations of vitamin E acetate which is used to dilute, “cut” THC [210].
Furthermore, studies on humans have shown that vaping altered nasal mucosa genes towards immune suppression [211], levels and expression of >200 bronchial epithelium proteins associated with membrane functionality, possibly through PG/VG [212], in addition to pulmonary lipid homeostasis and immunity alterations, through ingredients other than nicotine [213].
As of January 2020, 2668 hospitalized EVALI cases had been reported, with 82% of those having reported use of THC containing e-liquid [87]; vitamin E acetate was strongly associated with EVALI, however potential toxicant role of other ingredients has not yet been ruled out [83]. The causal role of each of the e-liquid ingredients, their thermolysis byproducts, potential interactions and additive effect, as well as the role of the patients’ immunological response to the ultimate injury expression and outcome, is the subject of ongoing scientific research [67]. Currently CDC recommends avoidance of THC-containing e-liquids and especially those originating from unauthorized/illicit sources [87].
4.2. Accidents
The majority of reviewed cases presented accidents leading to traumatic, chemical, and thermal injuries, mainly caused by technical/safety flaws, as the device and or the battery could self-explode. Self-explosion of the assembled device, or the “in pocket kept” battery, may explain why the majority of cases were men with thigh and or hand injuries; the warm and humid pocket conditions and the presence of metallic objects usually keys have been proposed as possible causes.
Patterson [90] proposed a dual classification of burn injuries from e-cigarette explosions: direct/indirect and an additional arithmetic classification in types 1–5b: types 1, 2, and 3 were defined by the body area affected (hand, face, waist, and groin), type 4 included injuries from house fire, while type 5 included inhalation injuries from device on fire further subclassified in—5a (upper airway injuries from direct flash or explosion of the e-cigarette), and 5b (chemical, subglottic smoke inhalation injury). Types 1, 2, 3, and 5a were the direct injuries, whereas type 4 and 5b were the indirect ones from house or vehicle fire. Patterson then proceeded to indicate preventive measures to fit each specific type.
4.3. Poisonings
In the pre-e-cigarette era, nicotine intoxication was rarely reported in humans, with the exception of tobacco manufacturing workers [214]. E-cigarette exposes user to novel risk factors for poisonings involving both adults and accidentally children and has brought to light the underestimated, often times ignored, nicotine, PG, and cannabis toxicity. In the cardiovascular category of this review the cases of two young adults were presented who experienced acute myocardial ischemia; as the presence of other risk factors was excluded, it was suggested that the cause was the effect of cannabis and nicotine in the e-liquid used respectively.
Case reports of nicotine poisonings gave a new insight in the metabolism, bioavailability and the dose/effect relationship of the substance [19,151], adding to the existing controversies regarding the level of fatal nicotine concentrations. The fatal dose of 60 mg indicated by Lazutka et al. in 1969 [215] was based on studies in mice, while more recent studies suggest an oral lethal dose of 0.5–1 gr [216].
Injection of nicotine and PG as contained in the e-liquid mixture, leads to a new type of acute intoxication, physicians should be aware of. PG intoxication leads to lactic acidosis with elevated anion gap, while acute nicotine intoxication presents in two progressively aggravating clinical phases; until the second more severe phase of Central Nervous System depression and respiratory failure supervenes, there is a 3-hour window opportunity for the physician to intervene [161].
E-liquid storage together with medications, or in empty bottles previously containing other products was the main reason for misuse, as it has been mistaken for eye drops or medical syrup. Opthalmologists and emergency personnel should be aware of the variable e-liquid pH which can be acidic [180] or alkaline [179] and treat the eye as indicated to restore a normal eye pH.
Pediatric poisonings in particular, have increased alarmingly within the period 2010–2018 following the similarly increasing trend of e-cig use. Pediatric poisonings were accidental and majority of cases were treated without complications.
In contrast, adult poisonings primarily represented intentional suicidal attempts by ingestion and or injection; 1/3 of those suicidal attempts were successful.
Unmet device and package safety requirements such as child proof e-liquid bottle cap, the sweet, fruity e-liquid flavors, the various sizes of e-liquid vials and high nicotine concentrations, lie behind the accidental ingestion. Since 2016, in the EU and UK regulations regarding packaging with enhanced child-proof features protect the childhood population from undesired poisonings. Under the EU TPD, e-liquid vials should be limited to a maximum of 20mg/mL nicotine concentration, refill containers to a maximum of 10 mL size and tanks to a 2 mL size, thus containing 200 mg and 40 mg total nicotine quantity respectively [217]. However, outside the EU and through unauthorized sources over the web, refill mixtures may contain nicotine concentrations ranging from 18 mg/mL to 59 mg/mL, with the highest concentrations being contained by the newer device generations, especially those using protonated nicotine salts; moreover manipulation of the device’s wattage and voltage by the user may enhance the nicotine concentration in the delivered aerosol [8].
4.4. Substance Abuse
E-cigarette is used as a vehicle for inhalation of nicotine, cannabis, fentanyl and other psychoactive substances, either pure or combined in various mixtures; considering the addictive nature of those substances it becomes evident the potential of e-cig to function as a gateway to nicotine and various other addictive substances/drugs [17,218].
4.5. Seizures and Effect on Medication Metabolism
Rong et al., 2014 [219] concluded that smoking and epilepsy relationship is unclear, while Iha et al. [220] in their study in mice, suggested that nicotine activates certain brain regions such as the epileptogenic amygdala; indeed, nicotine injection to amygdala led to convulsive seizures, therefore supporting their hypothesis. Seizures are a known clinical feature of the acute nicotine intoxication. Cases reporting seizures in the current review, suggest that vaping induces seizures either through nicotine, either through PG and glycerol (G) induced circadian rhythm alterations [176]; the potential effect on medication levels should be also considered and therefore patients with epilepsy be informed accordingly.
Increased clozapine levels reported in the reviewed cases, practically highlight the effect of nicotine on medication plasma levels and raise wider concerns regarding potential interactions with other medications as well. Patients’ smoking and or vaping behavior should be extensively asked and specifically sought for by clinicians during history interview and more importantly, taken into account whenever changes of dosage or medication regimen are attempted. Furthermore, patients should be aware that even when switching between different tobacco products (i.e., combustible/e-cigarette and vice versa), or are planning smoking cessation, serum levels of their medication might be altered [221].
4.6. Regulatory Gap
The current review presents the emergence of a novel public health risk that although associated with the use of tobacco products, goes beyond pulmonology and expands across several medical specialties, previously unlikely to be implicated. In addition, it reveals the existent regulatory gap [222] regarding the ENDS and highlights the need for more efficient, universal, protective and preventive measures. Majority of the ENDS regulations to date are limited to simple recommendations. Multilevel, universal regulations should be placed regarding the design, development and safety of the device and its components, the ingredients/flavors/additives, including their package safety and quantity limit, especially for toxic compounds such as nicotine.
4.7. Limitations
A limited number of e-cigarette-related cases was excluded during the screening process because of insufficient information provided by the authors of the respective publications. The claimed health-effect was not clearly related to the e-cigarette use.
Another limitation derives from the fact that the current review focused on the e-cigarette-related clinical cases and published case reports, therefore experimental, observational studies and clinical trials were not included. Finally, worldwide public health organizations stands regarding e-cigarette regulation and regulation gaps as well as the harm reduction product and cessation tool approach regarding its use were not examined.
5. Conclusions
The current review is the first to show the full range of the e-cigarette-related injury which extends beyond the plausible respiratory disorder; in addition to the acute lung injury syndromes, it is also associated with accidents leading to traumatic and thermal injuries and severe, potentially fatal, acute intoxications. Physicians should be aware of the distinct clinical presentations and trained to respond and treat effectively. To protect and promote public health, regulators and public health authorities such as the European Commission, CDC, FDA, and WHO should address the regulatory gap regarding ENDS and novel tobacco products, aiming to protectively cover the global population.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/7/2248/s1, Table S1: e-cigarette-related case reports by type of injury.xlsx.
