Exposure to Tobacco, Environmental Tobacco Smoke and Nicotine in Pregnancy: A Pragmatic Overview of Reviews of Maternal and Child Outcomes, Effectiveness of Interventions and Barriers and Facilitators to Quitting
Abstract
1. Introduction
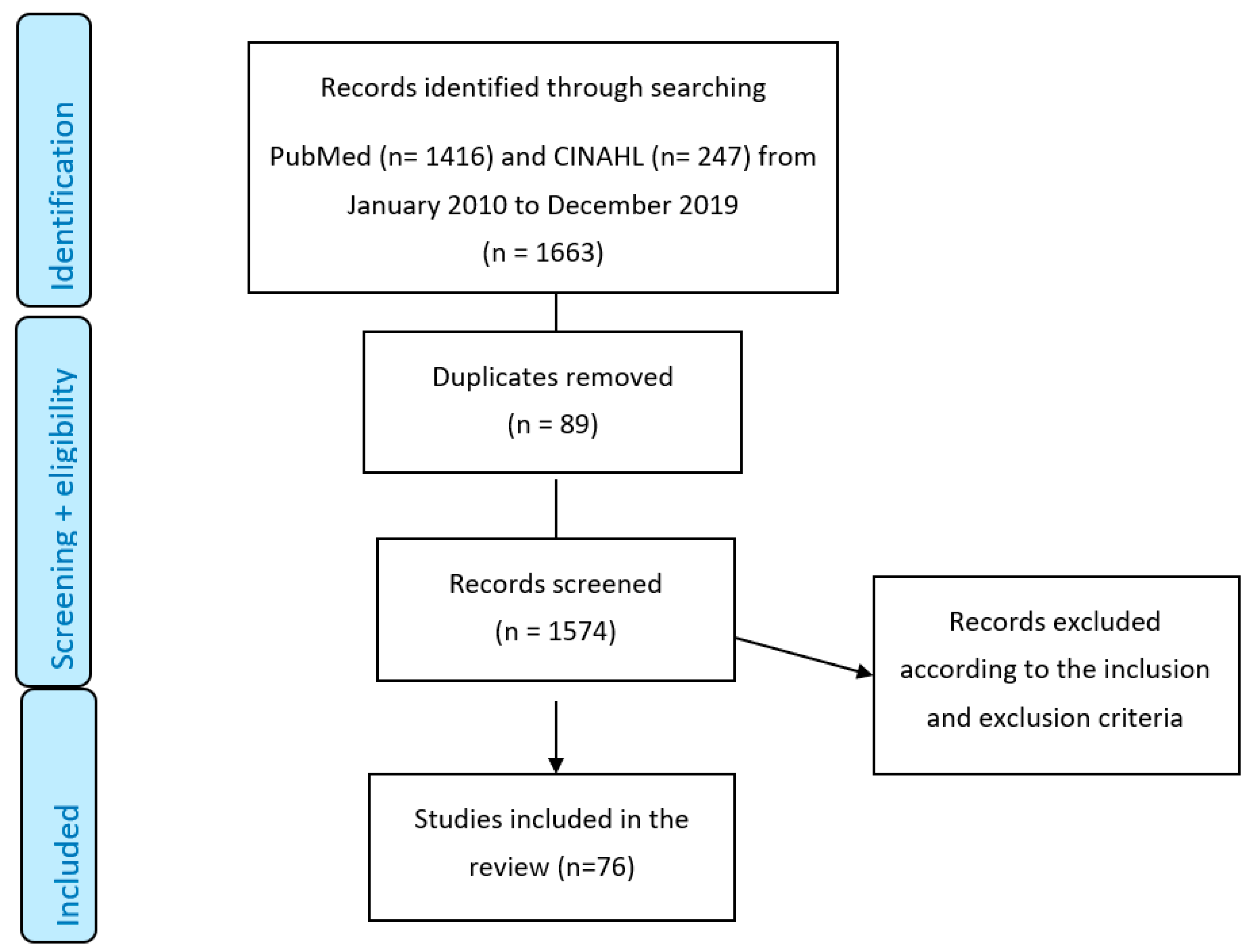
2. Materials and Methods
2.1. Research Questions
2.2. Literature Search and Search Strategy
2.3. Screening and Data Extraction
2.4. Inclusion and Exclusion Criteria
2.5. Types of Outcome Measures
- Neonatal, infant, and child outcomes (0–2 years age) of mothers who smoked during pregnancy;
- Maternal obstetric outcomes;
- Smoking cessation (self-reported and bio-chemically validated);
- Prevention of second-hand smoke exposure of pregnant women;
- Barriers and facilitators to smoking cessation;
- Studies were excluded if papers lacked a systematic methodology as above, were primary or empirical studies or were animal studies.