Author Contributions
All authors had substantially contributed to the manuscript; in particular A.T. was responsible for conceptualization and contributed in the screening process, critical review of publications, writing of the manuscript and supervision of the whole process. M.K. was responsible for databases search, methodology design and statistical analysis while she contributed to the screening process and writing of the manuscript. V.E. and P.B. contributed in the final decision regarding the inclusion or rejection of the manuscripts that were deemed of low quality and they provided critical review of all draft versions of the manuscript. All authors have read and agreed to the final version of the manuscript.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patel, D.; Davis, K.; Cox, S.; Brian, B.; King, B.A.; Sfaher, P.; Caraballo, R.; Rebecca, B. Reasons for Current E-Cigarette Use among U.S. Adults. Prev. Med. 2016, 93, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pinkston, R.; McLemore, B.; Dorsey, W.C.; Batra, S. Immunological and Toxicological Risk Assessment of E-Cigarettes. Eur. Respir. Rev. 2018, 27, 170119. [Google Scholar] [CrossRef] [PubMed]
- Kaisar, M.A.; Prasad, S.; Liles, T.; Cucullo, L. A Decade of E-Cigarettes: Limited Research & Unresolved Safety Concerns. Physiol. Behav. 2016, 365, 67–75. [Google Scholar] [CrossRef]
- Fernández, E.; Fu, M.; Martinez-Sanchez, J. Exposure to Aerosol from Smoking-Proxy Electronic Inhaling Systems: A Systematic Review. World Heal. Organ. Tob. Free Initiave 2016, 1–70. [Google Scholar]
- Fernández, E.; Ballbè, M.; Sureda, X.; Fu, M.; Saltó, E.; Martínez-Sánchez, J.M. Particulate Matter from Electronic Cigarettes and Conventional Cigarettes: A Systematic Review and Observational Study. Curr. Environ. Heal. Rep. 2015, 2, 423–429. [Google Scholar] [CrossRef]
- Ghinai, I.; Pray, I.W.; Navon, L.; Laughlin, K.O.; Saathoff-huber, L.; Hoots, B.; Kimball, A.; Tenforde, M.W.; Chevinsky, J.R.; Layer, M.; et al. E-Cigarette Product Use, or Vaping, Among Persons with Associated Lung Injury—Illinois and Wisconsin, April—September 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Stratton, K.; Kwan, L.Y.; Eaton, D.L. Public Health Consequences of E-Cigarettes: A Consesus Study Report of the National Academies of Sciences, Health, Medicine. J. Public Health Policy 2018, 39, 379–381. [Google Scholar] [CrossRef]
- Jenssen, B.P.; Boykan, R. Electronic Cigarettes and Youth in the United States: A Call to Action (at the Local, National and Global Levels). Children 2019, 6, 30. [Google Scholar] [CrossRef]
- Pisinger, C.; Døssing, M. A Systematic Review of Health Effects of Electronic Cigarettes. Prev. Med. 2014, 69, 248–260. [Google Scholar] [CrossRef]
- Hua, M.; Talbot, P. Potential Health Effects of Electronic Cigarettes: A Systematic Review of Case Reports. Prev. Med. Rep. 2016, 4, 169–178. [Google Scholar] [CrossRef]
- McCauley, L.; Markin, C.; Hosmer, D. An Unexpected Consequence of Electronic Cigarette Use. Chest 2012, 141, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Vardavas, C.I.; Girvalaki, C.; Filippidis, F.T.; Oder, M.; Kastanje, R.; De Vries, I.; Scholtens, L.; Annas, A.; Plackova, S.; Turk, R.; et al. Characteristics and Outcomes of E-Cigarette Exposure Incidents Reported to 10 European Poison Centers: A Retrospective Data Analysis. Tob. Induc. Dis. 2017, 15, 2010–2013. [Google Scholar] [CrossRef] [PubMed]
- Vakkalanka, J.P.; Hardison, L.S.; Holstege, C.P. Epidemiological Trends in Electronic Cigarette Exposures Reported to U.S. Poison Centers. Clin. Toxicol. 2014, 52, 542–548. [Google Scholar] [CrossRef]
- ICD-10-CM Official Coding Guidelines—Supplement Coding Encounters Related to E-Cigarette, or Vaping, Product Use; ICD-10-CM Coding Guidance Vaping related disorders; Centers for Disease Control and Prevention; Available online: https://www.cdc.gov/nchs/data/icd/Vapingcodingguidance2019_10_17_2019.pdf (accessed on 17 October 2019).
- Siegel, D.A.; Jatlaoui, T.C.; Koumans, E.H.; Kiernan, E.A.; Layer, M.; Cates, J.E.; Kimball, A.; Weissman, D.N.; Petersen, E.E.; Reagan-Steiner, S.; et al. Update: Interim Guidance for Health Care Providers Evaluating and Caring for Patients with Suspected E-Cigarette, or Vaping, Product Use Associated Lung Injury—United States, October 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 919–927. [Google Scholar] [CrossRef] [PubMed]
- 2019 Lung Injury Surveillance Primary Case Definitions; U.S. Department of Health and Human Services: Washington, DC, USA; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Ghinai, I.; Navon, L.; Gunn, J.K.L.; Duca, L.M.; Brister, S.; Love, S.; Brink, R. Characteristics of Persons Who Report Using Only Nicotine-Containing Products Among Interviewed Patients with E-Cigarette, or Vaping, Product Use—Associated Lung Injury—Illinois, August—December 2019. Morb. Mortal. Wkly. Rep. 2020, 69, 84–89. [Google Scholar] [CrossRef]
- Chalmers, S.; Von Buchwald, C.L.; Gajic, O. VpALI—Vaping-Related Acute Lung Injury: A New Killer Around the Block. Mayo Clin. Proc. 2019, 1–12. [Google Scholar] [CrossRef]
- Gotts, J.E.; Jordt, S.-E.; McConnell, R.; Tarran, R. What Are the Respiratory Effects of E-Cigarettes? BMJ 2019, 366, l5275. [Google Scholar] [CrossRef]
- Maessen, G.C.; Wijnhoven, A.M.; Neijzen, R.L.; Paulus, M.C.; van Heel, D.A.M.; Bomers, B.H.A.; Boersma, L.E.; Konya, B.; van der Heyden, M.A.G. Nicotine Intoxication by E-Cigarette Liquids: A Study of Case Reports and Pathophysiology. Clin. Toxicol. 2019, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seitz, C.M.S.; Kabir, Z. Burn Injuries Caused by E-Cigarette Explosions: A Systematic Review of Published Cases. Tob. Prev. Cessat. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Arnaout, A.; Khashaba, H.; Dobbs, T.; Dewi, F.; Pope-Jones, S.; Sack, A.; Estela, C.; Nguyen, D. The Southwest UK Burns Network (SWUK) Experience of Electronic Cigarette Explosions and Review of Literature. Burns 2017, 43, e1–e6. [Google Scholar] [CrossRef]
- Henry, T.S.; Kligerman, S.J.; Raptis, C.A.; Mann, H.; Sechrist, J.W.; Kanne, J.P. Imaging Findings of Vaping-Associated Lung Injury. Am. J. Roentgenol. 2020, 214, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Butt, Y.M.; Smith, M.L.; Tazelaar, H.D.; Vaszar, L.T.; Swanson, K.L.; Cecchini, M.J.; Boland, J.M.; Bois, M.C.; Boyum, J.H.; Froemming, A.T.; et al. Pathology of Vaping-Associated Lung Injury. N. Engl. J. Med. 2019, 381, 1780–1781. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef]
- Boloña, E.; Felix, M.; Vanegas, E.; Vera Paz, C.; Cherrez Ojeda, I. A Case of Vaping Associated Pulmonary Illness in South America: Highlighting the Need for Awareness and Surveillance Programs in the Region. Am. J. Respir. Crit. Care Med. Ja 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Saccone, N.M.; Lindner, D.H. A Case of Vaping TCH Oil Leading to Vaping Associated Pulmonary Injury: Our Approach to Its Diagnosis, Management, and Recommendations. Case Rep. Pulmonol. 2020. [Google Scholar] [CrossRef]
- Abeles, M.; Popofsky, S.; Wen, A.; Valsamis, C.; Webb, A.; Halaby, C.; Pirzada, M. Vaping-Associated Lung Injury Caused by Inhalation of Cannabis Oil. Pediatr. Pulmonol. 2019, 55, 226–228. [Google Scholar] [CrossRef]
- Buus, D.; Alzoubaidi, M.; Jamous, F. Vaping Induced Lung Injury: A Case Report. South Dakota Med. 2019, 72, 446–449. [Google Scholar]
- Casanova, G.S.; Amaro, R.; Soler, N.; Sánchez, M.; Badía, J.R.; Barberà, A.; Agustí, A. An Imported Case of E-Cigarette or Vaping Associated Lung Injury (EVALI) in Barcelona. Eur. Respir. J. 2019, 55, 1902076. [Google Scholar] [CrossRef]
- Pokhrel, K.; Goldman, C.; Smith, T.C.; Smith, T.C. Cannabinoid Oil Vaping Associated Lung Injury and Its Radiographic Appearance. Am. J. Med. 2019. [Google Scholar] [CrossRef]
- Ocampo-Gonzalez, F.A.; Park, J.W. Cytologic Features of Vaping-Induced Lung Injury: A Case Report. Diagn. Cytopathol. 2019, 1–3. [Google Scholar] [CrossRef]
- Itoh, M.; Aoshiba, K.; Herai, Y.; Nakamura, H.; Takemura, T. Lung Injury Associated with Electronic Cigarettes Inhalation Diagnosed by Transbronchial Lung Biopsy. Respirol. Case Rep. 2018, 6, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Qarajeh, R.; Kitchen, J. THC Vaping-Induced Acute Respiratory Distress Syndrome. Am. J. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Aftab, G.; Mudassar, A.; Douglas, F. Vaping-Associated Lung Injury. Cureus 2019. [Google Scholar] [CrossRef]
- Sakla, N.; Gattu, R.; Singh, G.; Sadler, M. Vaping-Associated Acute Respiratory Distress Syndrome. Emerg. Radiol. 2019, 27, 103–106. [Google Scholar] [CrossRef]
- Sharma, M.; Anjum, H.; Bulathsinghala, C.P.; Buch, M.; Surani, S.R. A Case Report of Secondary Spontaneous Pneumothorax Induced by Vape. Cureous 2019, 11. [Google Scholar] [CrossRef]
- He, T.; Oks, M.; Esposito, M.; Steinberg, H.; Makaryus, M. “Tree-in-Bloom”: Severe Acute Lung Injury Induced by Vaping Cannabis Oil. Ann. Am. Thoracic Soc. 2017, 14, 468–470. [Google Scholar] [CrossRef]
- Landman, S.T.; Dhaliwal, I.; Mackenzie, C.A.; Martinu, T.; Steele, A.; Bosma, K.J. Life-Threatening Bronchiolitis Related to Electronic Cigarette Use in a Canadian Youth. CMAJ 2019, 191, E1321–E1331. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mehrad, M.; Dammert, P.; Arrossi, A.V.; Sarda, R.; Brenner, D.S.; Maldonado, F.; Choi, H.; Ghobrial, M. Lung Biopsy Findings in Severe Pulmonary Illness Associated with E-Cigarette Use (Vaping). Am. J. Clin. Pathol. 2019, 153, 30–39. [Google Scholar] [CrossRef]
- Khan, M.S.; Khateeb, F.; Akhtar, J.; Khan, Z.; Lal, A.; Kholodovych, V.; Hammersley, J. Organizing Pneumonia Related to Electronic Cigarette Use: A Case Report and Review of Literature. Clin. Respir. J. 2018, 12, 1295–1299. [Google Scholar] [CrossRef]
- Layden, J.E.; Ghinai, I.; Pray, I.; Kimball, A.; Layer, M.; Tenforde, M.; Navon, L.; Hoots, B.; Salvatore, P.P.; Elderbrook, M.; et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin—Preliminary Report. N. Engl. J. Med. 2019, 1–14. [Google Scholar] [CrossRef]
- Flower, M.; Nandakumar, L.; Singh, M.; Wyld, D.; Windsor, M.; Fielding, D. Respiratory Bronchiolitis-Associated Interstitial Lung Disease Secondary to Electronic Nicotine Delivery System Use Confirmed with Open Lung Biopsy. Respirol. Case Rep. 2017, 5, 3–5. [Google Scholar] [CrossRef]
- Lu, M.A.; Jabre, N.A.; Mogayzel, P.J., Jr. Vaping-Related Lung Injury in an Adolescent. Am. J. Respir. Crit. Care Med. 2020. [Google Scholar] [CrossRef]
- Mantilla, R.D.; Darnell, R.T.; Sofi, U. Vapor Lung: Bronchiolitis Obliterans Organizing Pneumonia (BOOP) in Patient with E-Cigarette Use; American Thoracic Society Conferences, USA Publisher: Philadelphia, PA, USA, 2016. [Google Scholar]
- Ansari-Gilani, K.; Petraszko, A.M.; Teba, C.V.; Reeves, A.R.; Gupta, A.; Gupta, A.; Ramaiya, N.H.; Gilkeson, R.C. E-Cigarette Use Related Lung Disease, Review of Clinical and Imaging Findings in 3 Cases. Hear. Lung 2020, 1–5. [Google Scholar] [CrossRef]
- Modi, S.; Sangani, R.; Alhajhusain, A. Acute Lipoid Pneumonia Secondary to E-Cigarettes Use: An Unlikely Replacement for Cigarettes. Chest 2015, 148, 382A. [Google Scholar] [CrossRef]
- Abbara, S.; Fernando, M.; Kay, U. Electronic Cigarette or Vaping-Associated Lung Injury (EVALI): The Tip of the Iceberg. Radiol. Cardiothorac. Imaging 2019, 1, e190212. [Google Scholar] [CrossRef]
- Maddock, S.D.; Cirulis, M.M.; Callahan, S.J.; Keenan, L.M.; Pirozzi, C.S.; Raman, S.M.; Aberegg, S.K. Pulmonary Lipid-Laden Macrophages and Vaping. N. E. J. Med. 2019, 381, 1488–1489. [Google Scholar] [CrossRef]
- Viswam, D.; Trotter, S.; Burge, P.S.; Walters, G.I. Respiratory Failure Caused by Lipoid Pneumonia from Vaping E-Cigarettes. BMJ Case Rep. 2018, 2018, 1–4. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V.; Trachuk, P.; Fakier, F.; Teka, M.; Suhrland, M.J. Vaping - Associated Acute Respiratory Failure Due to Acute Lipoid Pneumonia. Lung 2019, 10–12. [Google Scholar] [CrossRef]
- Bonilla, A.; Blair, A.J.; Alamro, S.M.; Ward, R.A.; Feldman, M.B.; Dutko, R.A.; Karagounis, T.K.; Johnson, A.L.; Folch, E.E.; Vyas, J.M. Recurrent Spontaneous Pneumothoraces and Vaping in an 18-Year-Old Man: A Case Report and Review of the Literature. J. Med. Case Rep. 2019, 13, 4–9. [Google Scholar] [CrossRef]
- Lo, T. Vaping and Tension Pneumothorax: A Life-Threatening Association; American Thoracic Society Conferences: Philadelphia, PA, USA, 2017. [Google Scholar]
- Skertich, N.J.; Sullivan, G.A.; Madonna, M.B.; Shah, A.N. Vaping Is a Risk Factor for Spontaneous Pneumothorax: Two Cases. J. Pediatr. Surg. Case Rep. 2019, 50, 101305. [Google Scholar] [CrossRef]
- Bradford, L.E.; Rebuli, M.E.; Ring, B.J.; Jaspers, I.; Clement, K.C.; Loughlin, C.E. Danger in the Vapor? ECMO for Adolescents with Status Asthmaticus after Vaping. J. Asthma 2019, 1–5. [Google Scholar] [CrossRef]
- Arter, Z.L.; Wiggins, A.; Hudspath, C.; Kisling, A.; Hostler, D.C.; Hostler, J.M. Acute Eosinophilic Pneumonia Following Electronic Cigarette Use. Respir. Med. Case Rep. 2019, 27, 100825. [Google Scholar] [CrossRef]
- Thota, D.; Latham, E. Case Report of Electronic Cigarettes Possibly Associated with Eosinophilic Pneumonitis in a Previously Healthy Active-Duty Sailor. J. Emerg. Med. 2014, 47, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Salzman, G.A.; Alqawasma, M.; Asad, H. Vaping Associated Lung Injury (EVALi): An Explosive United States Epidemic. Mo. Med. 2019, 116, 492–496. [Google Scholar] [PubMed]
- Antwi-Amoabeng, D.; Islam, R. Vaping Is Not Safe: A Case of Acute Eosinophilic Pneumonia Following Cannabis Vapor Inhalation. Case Rep. Pulmonol. 2020. [Google Scholar] [CrossRef]
- Sommerfeld, C.G.; Weiner, D.J.; Nowalk, A.; Larkin, A. Hypersensitivity Pneumonitis and Acute Respiratory Distress Syndrome from E-Cigarette Use. Pediatrics 2018, 141. [Google Scholar] [CrossRef]
- Attis, M.; King, J.; Hardison, D.; Bridges, B. The Journey to ECMO Could Start with a Single Vape: A Case of Severe Hypersensitivity Pneumonitis in a Pediatric Patient. ASAIO 2018, 14. [Google Scholar] [CrossRef]