2.6. Quality Assessment
2.7. Data Synthesis
3. Results
3.1. Risks of Tobacco Exposure during Pregnancy
3.1.1. Risks of Smoking for Pregnant Women
3.1.2. Risks for Foetus and Child
3.2. Risks Associated with Exposure to Tobacco and Nicotine in Other Forms, E-Cigarettes, and Second-Hand Smoke
3.3. Interventions to Reduce Tobacco Exposure in Pregnancy
3.3.1. Behavioral Interventions
3.3.2. Pharmacological Interventions
3.3.3. Interventions to Reduce SHS
3.4. Predictors of Smoking and Barriers to Cessation
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rogers, J.M. Tobacco and pregnancy. Reprod. Toxicol. 2009, 28, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Rosenthal, D.G.; Sherman, S.; Zelikoff, J.; Gordon, T.; Weitzman, M. Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Veisani, Y.; Jenabi, E.; Delpisheh, A.; Khazaei, S. Effect of prenatal smoking cessation interventions on birth weight: Meta-analysis. J. Matern. -Fetal Neonatal Med. 2019, 32, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Marufu, T.C.; Ahankari, A.; Coleman, T.; Lewis, S. Maternal smoking and the risk of still birth: Systematic review and meta-analysis. BMC Public Health 2015, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, X. Maternal smoking and increased risk of sudden infant death syndrome: A meta-analysis. Leg. Med. 2013, 15, 115–121. [Google Scholar] [CrossRef]
- Prabhu, N.; Smith, N.; Campbell, D.; Craig, L.C.; Seaton, A.; Helms, P.J.; Devereux, G.; Turner, S.W. First trimester maternal tobacco smoking habits and fetal growth. Thorax 2010, 65, 235–240. [Google Scholar] [CrossRef]
- Bar-Zeev, Y.; Bovill, M.; Bonevski, B.; Gruppetta, M.; Reath, J.; Gould, G.S. Assessing and Validating an Educational Resource Package for Health Professionals to Improve Smoking Cessation Care in Aboriginal and Torres Strait Islander Pregnant Women. Int. J. Environ. Res. Public Health 2017, 14, 1148. [Google Scholar] [CrossRef]
- Zeev, Y.B.; Bonevski, B.; Twyman, L.; Watt, K.; Atkins, L.; Palazzi, K.; Oldmeadow, C.; Gould, G.S. Opportunities Missed: A Cross-Sectional Survey of the Provision of Smoking Cessation Care to Pregnant Women by Australian General Practitioners and Obstetricians. Nicotine Tob. Res. 2017, 19, 636–641. [Google Scholar] [CrossRef]
- Gould, G.S.; Zeev, Y.B.; Tywman, L.; Oldmeadow, C.; Chiu, S.; Clarke, M.; Bonevski, B. Do Clinicians Ask Pregnant Women about Exposures to Tobacco and Cannabis Smoking, Second-Hand-Smoke and E-Cigarettes? An Australian National Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2017, 14, 1585. [Google Scholar] [CrossRef]
- Daudt, H.M.L.; van Mossel, C.; Scott, S.J. Enhancing the scoping study methodology: A large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med. Res. Methodol. 2013, 13, 48. [Google Scholar] [CrossRef]
- Shobeiri, F.; Masoumi, S.Z.; Jenabi, E. The association between maternal smoking and placenta abruption: A meta-analysis. J. Matern. -Fetal Neonatal Med. 2017, 30, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Shobeiri, F.; Jenabi, E. Smoking and placenta previa: A meta-analysis. J. Matern. -Fetal Neonatal Med. 2017, 30, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Jenabi, E.; Fereidooni, B. The association between maternal smoking and hyperemesis gravidarum: A meta-analysis. J. Matern. -Fetal Neonatal Med. 2017, 30, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Damron, K.R. Review of the Relationships Among Psychosocial Stress, Secondhand Smoke, and Perinatal Smoking. J Obs. Gynecol Neonatal Nurs 2017, 46, 325–333. [Google Scholar] [CrossRef]
- Tuenter, A.; Bautista Nino, P.K.; Vitezova, A.; Pantavos, A.; Bramer, W.M.; Franco, O.H.; Felix, J.F. Folate, vitamin B12, and homocysteine in smoking-exposed pregnant women: A systematic review. Matern. Child Nutr. 2019, 15, e12675. [Google Scholar] [CrossRef]
- Budani, M.C.; Fensore, S.; Di Marzio, M.; Tiboni, G.M. Cigarette smoking impairs clinical outcomes of assisted reproductive technologies: A meta-analysis of the literature. Reprod. Toxicol. 2018, 80, 49–59. [Google Scholar] [CrossRef]
- Purewal, S.; Chapman, S.C.E.; van den Akker, O.B.A. A systematic review and meta-analysis of lifestyle and body mass index predictors of successful assisted reproductive technologies. J. Psychosom. Obstet. Gynaecol. 2019, 40, 2–18. [Google Scholar] [CrossRef]
- Antonopoulos, C.N.; Sergentanis, T.N.; Papadopoulou, C.; Andrie, E.; Dessypris, N.; Panagopoulou, P.; Polychronopoulou, S.; Pourtsidis, A.; Athanasiadou-Piperopoulou, F.; Kalmanti, M.; et al. Maternal smoking during pregnancy and childhood lymphoma: A meta-analysis. Int. J. Cancer 2011, 129, 2694–2703. [Google Scholar] [CrossRef]
- Burke, H.; Leonardi-Bee, J.; Hashim, A.; Pine-Abata, H.; Chen, Y.; Cook, D.G.; Britton, J.R.; McKeever, T.M. Prenatal and passive smoke exposure and incidence of asthma and wheeze: Systematic review and meta-analysis. Pediatrics 2012, 129, 735–744. [Google Scholar] [CrossRef]
- Hackshaw, A.; Rodeck, C.; Boniface, S. Maternal smoking in pregnancy and birth defects: A systematic review based on 173 687 malformed cases and 11.7 million controls. Hum. Reprod. Update 2011, 17, 589–604. [Google Scholar] [CrossRef]