- Agustin, M.; Yamamoto, M.; Cabrera, F.; Eusebio, R. Diffuse Alveolar Hemorrhage Induced by Vaping. Case Rep. Pulmonol. 2018, 2018, 1–3. [Google Scholar] [CrossRef]
- Gutsche, J.; Pasternak, R.; Campbell, D.; Schili, J.L.; Boyle, P.J.; Tilney, P. A 19-Year-Old Man with Vaping-Associated Lung Injury. Air Med. J. 2020, 39, 6–8. [Google Scholar] [CrossRef]
- Youmans, A.J.; Harwood, J. Gross and Histopathological Findings in the First Reported Vaping-Induced Lung Injury Death in the United States. Am. J. ForensicMed. Pathol. 2020, 41, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bozzella, M.J.; Magyar, M.; DeBiasi, R.L.; Ferrer, K. Epiglottitis Associated With Intermittent E-Cigarette Use: The Vagaries of Vaping Toxicity. Pediatrics 2020, 145, e20192399. [Google Scholar] [CrossRef] [PubMed]
- Thakrar, P.D.; Boyd, K.P.; Swanson, C.P.; Wideburg, E.; Kumbhar, S.S. E-Cigarette, or Vaping, Product Use-Associated Lung Injury in Adolescents: A Review of Imaging Features. Pediatr. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, G.A.; Tiberio, P.J.; Zou, R.H.; Lamberty, P.E.; Lynch, M.J.; Kreit, J.W.; Gladwin, M.T.; Morris, A.; Chiarchiaro, J. Vaping-Associated Acute Lung Injury: A Case Series. Am. J. Respir. Crit. Care Med. 2019, 200, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Kalininskiy, A.; Bach, C.T.; Nacca, N.E.; Ginsberg, G.; Marraffa, J.; Navarette, K.A.; McGraw, M.D.; Croft, D.P. E-Cigarette, or Vaping, Product Use Associated Lung Injury (EVALI): Case Series and Diagnostic Approach. Lancet. Respir. Med. 2019, 7, 1017–1026. [Google Scholar] [CrossRef]
- Henry, T.S.; Kanne, J.P.; Kligerman, S.J. Imaging of Vaping-Associated Lung Disease. N. Engl. J. Med. 2019, 381, 1486–1487. [Google Scholar] [CrossRef]
- Zou, R.H.; Tiberio, P.J.; Ph, D.; Kreit, J.W. Clinical Characterization of E-Cigarette, or Vaping, Product Use Associated Lung Injury in 36 Patients in Pittsburgh, PA. Am. J. Respir. Crit. Care Med. 2020. [Google Scholar] [CrossRef]
- Blagev, D.P.; Harris, D.; Dunn, A.C.; Guidry, D.W.; Grissom, C.K.; Lanspa, M.J. Clinical Presentation, Treatment, and Short-Term Outcomes of Lung Injury Associated with e-Cigarettes or Vaping: A Prospective Observational Cohort Study. Lancet 2019, 6736, 1–11. [Google Scholar] [CrossRef]
- Schier, J.G.; Meiman, J.G.; Layden, J.; Mikosz, C.A.; Vanfrank, B.; King, B.A. Severe Pulmonary Disease Associated with Electronic-Cigarette–Product Use—Interim Guidance. Morb. Mortal. Wkly. Rep. Sev. 2019, 68, 787–790. [Google Scholar] [CrossRef]
- Siegel, D.A.; Jatlaoui, T.C.; Koumans, E.H.; Kiernan, E.A.; Layer, M.; Cates, J.E.; Kimball, A.; Weissman, D.N.; Petersen, E.E.; Reagan-Steiner, S.; et al. Update: Interim Guidance for Health Care Providers for Managing Patients with Suspected E-Cigarette, or Vaping, Product Use—Associated Lung Injury—United States, November 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 1081–1086. [Google Scholar] [CrossRef]
- Evans, M.E.; Twentyman, E.; Click, E.S.; Goodman, A.B. Update: Interim Guidance for Health Care Professionals Evaluating and Caring for Patients with Suspected E-Cigarette, or Vaping, Product Use—Associated Lung Injury and for Reducing the Risk for Rehospitalization and Death Following Hospital Discharge. Morb. Mortal. Wkly. Rep. 2020, 68, 1189–1194. [Google Scholar] [CrossRef]
- Perrine, C.G.; Pickens, C.M.; Boehmer, T.K.; King, B.A.; Jones, C.M. Characteristics of a Multistate Outbreak of Lung Injury Associated with E-Cigarette Use, or Vaping—United States, 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.; McCaffrey, K.; Sage, K.; Cheng, C.J.; Green, J.; Goldstein, L.; Campbell, H.; Ferrell, D.; Malan, N.; LaCross, N.; et al. E-Cigarette Use, or Vaping, Practices and Characteristics Among Persons with Associated Lung Injury—Utah, April-October 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Moritz, E.D.; Zapata, L.B.; Lekiachvili, A.; Glidden, E.; Annor, F.B.; Werner, A.K.; Ussery, E.N.; Hughes, M.M.; Kimball, A.; DeSisto, C.L.; et al. Update: Characteristics of Patients in a National Outbreak of E-Cigarette, or Vaping, Product Use-Associated Lung Injuries—United States, October 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 985–989. [Google Scholar] [CrossRef]
- Chatham-Stephens, K.; Roguski, K.; Jang, Y.; Cho, P.; Jatlaoui, T.C.; Kabbani, S.; Glidden, E.; Ussery, E.N.; Trivers, K.F.; Evans, M.E.; et al. Characteristics of Hospitalized and Nonhospitalized Patients in a Nationwide Outbreak of E-Cigarette, or Vaping, Product Use–Associated Lung Injury—United States, November 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Wiens, T.; Peterson, J.; Saravia, S.; Lunda, M.; Hanson, K.; Wogen, M.; D’Heilly, P.; Margetta, J.; Bye, M.; et al. Characteristics of E-Cigarette, or Vaping, Products Used by Patients with Associated Lung Injury and Products Seized by Law Enforcement—Minnesota, 2018 and 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Lozier, M.J.; Wallace, B.; Anderson, K.; Ellington, S.; Jones, C.M. Update: Demographic, Product, and Substance-Use Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-Cigarette, or Vaping, Product Use—Associated Lung Injuries—United States, December 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 1142–1148. [Google Scholar] [CrossRef]
- Gaub, K.L.; Hallyburton, S.; Samanic, C.; Paddack, D.; Clark, C.R.; Pence, S.; Brown, J.A.; Hawkins, E. Patient Characteristics and Product Use Behaviors Among Persons with E-Cigarette, or Vaping, Product Use-Associated Lung Injury-Indiana, June–October 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 1139–1141. [Google Scholar] [CrossRef]
- Mikosz, C.A.; Danielson, M.; Anderson, K.N.; Pollack, L.A.; Currie, D.W.; Njai, R. Characteristics of Patients Experiencing Rehospitalization or Death After Hospital Discharge in a Nationwide Outbreak of E-Cigarette, or Vaping, Product Use—Associated Lung Injury—United States, 2019. Morb. Mortal. Wkly. Rep. 2020, 68, 1183–1188. [Google Scholar] [CrossRef]
- Lozier, M.J.; Wallace, B.; Anderson, K.; Ellington, S.; Jones, C.M.; Rose, D.; Baldwin, G.; King, B.A.; Briss, P.; Mikosz, C.A. Update: Product, Substance-Use, and Demographic Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-Cigarette, or Vaping, Product Use—Associated Lung Injury—United States, August 2019–January 2020. Morb. Mortal. Wkly. Rep. 2019, 69, 44–49. [Google Scholar] [CrossRef]
- He, T.; Oks, M.; Esposito, M.; Steinberg, H.; Makaryus, M. Outbreak of Electronic-Cigarette—Associated Acute Lipoid Pneumonia—North Carolina, July–August 2019. Morb. Mortal. Wkly. Rep. 2019, 68. [Google Scholar] [CrossRef]
- Navon, L.; Jones, C.M.; Ghinai, I.; King, B.A.; Briss, P.A.; Hacker, K.A.; Layden, J.E. Risk Factors for E-Cigarette, or Vaping, Product Use-Associated Lung Injury (EVALI) Among Adults Who Use E-Cigarette, or Vaping, Products-Illinois, July-October 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Karwowski, M.P.; Morel-Espinosa, M.; Rees, J.; Sosnoff, C.; Cowan, E.; Gardner, M.; Wang, L.; Valentin-Blasini, L.; Silva, L.; et al. Evaluation of Bronchoalveolar Lavage Fluid from Patients in an Outbreak of E-Cigarette, or Vaping, Product Use-Associated Lung Injury-10 States, August-October 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 1040–1041. [Google Scholar] [CrossRef]
- Krishnasamy, V.P.; Hallowell, B.D.; Ko, J.Y.; Board, A.; Hartnett, K.P.; Salvatore, P.P.; Melstrom, P.; Haag, B.; King, B.A.; Briss, P.; et al. Update: Characteristics of a Nationwide Outbreak of E-Cigarette, or Vaping, Product Use—Associated Lung Injury—United States, August 2019–January 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 90–94. [Google Scholar] [CrossRef]
- Moore, J.; Mihalache, G.; Messahel, A. “Exploding” Electronic Cigarette: A Case Report. Br. J. Oral Maxillofac. Surg. 2016, 54, 1056–1057. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.J.S.; Eshraghi, N.; Kemalyan, N.A.; Mueller, C. Electronic Cigarette Burns: A Case Series. Trauma 2018, 21, 103–106. [Google Scholar] [CrossRef]
- Brooks, J.K.; Kleinman, J.W.; Brooks, J.B.; Reynolds, M.A. Electronic Cigarette Explosion Associated with Extensive Intraoral Injuries. Dent. Traumatol. 2016, 33, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Kumetz, E.A.; Hurst, N.D.; Cudnik, R.J.; Rudinsky, S.L. Electronic Cigarette Explosion Injuries. Am. J. Emerg. Med. 2016, 34, 2252.e1–2252.e3. [Google Scholar] [CrossRef] [PubMed]
- Norii, T.; Plate, A. Electronic Cigarette Explosion Resulting in a C1 and C2 Fracture: A Case Report. J. Emerg. Med. 2016, 52, 86–88. [Google Scholar] [CrossRef]
- Harrison, R.; Hicklin, D. Electronic Cigarette Explosions Involving the Oral Cavity. J. Am. Dent. Assoc. 2016, 147, 891–896. [Google Scholar] [CrossRef]
- Smith, S.L.; Smith, C.; Cheatham, M.; Smith, H.G. Electronic Cigarettes: A Burn Case Series. J. Nurse Pract. 2017, 13, 693–699. [Google Scholar] [CrossRef]
- Shastry, S.; Langdorf, M.I. Electronic Vapor Cigarette Battery Explosion Causing Shotgun-like Superficial Wounds and Contusion. West. J. Emerg. Med. 2016, 17, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Vaught, B.; Spellman, J.; Shah, A.; Stewart, A.; Mullin, D. Facial Trauma Caused by Electronic Cigarette Explosion. Ear Nose Throat J. 2017, 96, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Khairudin, M.N.; Zahidin, A.Z.M.; Bastion, M.L.C. Front to Back Ocular Injury from a Vaping-Related Explosion. BMJ Case Rep. 2016, 2016, 1–4. [Google Scholar] [CrossRef]
- Foran, I.; Oak, N.R.; Meunier, M.J. High-Pressure Injection Injury Caused by Electronic Cigarette Explosion: A Case Report. JBJS Case Connect. 2017, 7, e36. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, N.; Beavers, K.; Yu, A.; Gardner, K. 2016: ELECTRONIC CIGARETTES AND THERMAL INJURY: A CASE REPORT ON THE DANGERS OF VAPING. Crit. Care Med. 2016, 44, 578. [Google Scholar] [CrossRef]
- Colaianni, C.A.; Tapias, L.F.; Cauley, R.; Sheridan, R.; Schulz, J.T.; Goverman, J. Injuries Caused by Explosion of Electronic Cigarette Devices; Interesting Case, 2016; 16: ic9; 2016; Available online: www.ePlasty.com (accessed on 29 February 2016).
- Cason, D.E.; Morgan, D.E.; Pietryga, J.A. Injuries from an Exploding E-Cigarette: A Case Report. Ann. Intern. Med. 2016, 165, 678–679. [Google Scholar] [CrossRef]
- Katz, M.G.; Russell, K.W. Injury from E-Cigarette Explosion. N. Engl. J. Med. 2019, 380, 2460. [Google Scholar] [CrossRef]
- Rogér, J.M.; Abayon, M.; Elad, S.; Kolokythas, A. Oral Trauma and Tooth Avulsion Following Explosion of E-Cigarette. J. Oral Maxillofac. Surg. 2016, 74, 1181–1185. [Google Scholar] [CrossRef]
- Jiwani, A.Z.; Williams, J.F.; Rizzo, J.A.; Chung, K.K.; King, B.T.; Cancio, L.C. Thermal Injury Patterns Associated with Electronic Cigarettes. Int. J. Burns Trauma 2017, 7, 1–5. [Google Scholar]
- Maraqa, T.; Mohamed, M.A.T.; Salib, M.; Morris, S.; Mercer, L.; Sachwani-Daswani, G.R. Too Hot for Your Pocket! Burns from E-Cigarette Lithium Battery Explosions: A Case Series. J. Burn Care Res. 2018, 39, 1043–1047. [Google Scholar] [CrossRef]
- Ackley, E.; Williams, J.T.B.; Kunrath, C.; Monson, M.; Ignatiuk, A.; Gaensbauer, J. Too Hot to Handle? When Vaporizers Explode. J. Pediatr. 2018, 196, 320.e1. [Google Scholar] [CrossRef]
- Chi, A.C.; Neville, B.W.; Ravenel, M. Electronic Cigarette Explosion: Case Report of an Emerging Cause of Orofacial Trauma. Trauma 2018, 20, 62–67. [Google Scholar] [CrossRef]
- Patterson, S.B.; Beckett, A.R.; Lintner, A.; Leahey, C.; Greer, A.; Brevard, S.B.; Simmons, J.D.; Kahn, S.A. A Novel Classification System for Injuries after Electronic Cigarette Explosions. J. Burn Care Res. 2016, 38, e95–e100. [Google Scholar] [CrossRef]
- Anderson, H.; Richie, C.; Bernard, A. A Surprisingly Volatile Smoking Alternative: Explosion and Burns as Risks of E-Cigarette Use. J. Burn Care Res. 2017, 38, e884. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.; Krishnan, D.G.; Abdallah, Y. Ballistic Trauma from an Exploding Electronic Cigarette: Case Report. Oral Maxillofac. Surg. Cases 2017, 3, 61–63. [Google Scholar] [CrossRef]
- Kite, A.C.; Le, B.Q.; Cumpston, K.L.; Hieger, M.A.; Feldman, M.J.; Pozez, A.L. Blast Injuries Caused by Vape Devices 2 Case Reports. Ann. Plast. Surg. 2016, 77, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Paley, G.L.; Echalier, E.; Eck, T.W.; Hong, A.R.; Farooq, A.V.; Gregory, D.G.; Lubniewski, A.J. Corneoscleral Laceration and Ocular Burns Caused by Electronic Cigarette Explosions. Cornea 2016, 35, 1015–1018. [Google Scholar] [CrossRef]
- Herlin, C.; Bekara, F.; Bertheuil, N.; Frobert, P.; Carloni, R.; Chaput, B. Deep Burns Caused by Electronic Vaping Devices Explosion. Burns 2016, 42, 1875–1877. [Google Scholar] [CrossRef]
- Archambeau, B.A.; Young, S.; Lee, C.; Pennington, T.; Vanderbeek, C.; Miulli, D.; Culhane, J.; Neeki, M. E-Cigarette Blast Injury: Complex Facial Fractures and Pneumocephalus. West. J. Emerg. Med. 2016, 17, 805–807. [Google Scholar] [CrossRef]
- Quiroga, L.; Asif, M.; Lagziel, T.; Bhat, D.; Caffrey, J. E-Cigarette Battery Explosions: Review of the Acute Management of the Burns and the Impact on Our Population. Cureus 2019, 11. [Google Scholar] [CrossRef]
- Treitl, D.; Solomon, R.; Davare, D.L.; Sanchez, R.; Kiffin, C. Full and Partial Thickness Burns from Spontaneous Combustion of E-Cigarette Lithium-Ion Batteries with Review of Literature. J. Emerg. Med. 2017, 53, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, K.J.; Rose, A.M.; Khan, M.A.A.; Quaba, O.; Lowrie, A.G. Thigh Burns from Exploding E-Cigarette Lithium Ion Batteries: First Case Series. Burns 2016, 42, e42–e46. [Google Scholar] [CrossRef] [PubMed]
- Hickey, S.; Goverman, J.; Friedstat, J.; Sheridan, R.; Schulz, J. Thermal Injuries from Exploding Electronic Cigarettes. Burns 2018, 44, 1294–1301. [Google Scholar] [CrossRef]
- Harshman, J.; Vojvodic, M.; Rogers, A.D. Burns Associated with E-Cigarette Batteries: A Case Series and Literature Review. Can. J. Emerg. Med. 2018, 20, S20–S28. [Google Scholar] [CrossRef]