- Lee, L.J.; Lupo, P.J. Maternal smoking during pregnancy and the risk of congenital heart defects in offspring: A systematic review and metaanalysis. Pediatric Cardiol. 2013, 34, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, D.; Appel, L.D.; Siedersberger Neto, P.; Guimaraes, G.W.; Zhang, L. Maternal smoking during pregnancy and birth defects in children: A systematic review with meta-analysis. Cad. De Saude Publica 2014, 30, 2491–2529. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Z.P.; Gong, R.; Zhao, Z.T. Maternal smoking during pregnancy and neural tube defects in offspring: A meta-analysis. Childs Nerv. Syst. 2014, 30, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Yang, X.; Li, J.Y.; Cheikh Ismail, L. Smoking during pregnancy and vision difficulties in children: A systematic review. Acta Ophthalmol. 2015, 93, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.; Tarabulsy, G.M.; Bussieres, E.L. Foetal programming and cortisol secretion in early childhood: A meta-analysis of different programming variables. Infant Behav. Dev. 2015, 40, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, M.; Franchi, S.; Pistorio, A.; Petecchia, L.; Rusconi, F. Smoke exposure, wheezing, and asthma development: A systematic review and meta-analysis in unselected birth cohorts. Pediatric Pulmonol. 2015, 50, 353–362. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Y.; Gong, X.; Wang, G. A Meta-Analysis of Maternal Smoking during Pregnancy and Autism Spectrum Disorder Risk in Offspring. Int. J. Environ. Res. Public Health 2015, 12, 10418–10431. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.H.; Zheng, X.M.; Liu, T.Z.; Zhang, W.B.; Zheng, H.; Chen, M.F. Maternal gestational smoking, diabetes, alcohol drinking, pre-pregnancy obesity and the risk of cryptorchidism: A systematic review and meta-analysis of observational studies. PLoS ONE 2015, 10, e0119006. [Google Scholar] [CrossRef]
- Xuan, Z.; Zhongpeng, Y.; Yanjun, G.; Jiaqi, D.; Yuchi, Z.; Bing, S.; Chenghao, L. Maternal active smoking and risk of oral clefts: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 680–690. [Google Scholar] [CrossRef]
- Pineles, B.L.; Hsu, S.; Park, E.; Samet, J.M. Systematic Review and Meta-Analyses of Perinatal Death and Maternal Exposure to Tobacco Smoke During Pregnancy. Am. J. Epidemiol. 2016, 184, 87–97. [Google Scholar] [CrossRef]
- Yan, K.; Xu, X.; Liu, X.; Wang, X.; Hua, S.; Wang, C.; Liu, X. The associations between maternal factors during pregnancy and the risk of childhood acute lymphoblastic leukemia: A meta-analysis. Pediatric Blood Cancer 2015, 62, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cui, H.; Zhang, L.; Huang, Y.; Zhu, J.; Li, X. Is maternal smoking during pregnancy associated with an increased risk of congenital heart defects among offspring? A systematic review and meta-analysis of observational studies. J. Matern. -Fetal Neonatal Med. 2017, 30, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.P.; Da Mata, F.A.; Figueiredo, A.C.; de Andrade, K.R.; Pereira, M.G. Maternal Active Smoking During Pregnancy and Low Birth Weight in the Americas: A Systematic Review and Meta-analysis. Nicotine Tob. Res. 2017, 19, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Alramadhan, S.; Iniguez, C.; Duijts, L.; Jaddoe, V.W.; Den Dekker, H.T.; Crozier, S.; Godfrey, K.M.; Hindmarsh, P.; Vik, T.; et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS ONE 2017, 12, e0170946. [Google Scholar] [CrossRef] [PubMed]
- Koning, I.V.; Tielemans, M.J.; Hoebeek, F.E.; Ecury-Goossen, G.M.; Reiss, I.K.M.; Steegers-Theunissen, R.P.M.; Dudink, J. Impacts on prenatal development of the human cerebellum: A systematic review. J. Matern. -Fetal Neonatal Med. 2017, 30, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Sun, Y.; Duan, W.; Jia, C. Meta-analysis of the association of maternal smoking and passive smoking during pregnancy with neural tube defects. Int. J. Gynaecol. Obstet. 2018, 140, 18–25. [Google Scholar] [CrossRef]
- Quelhas, D.; Kompala, C.; Wittenbrink, B.; Han, Z.; Parker, M.; Shapiro, M.; Downs, S.; Kraemer, K.; Fanzo, J.; Morris, S.; et al. The association between active tobacco use during pregnancy and growth outcomes of children under five years of age: A systematic review and meta-analysis. BMC Public Health 2018, 18, 1372. [Google Scholar] [CrossRef]
- Muller-Schulte, E.; Kurlemann, G.; Harder, A. Tobacco, alcohol and illicit drugs during pregnancy and risk of neuroblastoma: Systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F467–F473. [Google Scholar] [CrossRef]
- Palma-Gudiel, H.; Cirera, F.; Crispi, F.; Eixarch, E.; Fananas, L. The impact of prenatal insults on the human placental epigenome: A systematic review. Neurotoxicology Teratol. 2018, 66, 80–93. [Google Scholar] [CrossRef]
- Yu, C.; Wei, Y.; Tang, X.; Liu, B.; Shen, L.; Long, C.; Lin, T.; He, D.; Wu, S.; Wei, G. Maternal smoking during pregnancy and risk of cryptorchidism: A systematic review and meta-analysis. Eur. J. Pediatrics 2019, 178, 287–297. [Google Scholar] [CrossRef]