- Bauman, Z.M.; Roman, J.; Singer, M.; Vercruysse, G.A. Canary in the Coal Mine—Initial Reports of Thermal Injury Secondary to Electronic Cigarettes. Burns 2017, 43, e38–e42. [Google Scholar] [CrossRef]
- Walsh, K.; Sheikh, Z.; Johal, K.; Khwaja, N. Rare Case of Accidental Fire and Burns Caused by E-Cigarette Batteries. BMJ Case Rep. 2016, 2016, 2015–2017. [Google Scholar] [CrossRef]
- Jablow, L.M.; Sexton, R.J. Spontaneous Electronic Cigarette Explosion: A Case Report. Am. J. Med. Case Reports 2015, 3, 93–94. [Google Scholar] [CrossRef][Green Version]
- Sheckter, C.; Chattopadhyay, A.; Paro, J.; Karanas, Y. Burns Resulting from Spontaneous Combustion of Electronic Cigarettes: A Case Series. Burn. Trauma 2016, 4, 4–7. [Google Scholar] [CrossRef]
- Michael, R.; Ebraheim, N.; Maier, J.; Tanios, M.; Kouri, A. Electronic Cigarette Burns: A Case Report and Review of Current Literature. Case Rep. Orthop. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Serror, K.; Chaouat, M.; De Runz, A.; Mimoun, M.; Boccara, D. Thigh Deep Burns Caused by Electronic Vaping Devices (e-Cigarettes): A New Mechanism. Burns 2017, 43, 1133–1135. [Google Scholar] [CrossRef]
- Bohr, S.; Almarzouqi, F.; Pallua, N. Extensive Burn Injury Caused by Fundamental Electronic Cigarette Design Flaw. Ann. Burns Fire Disasters 2016, 29, 231–233. [Google Scholar] [PubMed]
- Satteson, E.S.; Walker, N.J.; Tuohy, C.J.; Molnar, J.A. Extensive Hand Thermal and Blast Injury from Electronic Cigarette Explosion: A Case Report. Hand 2018. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, A.; Dewi, F.; Nguyen, D. Re: Burn Injuries from Exploding Electronic Cigarette Batteries: An Emerging Public Health Hazard. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 981–982. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Anwar, M.U.; Muthayya, P.; Jivan, S. Burn Injuries from Exploding Electronic Cigarette Batteries: An Emerging Public Health Hazard. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 1716–1718. [Google Scholar] [CrossRef]
- Ramirez, J.I.; Ridgway, C.A.; Lee, J.G.; Potenza, B.M.; Sen, S.; Palmieri, T.L.; Greenhalgh, D.G.; Maguina, P. The Unrecognized Epidemic of Electronic Cigarette Burns. J. Burn Care Res. 2017, 38, 220–224. [Google Scholar] [CrossRef]
- Toy, J.; Dong, F.; Lee, C.; Zappa, D.; Le, T.; Archambeau, B.; Culhane, J.T.; Neeki, M.M. Alarming Increase in Electronic Nicotine Delivery Systems-Related Burn Injuries: A Serious Unregulated Public Health Issue. Am. J. Emerg. Med. 2017, 35, 1781–1782. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Topol, E.J. Explosion Injuries from E-Cigarettes. N. Engl. J. Med. 2016, 375, 1400. [Google Scholar] [CrossRef]
- Serror, K.; Chaouat, M.; Legrand, M.M.; Depret, F.; Haddad, J.; Malca, N.; Mimoun, M.; Boccara, D. Burns Caused by Electronic Vaping Devices (e-Cigarettes): A New Classification Proposal Based on Mechanisms. Burns 2018, 44, 544–548. [Google Scholar] [CrossRef]
- Rudy, S.F.; Durmowicz, E.L. Electronic Nicotine Delivery Systems: Overheating, Fires and Explosions. Tob. Control 2017, 26, 10–18. [Google Scholar] [CrossRef]
- McKenna, L.A., Jr. Electronic Cigarette Fires and Explosions in the United States 2009–2016; US Fire Administration: Emmitsburg, MD, USA, 2017. [Google Scholar]
- Rossheim, M.E.; Livingston, M.D.; Soule, E.K.; Zeraye, H.A.; Thombs, D.L. Electronic Cigarette Explosion and Burn Injuries, US Emergency Departments 2015–2017. Tob. Control 2019, 28, 472–474. [Google Scholar] [CrossRef]
- Corey, C.G.; Chang, J.T.; Rostron, B.L. Electronic Nicotine Delivery System (ENDS) Battery-Related Burns Presenting to US Emergency Departments, 2016. Inj. Epidemiol. 2018, 5, 1–4. [Google Scholar] [CrossRef]
- Howard, C. A New Source for Nicotine Exposures in Pediatric Patients: Electronic Cigarettes. J. Emerg. Nurs. 2016, 42, 451–453. [Google Scholar] [CrossRef]
- Seo, A.D.; Kim, D.C.; Yu, H.J.; Kang, M.J. Accidental Ingestion of E-Cigarette Liquid Nicotine in a 15-Month-Old Child: An Infant Mortality Case of Nicotine Intoxication. Korean J. Pediatr. 2016, 59, 490–493. [Google Scholar] [CrossRef]
- Gupta, S.; Gandhi, A.; Manikonda, R. Accidental Nicotine Liquid Ingestion: Emerging Paediatric Problem. Arch. Dis. Child. Educ. Pract. Ed. 2014, 99, 1149. [Google Scholar] [CrossRef]
- Gill, N.; Sangha, G.; Poonai, N.; Lim, R. E-Cigarette Liquid Nicotine Ingestion in a Child: Case Report and Discussion. Can. J. Emerg. Med. 2015, 17, 699–703. [Google Scholar] [CrossRef]
- De Pieri, C.; Brisotto, S.; Marzona, F.; Dolcemascolo, V.; Cogo, P.E. Liquid Nicotine Intoxication Due to Dangerous Packaging. Pediatr. Emerg. Care 2019, 1. [Google Scholar] [CrossRef]
- Yuji, K.; Tanimoto, T.; Oshima, Y. Nicotine Poisoning in an Infant. N. Engl. J. Med. 2014, 370, 2248. [Google Scholar] [CrossRef]
- Eggleston, W.; Nacca, N.; Stork, C.M.; Marraffa, J.M. Pediatric Death after Unintentional Exposure to Liquid Nicotine for an Electronic Cigarette. Clin. Toxicol. 2016, 54, 890–891. [Google Scholar] [CrossRef]
- Demir, E.; Topal, S. Sudden Sensorineural Hearing Loss Associated with Electronic Cigarette Liquid: The First Case in the Literature. Int. J. Pediatr. Otorhinolaryngol. 2018, 114, 26–28. [Google Scholar] [CrossRef]
- Noble, M.J.; Longstreet, B.; Hendrickson, R.G.; Gerona, R. Unintentional Pediatric Ingestion of Electronic Cigarette Nicotine Refill Liquid Necessitating Intubation. Ann. Emerg. Med. 2017, 69, 94–97. [Google Scholar] [CrossRef]
- Schipper, E.M.; De Graaff, L.C.G.; Koch, B.C.P.; Brkic, Z.; Wilms, E.B.; Alsma, J.; Schuit, S.C.E. A New Challenge: Suicide Attempt Using Nicotine Fillings for Electronic Cigarettes. Br. J. Clin. Pharmacol. 2014, 78, 1469–1471. [Google Scholar] [CrossRef]
- Chen, B.C.; Bright, S.B.; Trivedi, A.R.; Valento, M. Death Following Intentional Ingestion of E-Liquid. Clin. Toxicol. 2015, 53, 914–916. [Google Scholar] [CrossRef]
- Park, E.J.; Min, Y.G. The Emerging Method of Suicide by Electronic Cigarette Liquid: A Case Report. J. Korean Med. Sci. 2018, 33, e52. [Google Scholar] [CrossRef]
- Christensen, L.B.; Van’t Veen, T.; Bang, J. Three Cases of Attempted Suicide by Ingestion of Nicotine Liquid Used in E-Cigarettes. Clin. Toxicol. 2013, 51, 290. [Google Scholar]
- Morley, S.; Slaughter, J.; Smith, P.R. Death from Ingestion of E-Liquid. J. Emerg. Med. 2017, 53, 862–864. [Google Scholar] [CrossRef]
- You, G.; Rhee, J.; Park, Y.; Park, S. Determination of Nicotine, Cotinine and Trans-3′-Hydroxycotinine Using LC/MS/MS in Forensic Samples of a Nicotine Fatal Case by Oral Ingestion of e-Cigarette Liquid. J. Forensic Sci. 2016, 61, 1149–1154. [Google Scholar] [CrossRef]
- Sommerfeld, K.; Łukasik-Głebocka, M.; Kulza, M.; Druzdz, A.; Panieński, P.; Florek, E.; Zielińska-Psuja, B. Intravenous and Oral Suicidal E-Liquid Poisonings with Confirmed Nicotine and Cotinine Concentrations. Forensic Sci. Int. 2016, 262, e15–e20. [Google Scholar] [CrossRef]
- Garat, A.; Nisse, P.; Kauv, M.; Mathieu-Nolf, M.; Allorge, D.; Mathieu, D. Lactic Acidosis Due to Voluntary E-Liquid Ingestion. Toxicol. Anal. Clin. 2016, 28, 329–332. [Google Scholar] [CrossRef]