- Wantanabe, H.; Fukuoka, H. Maternal Smoking and Perinatal Outcomes. Austin. J. Drug Abus. Addict. 2016, 3, 1007. [Google Scholar]
- Salmasi, G.; Grady, R.; Jones, J.; McDonald, S.D.; Knowledge Synthesis, G. Environmental tobacco smoke exposure and perinatal outcomes: A systematic review and meta-analyses. Acta Obstet. Et Gynecol. Scand. 2010, 89, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Suliankatchi, R.A.; Sinha, D.N. The Human Cost of Tobacco Chewing Among Pregnant Women in India: A Systematic Review and Meta-analysis. J. Obstet. Gynaecol. India 2016, 66, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, A.S.; Croucher, R.E.; Chokhandre, M.K.; Mashyakhy, M.H.; Marinho, V.C. Maternal Smokeless Tobacco Use in Pregnancy and Adverse Health Outcomes in Newborns: A Systematic Review. Nicotine Tob. Res. 2015, 17, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Chen, M.H.; Lin, C.C.; Ng, S.; Hsieh, C.J.; Liu, C.Y.; Hsieh, W.S.; Chen, P.C. Children’s environmental health based on birth cohort studies of Asia. Sci. Total Environ. 2017, 609, 396–409. [Google Scholar] [CrossRef] [PubMed]
- El-Zaatari, Z.M.; Chami, H.A.; Zaatari, G.S. Health effects associated with waterpipe smoking. Tob. Control 2015, 24 (Suppl 1), i31–i43. [Google Scholar] [CrossRef]
- Jones, M.; Lewis, S.; Parrott, S.; Coleman, T. Systematic critical review of previous economic evaluations of smoking cessation during pregnancy. BMJ Open 2015, 5, e008998. [Google Scholar] [CrossRef]
- Leonardi-Bee, J.; Britton, J.; Venn, A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: A meta-analysis. Pediatrics 2011, 127, 734–741. [Google Scholar] [CrossRef]
- Jones, L.L.; Hashim, A.; McKeever, T.; Cook, D.G.; Britton, J.; Leonardi-Bee, J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: Systematic review and meta-analysis. Respir. Res. 2011, 12, 5. [Google Scholar] [CrossRef]
- Suzuki, D.; Wariki, W.M.V.; Suto, M.; Yamaji, N.; Takemoto, Y.; Rahman, M.; Ota, E. Secondhand Smoke Exposure During Pregnancy and Mothers’ Subsequent Breastfeeding Outcomes: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 8535. [Google Scholar] [CrossRef]
- Sabbagh, H.J.; Hassan, M.H.; Innes, N.P.; Elkodary, H.M.; Little, J.; Mossey, P.A. Passive smoking in the etiology of non-syndromic orofacial clefts: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0116963. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Gong, T.T.; Liu, C.X.; Wu, Q.J. Associations between Passive Maternal Smoking during Pregnancy and Preterm Birth: Evidence from a Meta-Analysis of Observational Studies. PLoS ONE 2016, 11, e0147848. [Google Scholar] [CrossRef]
- Suzuki, D.; Wariki, W.M.V.; Suto, M.; Yamaji, N.; Takemoto, Y.; Rahman, M.M.; Ota, E. Association of secondhand smoke and depressive symptoms in nonsmoking pregnant Women: A systematic review and meta-analysis. J. Affect. Disord. 2019, 245, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Ratsch, A.; Bogossian, F. Smokeless tobacco use in pregnancy: An integrative review of the literature. Int. J. Public Health 2014, 59, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.A.; Gaddam, S.; Gunukula, S.K.; Honeine, R.; Jaoude, P.A.; Irani, J. The effects of waterpipe tobacco smoking on health outcomes: A systematic review. Int. J. Epidemiol. 2010, 39, 834–857. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.C.; Brunette, M.F.; Green, A.I.; Goodman, D.J.; Blunt, H.B.; Heil, S.H. Treating tobacco use disorder in pregnant women in medication-assisted treatment for an opioid use disorder: A systematic review. J. Subst. Abus. Treat. 2015, 52, 40–47. [Google Scholar] [CrossRef]
- Filion, K.B.; Abenhaim, H.A.; Mottillo, S.; Joseph, L.; Gervais, A.; O’Loughlin, J.; Paradis, G.; Pihl, R.; Pilote, L.; Rinfret, S.; et al. The effect of smoking cessation counselling in pregnant women: A meta-analysis of randomised controlled trials. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1422–1428. [Google Scholar] [CrossRef]
- Myung, S.K.; Ju, W.; Jung, H.S.; Park, C.H.; Oh, S.W.; Seo, H.; Kim, H.; Korean Meta-Analysis Study, G. Efficacy and safety of pharmacotherapy for smoking cessation among pregnant smokers: A meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1029–1039. [Google Scholar] [CrossRef]
- Hemsing, N.; Greaves, L.; O’Leary, R.; Chan, K.; Okoli, C. Partner support for smoking cessation during pregnancy: A systematic review. Nicotine Tob. Res. 2012, 14, 767–776. [Google Scholar] [CrossRef]
- Chamberlain, C.; O’Mara-Eves, A.; Porter, J.; Coleman, T.; Perlen, S.M.; Thomas, J.; McKenzie, J.E. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst. Rev. 2017, 2, CD001055. [Google Scholar] [CrossRef]
- Passey, M.E.; Bryant, J.; Hall, A.E.; Sanson-Fisher, R.W. How will we close the gap in smoking rates for pregnant Indigenous women? Med. J. Aust. 2013, 199, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Washio, Y.; Cassey, H. Systematic Review of Interventions for Racial/Ethnic-Minority Pregnant Smokers. J. Smok. Cessat. 2016, 11, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Arden-Close, E.; McGrath, N. Health behaviour change interventions for couples: A systematic review. Br. J. Health Psychol. 2017, 22, 215–237. [Google Scholar] [CrossRef]
- Coleman, T.; Chamberlain, C.; Davey, M.A.; Cooper, S.E.; Leonardi-Bee, J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst. Rev. 2015, 12, CD010078. [Google Scholar] [CrossRef]
- Duckworth, A.L.; Chertok, I.R. Review of perinatal partner-focused smoking cessation interventions. Mcn. Am. J. Matern. Child Nurs. 2012, 37, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tong, V.T.; Dietz, P.M.; Rolle, I.V.; Kennedy, S.M.; Thomas, W.; England, L.J. Clinical interventions to reduce secondhand smoke exposure among pregnant women: A systematic review. Tob. Control 2015, 24, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Dherani, M.; Zehra, S.N.; Jackson, C.; Satyanaryana, V.; Huque, R.; Chandra, P.; Rahman, A.; Siddiqi, K. Behaviour change interventions to reduce second-hand smoke exposure at home in pregnant women—A systematic review and intervention appraisal. BMC Pregnancy Childbirth 2017, 17, 378. [Google Scholar] [CrossRef]
- Baxter, S.; Everson-Hock, E.; Messina, J.; Guillaume, L.; Burrows, J.; Goyder, E. Factors relating to the uptake of interventions for smoking cessation among pregnant women: A systematic review and qualitative synthesis. Nicotine Tob. Res. 2010, 12, 685–694. [Google Scholar] [CrossRef]
- Ingall, G.; Cropley, M. Exploring the barriers of quitting smoking during pregnancy: A systematic review of qualitative studies. Women Birth 2010, 23, 45–52. [Google Scholar] [CrossRef]
- Okoli, C.T.C.; Greaves, L.; Bottorff, J.L.; Marcellus, L.M. Health care providers’ engagement in smoking cessation with pregnant smokers. J. Obs. Gynecol. Neonatal Nurs. 2010, 39, 64–77. [Google Scholar] [CrossRef]
- Gould, G.S.; Munn, J.; Watters, T.; McEwen, A.; Clough, A.R. Knowledge and views about maternal tobacco smoking and barriers for cessation in Aboriginal and Torres Strait Islanders: A systematic review and meta-ethnography. Nicotine Tob. Res. 2013, 15, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Crane, C.A.; Hawes, S.W.; Weinberger, A.H. Intimate partner violence victimization and cigarette smoking: A meta-analytic review. Trauma Violence Abus. 2013, 14, 305–315. [Google Scholar] [CrossRef]
- Flemming, K.; Graham, H.; Heirs, M.; Fox, D.; Sowden, A. Smoking in pregnancy: A systematic review of qualitative research of women who commence pregnancy as smokers. J. Adv. Nurs. 2013, 69, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Bottorff, J.L.; Poole, N.; Kelly, M.T.; Greaves, L.; Marcellus, L.; Jung, M. Tobacco and alcohol use in the context of adolescent pregnancy and postpartum: A scoping review of the literature. Health Soc. Care Community 2014, 22, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Flemming, K.; McCaughan, D.; Angus, K.; Graham, H. Qualitative systematic review: Barriers and facilitators to smoking cessation experienced by women in pregnancy and following childbirth. J. Adv. Nurs. 2015, 71, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.; Flemming, K.; Fox, D.; Heirs, M.; Sowden, A. Cutting down: Insights from qualitative studies of smoking in pregnancy. Health Soc. Care Community 2014, 22, 259–267. [Google Scholar] [CrossRef]
- Rhodes-Keefe, J.M. Depression and smoking in the pregnant rural population: A literature review. Online J. Rural Nurs. Health Care 2015, 15, 60–73. [Google Scholar] [CrossRef]
- Bauld, L.; Graham, H.; Sinclair, L.; Flemming, K.; Naughton, F.; Ford, A.; McKell, J.; McCaughan, D.; Hopewell, S.; Angus, K.; et al. Barriers to and facilitators of smoking cessation in pregnancy and following childbirth: Literature review and qualitative study. Health Technol. Assess. 2017, 21, 1–158. [Google Scholar] [CrossRef]
- Riaz, M.; Lewis, S.; Naughton, F.; Ussher, M. Predictors of smoking cessation during pregnancy: A systematic review and meta-analysis. Addiction 2018, 113, 610–622. [Google Scholar] [CrossRef]
- Small, S.; Porr, C.; Swab, M.; Murray, C. Experiences and cessation needs of Indigenous women who smoke during pregnancy: A systematic review of qualitative evidence. JBI Database Syst. Rev Implement Rep 2018, 16, 385–452. [Google Scholar] [CrossRef]
- Harris, B.M.; Harris, M.L.; Rae, K.; Chojenta, C. Barriers and facilitators to smoking cessation within pregnant Aboriginal and/or Torres Strait Islander women: An integrative review. Midwifery 2019, 73, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Huy, C.; Schutz, J.; Diehl, K. Smoking cessation during pregnancy: A systematic literature review. Drug Alcohol Rev. 2010, 29, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Richmond, R.C.; Simpkin, A.J.; Woodward, G.; Gaunt, T.R.; Lyttleton, O.; McArdle, W.L.; Ring, S.M.; Smith, A.D.; Timpson, N.J.; Tilling, K.; et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum. Mol. Genet. 2015, 24, 2201–2217. [Google Scholar] [CrossRef] [PubMed]
- Clifford, A.; Lang, L.; Chen, R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: A literature review. Neurotoxicology Teratol. 2012, 34, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Meernik, C.; Goldstein, A.O. A critical review of smoking, cessation, relapse and emerging research in pregnancy and post-partum. Br. Med. Bull. 2015, 114, 135–146. [Google Scholar] [CrossRef]