- Bartschat, S.; Mercer-Chalmers-Bender, K.; Beike, J.; Rothschild, M.A.; Jübner, M. Not Only Smoking Is Deadly: Fatal Ingestion of e-Juice—A Case Report. Int. J. Legal Med. 2014, 129, 481–486. [Google Scholar] [CrossRef]
- Eberlein, C.K.; Frieling, H.; Köhnlein, T.; Hillemacher, T.; Bleich, S. Suicide Attempt by Poisoning Using Nicotine Liquid for Use in Electronic Cigarettes. Am. J. Psychiatry 2014, 171, 891. [Google Scholar] [CrossRef]
- Lam, R.P.K.; Tang, M.H.Y.; Leung, S.C.; Chong, Y.K.; Tsui, M.S.H.; Mak, T.W.L. Supraventricular Tachycardia and Acute Confusion Following Ingestion of E-Cigarette Fluid Containing AB-FUBINACA and ADB-FUBINACA: A Case Report with Quantitative Analysis of Serum Drug Concentrations. Clin. Toxicol. 2017, 55, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.H.; Kang, S.; Durey, A.; Kim, J.H.; Kim, A.J. Symptomatic Bradycardia Due to Nicotine Intoxication. Rev. Bras. Ter. Intensiva 2017, 30, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Ikeda, T.; Tani, N.; Shida, A.; Oritani, S.; Ishikawa, T. Evaluation of the Distribution of Nicotine Intravenous Injection: An Adult Autopsy Case Report with a Review of Literature. Int. J. Legal Med. 2019, 134, 243–249. [Google Scholar] [CrossRef]
- Caponnetto, P.; Campagna, D.; Papale, G.; Russo, C.; Polosa, R. Fatal Intravenous Injection of Electronic Nicotine Delivery System Refilling Solution. J. Med. Toxicol. 2014, 10, 202–204. [Google Scholar] [CrossRef]
- Belkoniene, M.; Socquet, J.; Njemba-Freiburghaus, D.; Pellaton, C. Near Fatal Intoxication by Nicotine and Propylene Glycol Injection: A Case Report of an e-Liquid Poisoning. BMC Pharmacol. Toxicol. 2019, 20, 14–18. [Google Scholar] [CrossRef]
- Cervellin, G.; Luci, M.; Bellini, C.; Lippi, G. Bad News about an Old Poison. A Case of Nicotine Poisoning Due to Both Ingestion and Injection of the Content of an Electronic Cigarette Refill. Emerg. Care J. 2013, 9, 18. [Google Scholar] [CrossRef]
- Asma, S.; Song, Y.; Cohen, J.; Eriksen, M.; Pechacek, T.; Cohen, N.; Iskander, J. Notes from the Field: Calls to Poison Centers for Exposures to Electronic Cigarettes--United States, September 2010–February 2014. Morb. Mortal. Wkly. Rep. 2014, 63, 277–280. [Google Scholar]
- Wang, B.; Liu, S.; Persoskie, A. Poisoning Exposure Cases Involving E-Cigarettes and e-Liquid in the United States, 2010–2018. Clin. Toxicol. 2019, 1–7. [Google Scholar] [CrossRef]
- Ordonez, J.E.; Kleinschmidt, K.C.; Forrester, M.B. Electronic Cigarette Exposures Reported to Texas Poison Centers. Nicotine Tob. Res. 2015, 17, 209–211. [Google Scholar] [CrossRef]
- Horth, R.Z.; Crouch, B.; Horowitz, B.Z.; Prebish, A.; Slawson, M.; McNair, J.; Elsholz, C.; Gilley, S.; Robertson, J.; Risk, I.; et al. Acute Poisonings from a Synthetic Cannabinoid Sold as Cannabidiol—Utah, 2017–2018. Morb. Mortal. Wkly. Rep. 2018, 67, 587–588. [Google Scholar] [CrossRef]
- Ang, E.; Tuthill, D.; Thompson, J. E-Cigarette Liquid Ingestion: A Fast Growing Accidental Issue in Children. Arch. Dis. Child. 2018, 103, 1091. [Google Scholar] [CrossRef]
- Chang, J.T.; Wang, B.; Chang, C.M.; Ambrose, B.K. National Estimates of Poisoning Events Related to Liquid Nicotine in Young Children Treated in US Hospital Emergency Departments, 2013–2017. Inj. Epidemiol. 2019, 6, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Rostron, B.L. Electronic Nicotine Delivery System (ENDS) Liquid Nicotine Exposure in Young Children Presenting to US Emergency Departments, 2018. Inj. Epidemiol. 2019, 5, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Shim, T.N.; Kosztyuova, T. Allergic Contact Dermatitis to Electronic Cigarette. Dermatitis 2018, 29, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, E.; Stone, N. Contact Allergy and Electronic Cigarettes (and Eyelash Curlers). Clin. Exp. Dermatol. 2017, 42, 682–683. [Google Scholar] [CrossRef] [PubMed]
- Maridet, C.; Atge, B.; Amici, J.M.; Taïeb, A.; Milpied, B. The Electronic Cigarette: The New Source of Nickel Contact Allergy of the 21st Century? Contact Dermat. 2015, 73, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.E.; De La Feld, S. E-Cigarette Dermatitis. Dermatitis 2019, 30, 272. [Google Scholar] [CrossRef]
- Khorassani, F.; Kaufman, M.; Lopez, L. Supatherapeutic Serum Clozapine Concentration After Transition from Traditional to Electronic Cigarettes. Arthritis Rheum. 2018, 38, 391–392. [Google Scholar] [CrossRef]
- Kocar, T.; Freudenmann, R.W.; Spitzer, M.; Graf, H. G. Switching from Tobacco Smoking to Electronic Cigarettes and the Impact on Clozapine Levels. J. Clin. Psychopharmacol. 2018, 38, 528–529. [Google Scholar] [CrossRef]
- Wharton, J.D.; Kozek, L.K.; Carson, R.P. Increased Seizure Frequency Temporally Related to Vaping: Where There’s Vapor, There’s Seizures? Pediatr. Neurol. 2019. [Google Scholar] [CrossRef]
- Han, C.; Yan, N.; Sun, C. E-Cigarettes as Salvage Therapy for Medically Refractory Ulcerative Colitis. Inflamm. Bowel Dis. 2013, (suppl.1). [Google Scholar] [CrossRef]
- Camus, M.; Gallois, G.; Marteau, P. Ulcerative Colitis and Electronic Cigarette: What’s the Matter? Am. J. Gastroenterol. 2014, 109, 608–609. [Google Scholar] [CrossRef] [PubMed]
- McCague, Y. Ocular Chemical Burns Secondary to Accidental Administration of E-Cigarette Liquid. Adv. Emerg. Nurs. J. 2018, 40, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Jamison, A.; Lockington, D. Ocular Chemical Injury Secondary to Electronic Cigarette Liquid Misuse. JAMA Ophthalmol. 2016, 134, 1443. [Google Scholar] [CrossRef] [PubMed]
- Andresen, N.S.; Lee, D.J.; Kowalski, C.E.; Bayon, R. Fall with E-Cigarette in Mouth Resulting in Pharyngeal and Esophageal Burns. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Farinha, H.; Martins, V. Lingua Villosa Nigra Associated with the Use of Electronic Cigarette. Acta Med. Port. 2015, 28, 393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bartram, A.; Jones, N.; Endersby, S. Lichenoid Eruption Associated with Use of an E-Cigarette. Br. J. Oral Maxillofac. Surg. 2016, 54, 475. [Google Scholar] [CrossRef]
- Cant, A.; Collard, B.; Cunliffe, D. Electronic Cigarettes: Necrotic Ulcer. Br. Dent. J. 2017, 222, 226. [Google Scholar] [CrossRef]
- Frossard, S.M.; Volansky, P.M.; Endara-Bravo, A.S. Acute Uvulitis Secondary to Electronic Cigarette Use. Am. J. Respir. Crit. Care Med. 2015, 191, A1556. [Google Scholar]
- Fracol, M.; Dorfman, R.; Janes, L.; Kulkarni, S.; Bethke, K.; Hansen, N.; Kim, J. The Surgical Impact of E-Cigarettes: A Case Report and Review of the Current Literature. Arch. Plast. Surg. 2017, 44, 477–481. [Google Scholar] [CrossRef]
- Agochukwu, N.; Liau, J.Y. Debunking the Myth of E-Cigarettes: A Case of Free Flap Compromise Due to e-Cigarette Use within the First 24 Hours. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, T.; Durmus, E.; Dervisova, R.; Sari, I.; Yesildag, O.; Sunbul, M. Acute Myocardial Infarction Due to Liquid Nicotine in a Young Man. Ther. Adv. Cardiovasc. Dis. 2014, 8, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.J.; Mahendran, A.K.; Bajaj, R.K.; Doshi, A.R. Myocardial Ischemia with Cannabinoid Use in an Adolescent. Cureus 2017, 2, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Gillen, S.; Saltzman, D. Antenatal Exposure to E-Cigarette Vapor as a Possible Etiology to Total Colonic Necrotizing Enterocolitits: A Case Report. J. Pediatr. Surg. Case Rep. 2014, 2, 536–537. [Google Scholar] [CrossRef][Green Version]