- Morgan, H.; Hoddinott, P.; Thomson, G.; Crossland, N.; Farrar, S.; Yi, D.; Hislop, J.; Moran, V.H.; MacLennan, G.; Dombrowski, S.U. Review of reviews of the barriers and facilitators experienced by women for smoking cessation in pregnancy and breastfeeding. In Benefits of Incentives for Breastfeeding and Smoking Cessation in Pregnancy (BIBS): A Mixed-Methods Study to Inform Trial Design; NIHR Journals Library: Southampton, UK, 2015. [Google Scholar]
- Greenhalgh, E.M.; Stillman, S.; Ford, C. 7.11.3 Predictors of Failure to Quit during and Post Pregnancy. In Tobacco in Australia: Facts and Issues; Cancer Council Victoria: Melbourne, Australia, 2019. [Google Scholar]
- Do, E.K.; Green, T.L.; Prom-Wormley, E.C.; Fuemmeler, B.F. Social determinants of smoke exposure during pregnancy: Findings from waves 1 & 2 of the Population Assessment of Tobacco and Health (PATH) Study. Prev. Med. Rep. 2018, 12, 312–320. [Google Scholar] [CrossRef]
- Boucher, J.; Konkle, A.T.M. Understanding Inequalities of Maternal Smoking-Bridging the Gap with Adapted Intervention Strategies. Int. J. Environ. Res. Public Health 2016, 13, 282. [Google Scholar] [CrossRef]
- Bowker, K.; Lewis, S.; Coleman, T.; Cooper, S. Changes in the rate of nicotine metabolism across pregnancy: A longitudinal study. Addiction 2015, 110, 1827–1832. [Google Scholar] [CrossRef]
- Higgins, S.T.; Solomon, L.J. Some Recent Developments on Financial Incentives for Smoking Cessation Among Pregnant and Newly Postpartum Women. Curr. Addict. Rep. 2016, 3, 9–18. [Google Scholar] [CrossRef]
- Notley, C.; Gentry, S.; Livingstone-Banks, J.; Bauld, L.; Perera, R.; Hartmann-Boyce, J. Incentives for smoking cessation. Cochrane Database Syst. Rev. 2019, 7, CD004307. [Google Scholar] [CrossRef]
- Ioakeimidis, N.; Vlachopoulos, C.; Katsi, V.; Tousoulis, D. Smoking cessation strategies in pregnancy: Current concepts and controversies. Hell. J. Cardiol. 2019, 60, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, Y.; Lim, L.L.; Bonevski, B.; Gruppetta, M.; Gould, G.S. Nicotine replacement therapy for smoking cessation during pregnancy. Med. J. Aust. 2018, 208, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.; Jones, M.; Vaz, L.R.; Coleman, T. Systematic Review and Meta-Analysis to Assess the Safety of Bupropion and Varenicline in Pregnancy. Nicotine Tob. Res. 2019, 21, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.B. E-Cigarettes: Introducing New Complexities and Controversies to the Field of Nicotine and Tobacco Research. Nicotine Tob. Res. 2015, 17, 1185–1186. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, V.M.; Fischbach, L.A.; Chowdhury, P. The use of electronic nicotine delivery systems during pregnancy and the reproductive outcomes: A systematic review of the literature. Tob. Induc. Dis. 2019, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- McRobbie, H.; Bullen, C.; Hartmann-Boyce, J.; Hajek, P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst. Rev. 2014, CD010216. [Google Scholar] [CrossRef]
- Suter, M.A.; Mastrobattista, J.; Sachs, M.; Aagaard, K. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth Defects Res. A Clin. Mol. Teratol. 2015, 103, 186–195. [Google Scholar] [CrossRef]
- Whittington, J.R.; Simmons, P.M.; Phillips, A.M.; Gammill, S.K.; Cen, R.; Magann, E.F.; Cardenas, V.M. The Use of Electronic Cigarettes in Pregnancy: A Review of the Literature. Obstet. Gynecol. Surv. 2018, 73, 544–549. [Google Scholar] [CrossRef]
- Bowker, K.; Orton, S.; Cooper, S.; Naughton, F.; Whitemore, R.; Lewis, S.; Bauld, L.; Sinclair, L.; Coleman, T.; Dickinson, A.; et al. Views on and experiences of electronic cigarettes: A qualitative study of women who are pregnant or have recently given birth. BMC Pregnancy Childbirth 2018, 18, 233. [Google Scholar] [CrossRef]
- Yong, H.-H.; Hitchman, S.C.; Cummings, K.M.; Borland, R.; Gravely, S.M.; McNeill, A.; Fong, G.T. Does the regulatory environment for e-cigarettes influence the effectiveness of e-cigarettes for smoking cessation?: Longitudinal findings from the ITC Four Country Survey. Nicotine Tob. Res. 2017, 19, 1268–1276. [Google Scholar] [CrossRef]
- Hunt, H.; Pollock, A.; Campbell, P.; Estcourt, L.; Brunton, G. An introduction to overviews of reviews: Planning a relevant research question and objective for an overview. Syst. Rev. 2018, 7, 39. [Google Scholar] [CrossRef] [PubMed]
| Study Number | Author Date | Included Studies | Outcomes Measured | Overall Results |
|---|---|---|---|---|
| Risks of smoking for pregnant women | ||||
| 1 | Shobeiri (2017) [11] | 27 | Placental abruption | Based on OR estimates obtained from case–control and cohort studies, there was a significant association between smoking and the risk of placental abruption (OR 1.80; 95% CI: 1.75–1.85; I2 = 78.1%, p < 0.001). Based on the results of cohort studies, smoking and placental abruption were significantly associated (RR 1.65; 95% CI: 1.51–1.80; I2 = 67.1%, p = 0.028). |
| 2 | Shobeiri (2017) [12] | 21 | Placenta previa | Based on the random effects model, compared to non-smokers, the estimated OR and RR of placenta previa among smokers was (OR 1.42, 95% CI: 1.30–1.54; I2 = 62.7%, p < 0.001) and (RR 1.27, 95% CI: 1.18–1.35; I2 = 34.6%, p = 0.205), respectively. |
| 3 | Jenabi (2017) [13] | 12 | Hyperemesis gravidarum | Compared to non-smokers, the OR of hyperemesis gravidarum among smokers was 0.40 (95% CI: 0.24–0.56; I2 = 93.5%, p < 0.001). |
| 4 | Damron (2017) [14] | 24 | Relationships among smoking and stress | Significant positive association between measures of stress (measured via subjective self- report measures, open responses in interviews and hair cortisol concentration) or the existence of stressors and the presence of smoking behaviors. |
| 5 | Tuenter (2018) [15] | 32 | levels of folate, Vitamin B12 and homocysteine | Smoking during pregnancy is associated with lower folate and vitamin B12 levels and higher homocysteine levels. |
| 6 | Budani (2018) [16] | 26 | Live birth rate per IVF cycle, clinical pregnancy rate, spontaneous miscarriage | Significant among women who smoke were a decrease in live birth rate per cycle (OR 0.59, 95% CI 0.44–0.79; I2 = 30.81%), a lower clinical pregnancy rate per cycle (OR 0.53, 95% CI 0.41–0.68; I2 = 49.75%), and an increase in terms of spontaneous miscarriage rate (OR 2.22, 95% CI 1.10–4.48; I2 = 53.89%). |
| 7 | Purewal (2019) [17] | 77 (overall); 28 for smoking | Live births and pregnancy | Women not smoking were significantly more likely to achieve a live birth or pregnancy than those who smoke (OR 1.457, 95% CI: 1.228–1.727, z = 4.324; I2 = 51.883; p = 0.001). |
| Study Number | Author Date | Included Studies | Outcomes Measured | Overall Results |
|---|---|---|---|---|
| 1 | Antonopoulos (2011) [18] | 12 | (i) non-Hodgkin lymphoma (NHL), (ii) Hodgkin lymphoma (HL) and (iii) any lymphoma category in children | Positive association between maternal smoking (any vs. none) during pregnancy and risk for childhood NHL (OR 1.22, 95% CI = 1.03–1.45, fixed effects model; I2 = 2.7%, p = 0.41). No association found for HL and any childhood lymphoma. |
| 2 | Burke (2011) [19] | 71 | wheeze and asthma in children | Maternal prenatal smoking: increase in risk of wheeze OR = 1.41, 95% CI = 1.20–1.67; I2 = 82.5 %), and asthma in children aged ≤2 years (OR = 1.85, 95% CI = 1.35–2.53; I2 = 41.9%) |
| 3 | Hackshaw (2011) [20] | 177 | Birth defects in children | Overall odds of birth defects in children: OR = 1.01, 95%CI = 0.96-1.07) Maternal smoking associated with a significant increased risk for defects of the cardiovascular (OR 1.09, 95% CI = 1.02–1.17), musculoskeletal (OR 1.16, 95% CI 1.05–1.27), CNS (OR = 1.10, 95% CI = 1.01–1.19) and gastrointestinal systems (OR 1.27, 95% CI 1.18–1.36; I2 = 36%, P = 0.02), the face (OR 1.19, 95% CI 1.06–1.35,) including orofacial clefts (OR = 1.28, 95% CI = 1.20–1.36), and cryptorchidism (OR = 1.13, 95% CI = 1.02–1.25). There appears to be a decreased risk for hypospadias and skin defects among babies born to women who smoked. |
| 4 | Zhang (2013) [5] | 35 | Sudden infant death syndrome (SIDS) risk with both prenatal and postnatal maternal smoking. | Prenatal and postnatal maternal smoking was associated with a significantly increased risk of SIDS for prenatal maternal smoking (OR = 2.25, 95% CI = 2.03–2.50, I2 = 76.6%, p < 0.001), and for postnatal maternal smoking (OR = 1.97, 95% CI = 1.77–2.19; I2 = 56.4%, p = 0.002) by random effects model. |
| 5 | Lee (2013) [21] | 35 | Congenital Heart Disease (CHD) and CHD subtypes. | Maternal smoking during pregnancy increases the risk of CHDs as a group (RR, 1.11; 95 % CI, 1.02–1.21). There was evidence of heterogeneity across studies (P < 0.001) for CHDs overall. Positive associations ranged from 1.02 (fixed effects) for double-outlet right ventricle (95 % CI, 0.72–1.46; n cases = 179) to 1.44 (random effects) for septal defects as a group (95 % CI, 1.16–1.79; n cases = 2977). |
| 6 | Nicoletti (2014) [22] | 188 | Birth defects including cardiovascular, digestive, musculoskeletal, and face and neck | Children of smoking mother had a higher chance of presenting any type of birth defects (OR = 1.18; 95%CI = 1.14–1.22; I2 = 77.2%). Significant positive associations between maternal smoking and birth defects in the following body systems: cardiovascular (OR: 1.11; 95%CI: 1.03–1.19), digestive (OR: 1.18; 95%CI: 1.07–1.30), musculoskeletal (OR: 1.27; 95%CI: 1.16–1.39) and face and neck (OR: 1.28; 95%CI: 1.19–1.37). |
| 7 | Wang (2014) [23] | 13 | Neural tube defects (NTDs) | The pooled OR of NTDs in offspring was 1.03 (95%CI = 0.80–1.33; I2 = 73.2%, p < 0.001) for maternal smoking during pregnancy. The overall effect was 1.55 (95 % CI = 1.06–2.26; I2 = 0.0%, p = 0.610) for NTDs subtype of spina bifida; the overall effect was 0.94 (95 % CI = 0.71–1.26; I2 = 79.2%, p < 0.001) for all NTDs subtypes together. |
| 8 | Fernandes (2015) [24] | 24 | Visual outcomes | Most studies (n = 18) reported foetal exposure to active or passive maternal cigarette smoking to be associated with an increased risk of adverse visual outcomes in children. In particular, higher rates of strabismus, refractive errors and retinopathy among children of women who smoked during pregnancy. |