- Okuni-Watanabe, M.; Kurata, K.; Yakushijin, K. The First Case of E-Cigarette-Induced Polycythemia. Case Rep. Hematol. 2019, 2019, 2084325. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Romagna, G. Chronic Idiopathic Neutrophilia in A Smoker, Relieved after Smoking Cessation with the Use of Electronic Cigarette: A Case Report. Clin. Med. Insights Case Rep. 2013, 6. [Google Scholar] [CrossRef]
- Miler, J.A.; Hajek, P. Resolution of Recurrent Tonsillitis in a Non-Smoker Who Became a Vaper. A Case Study and New Hypothesis. Med. Hypotheses 2017, 109, 17–18. [Google Scholar] [CrossRef]
- Miler, J.A.; Hajek, P. Resolution of Chronic Nasal Staphylococcus Aureus Infection in a Non-Smoker Who Started to Use Glycerine Based e-Cigarettes: Antibacterial Effects of Vaping? Med. Hypotheses 2018, 118, 42–43. [Google Scholar] [CrossRef]
- England, L.J.; Aagaard, K.; Bloch, M.; Conway, K.; Cosgrove, K.; Grana, R.; Gould, T.J.; Hatsukami, D.; Jensen, F.; Kandel, D.; et al. Developmental Toxicity of Nicotine: A Transdisciplinary Synthesis and Implications for Emerging Tobacco Products. Neurosci. Biobehav. Rev. 2017, 72, 176–189. [Google Scholar] [CrossRef]
- Christiani, D.C. Vaping-Induced Lung Injury. N. Engl. J. Med. 2019, 1–2. [Google Scholar] [CrossRef]
- Pambuccian, S.E. Testing for Lipid-Laden Macrophages in Bronchoalveolar Lavage Fluid to Diagnose Vaping-Associated Pulmonary Injury. Are We There Yet? J. Am. Soc. Cytopathy 2019. [Google Scholar] [CrossRef] [PubMed]
- Hadda, V.; Khilnani, G.C. Lipoid Pneumonia: An Overview. Expert Rev. Respir. Med. 2010, 4, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, S.L.; Martinez-Jimenez, S.; Rossi, S.E.; Truong, M.T.; Carrillo, J.; Erasmus, J.J. Lipoid Pneumonia: Spectrum of Clinical and Radiologic Manifestations. Am. J. Roentgenol. 2010, 194, 103–109. [Google Scholar] [CrossRef]
- De Giacomi, F.; Vassallo, R.; Yi, E.S.; Ryu, J.H. Acute Eosinophilic Pneumonia. Am. J. Respir. Crit. Care Med. 2018, 197, 728–736. [Google Scholar] [CrossRef] [PubMed]
- De Giacomi, F.; Decker, P.; Vassallo, R.; Ryu, J. Smoking-Related Acute Eosinophilic Pneumonia Compared to Idiopathic and Drug-Related Forms. A42. NEW DEVELOPMENTS IN ILD RESEARCH. Available online: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A1571 (accessed on 21 January 2020).
- Lappas, A.S.; Tzortzi, A.S.; Konstantinidi, E.M.; Teloniatis, S.I.; Tzavara, C.K.; Gennimata, S.A.; Koulouris, N.G.; Behrakis, P.K. Short-Term Respiratory Effects of e-Cigarettes in Healthy Individuals and Smokers with Asthma. Respirology 2018, 23, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Underner, M.; Urban, T.; Perriot, J.; Peiffer, G.; Harika-Germaneau, G.; Jaafari, N. Spontaneous Pneumothorax and Lung Emphysema in Cannabis Users. Rev. Pneumol. Clin. 2018, 74, 400–415. [Google Scholar] [CrossRef]
- Coetzee, C.; Levendal, R.A.; van de Venter, M.; Frost, C.L. Anticoagulant Effects of a Cannabis Extract in an Obese Rat Model. Phytomedicine 2007, 14, 333–337. [Google Scholar] [CrossRef]
- Cordier, J.F. Cryptogenic Organising Pneumonia. Eur. Respir. J. 2006, 28, 422–446. [Google Scholar] [CrossRef]
- Van Rooy, F.G.B.G.J.; Rooyackers, J.M.; Prokop, M.; Houba, R.; Smit, L.A.M.; Heederik, D.J.J. Bronchiolitis Obliterans Syndrome in Chemical Workers Producing Diacetyl for Food Flavorings. Am. J. Respir. Crit. Care Med. 2007, 176, 498–504. [Google Scholar] [CrossRef]
- Harber, P.; Saechao, K.; Boomus, C.; Reports, C. Diacetyl-Induced Lung Disease. Toxicol. Rev. 2006, 25, 261–272. [Google Scholar] [CrossRef]
- Hubbs, A.F.; Goldsmith, W.T.; Kashon, M.L.; Frazer, D.; Mercer, R.R.; Battelli, L.A.; Kullman, G.J.; Schwegler-Berry, D.; Friend, S.; Castranova, V. Respiratory Toxicologic Pathology of Inhaled Diacetyl in Sprague-Dawley Rats. Toxicol. Pathol. 2008, 36, 330–344. [Google Scholar] [CrossRef]
- Vas, C.A.; Porter, A.; McAdam, K. Acetoin Is a Precursor to Diacetyl in E-Cigarette Liquids. Food Chem. Toxicol. 2019, 133, 110727. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2019, 382, 697–705. [Google Scholar] [CrossRef]
- Martin, E.M.; Clapp, P.W.; Rebuli, M.E.; Pawlak, E.A.; Glista-Baker, E.; Benowitz, N.L.; Fry, R.C.; Jaspers, I. E-Cigarette Use Results in Suppression of Immune and Inflammatory-Response Genes in Nasal Epithelial Cells Similar to Cigarette Smoke. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L135–L144. [Google Scholar] [CrossRef]
- Ghosh, A.; Coakley, R.C.; Mascenik, T.; Rowell, T.R.; Davis, E.S.; Rogers, K.; Webster, M.J.; Dang, H.; Herring, L.E.; Sassano, M.F.; et al. Chronic E-Cigarette Exposure Alters the Human Bronchial Epithelial Proteome. Am. J. Respir. Crit. Care Med. 2018, 198, 67–76. [Google Scholar] [CrossRef]
- Madison, M.C.; Landers, C.T.; Gu, B.H.; Chang, C.Y.; Tung, H.Y.; You, R.; Hong, M.J.; Baghaei, N.; Song, L.Z.; Porter, P.; et al. Electronic Cigarettes Disrupt Lung Lipid Homeostasis and Innate Immunity Independent of Nicotine. J. Clin. Investig. 2019, 129, 4290–4304. [Google Scholar] [CrossRef] [PubMed]
- Franke, F.E.; Thomas, J.E. The treatment of acute nicotine poisoning. J. Am. Med. Assoc. 2015, 106, 509–512. [Google Scholar] [CrossRef]
- Lazutka, F.A.; Vasiliauskene, A.P.; Gefen, S.G. On the toxicological assessment of the insecticide nicotine sulfate TT-K toksikologicheskoĭ otsenke insektitsida nikotin-sul’fata. Gig. Sanit. 1969, 34, 30–33. [Google Scholar] [PubMed]
- Mayer, B. How Much Nicotine Kills a Human? Tracing Back the Generally Accepted Lethal Dose to Dubious Self-Experiments in the Nineteenth Century. Arch. Toxicol. 2014, 88, 5–7. [Google Scholar] [CrossRef]
- European Commission. Revision of the Tobacco Products Directive. Available online: https://ec.europa.eu/health/tobacco/products/revision_en (accessed on 1 March 2014).
- Doing, B.Y. Vaping-Induced Acute Lung Injury: An Epidemic That Could Have Been Prevented. AJRCCM 2019, 1–7. [Google Scholar] [CrossRef]
- Rong, L.; Frontera, A.T.; Benbadis, S.R. Tobacco Smoking, Epilepsy, and Seizures. Epilepsy Behav. 2014, 31, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Iha, H.A.; Kunisawa, N.; Shimizu, S.; Tokudome, K.; Mukai, T.; Kinboshi, M.; Ikeda, A.; Ito, H.; Serikawa, T.; Ohno, Y. Nicotine Elicits Convulsive Seizures by Activating Amygdalar Neurons. Front. Pharmacol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Blacker, C.J. Clinical Issues to Consider for Clozapine Patients Who Vape: A Case Illustration. Focus (Am. Psychiatr. Publ). 2020, 18, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Grigg, J. E-Cigarette Regulation: Getting It Wrong Costs Lives. Lancet Respir. Med. 2019, 7, 994–995. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).