| 9 | Marufu (2015) [4] | 34 | Stillbirth | Smoking during pregnancy was significantly associated with a 47% increase in the odds of stillbirth (OR 1.47, 95% CI 1.37–1.57; I2 = 79%). |
| 10 | Pearson (2015) [25] | 8 studies examined tobacco effect | Child cortisol secretion | Maternal smoking acts as a foetal programming factor that increases cortisol secretion in early childhood. The studies that examined prenatal smoking had a combined effect of (d = 0.21, p < 0.001, k = 17; Q = 6.04, p < 0.05). |
| 11 | Silvestri (2015) [26] | 43 | Asthma or wheezing in offspring of women who smoke during pregnancy | The pooled estimate of the effect of prenatal smoking on current wheezing was OR: 1.36 (95% CI: 1.19–1.55; I2 = 68.9%, p < 0.001). |
| 12 | Tang (2015) [27] | 14 | Autism Spectrum Disorder (ASD) | The pooled OR was 1.02 (95% CI: 0.93–1.13; I2 = 67.3%, p < 0.001) comparing mothers who smoked during pregnancy with those who did not. |
| 13 | Zhang (2015) [28] | 32 | Cryptorchidism | The meta-analysis showed that maternal smoking (OR: 1.17, 95% CI: 1.11–1.23; I2 = 28.30%, p = 0.10) during pregnancy was associated with increased risk of cryptorchidism. |
| 14 | Xuan (2016) [29] | 29 | Oro-facial clefts in children of women who smoke during pregnancy | The overall OR for oro-facial clefts was 1.39 (95% CI = 1.258–1.556; I2 = 53.1, p = 0.19). A modest but statistically significant association was found between maternal active smoking and cleft lip +/- palate (OR: 1.368; 95% CI: 1.259–1.486; I2 = 38.9%, p = 0.039) as well as cleft palate (OR 1.241; 95% CI 1.117–1.378; I2 = 35.1%, p = 0.066). |
| 15 | Pineles (2016) [30] | 142 | Stillbirths, neonatal death and perinatal death | Any active maternal smoking was associated with increased risks of stillbirth (summary relative risk (sRR) = 1.46, 95% CI: 1.38–1.54; I2 = 67%, P < 0.0001), neonatal death (sRR = 1.22, 95% CI: 1.14–1.30; I2 = 39%, P < 0.05), and perinatal death (sRR = 1.33, 95% CI: 1.25, 1.41; I2 = 60%, P < 0.0001). The risks of stillbirth, neonatal death, and perinatal death increased with the amount smoked by the mother. |
| 16 | Yan (2016) [31] | 49 | Acute lymphoblastic leukaemia (ALL) | The pooled ORs showed that there were associations between smoking and Acute lymphoblastic leukaemia (ALL): (Ever vs never, OR: 1.10, 95%CI = 1.02–1.19; I2 = 32.7%, p = 0.074). |
| 17 | Zhang (2017) [32] | 43 | Congenital heart defects (CHDs) among offspring of maternal smokers. | The pooled RR of any CHD was 1.11 (95% CI: 1.04, 1.18; I2 = 69.0%, p < 0.001). |
| 18 | Pereira (2017) [33] | 34 | Low birth weight among infants | Active maternal smoking was associated with low birth weight, OR: 2.00 (95% CI: 1.77–2.26; I2 = 66.3%). |
| 19 | Abraham (2017) [34] | 16 | Associations between maternal smoking during pregnancy and ultrasound measurements of foetal size | Maternal smoking was associated with reduced second trimester head size (mean reduction 0.09 SD [95% CI: 0.01, 0.16] I2 = 56%, p = 0.03) and femur length (0.06 [95% CI: 0.01, 0.10] I2 = 39%, p = 0.13) and reduced third trimester head size (0.18 SD [95% CI: 0.13, 0.23] I2 = 22%, p = 0.27), femur length (0.27 SD [95% CI: 0.21, 0.32] I2 = 30%, p = 0.22) and estimated foetal weight (0.18 SD [95% CI: 0.11, 0.24] I2 = 50%, p = 0.11). Foetal measurements were not reduced for those whose mothers quit before or after becoming pregnant compared to mothers who had never smoked. |
| 20 | Koning (2017) [35] | 15 (overall); 4 (smoking) | Transcerebellar diameter (TCD) and cellular outcomes in cerebellum | TCD is reduced in smoking compared to non-smoking mothers. Abnormal cytology and increased cell death in offspring of smoking mothers along with increased expression of nicotinic and muscarinic receptors |
| 21 | Meng (2018) [36] | 23 | Neural tube defects (NTDs) | The pooled OR for the risk of NTDs was 1.052 (95% CI = 0.907–1.220; I2 = 57.6%, P = 0.001) with maternal smoking |
| 22 | Quelhas (2018) [37] | 201 | Small for gestational age (SGA), length/height, and/or head circumference. | Active tobacco use during pregnancy associated with significantly higher rates of SGA (pooled adjusted odds ratio [AORs] = 1.95; 95% CI: 1.76–2.16; I2 = 99.2%, p < 0.001), shorter length (pooled weighted mean difference [WMD] = 0.43; 95% CI: 0.41–0.44; I2 = 93.9%, p < 0.001), and smaller head circumference (pooled WMD = 0.27; 95% CI: 0.25–0.29; I2 = 90.1%, p < 0.001) at birth. |
| 23 | Muller-Schulte (2018) [38] | 14 | Neuroblastoma | Meta-analysis of unadjusted estimates showed an association between tobacco (pooled OR: 1.22; 95% CI 1.04–1.44; I2 = 33%) and risk of neuroblastoma during childhood. |
| 24 | Palma-Gudiel (2018) [39] | 39 | DNA methylation, global methylation | Marked tendency towards placental hypomethylation in studies assessing tobacco use during pregnancy. Smoking during pregnancy seems to be associated with widespread hypomethylation. |
| 25 | Yu (2019) [40] | 20 | Cryptoorchidism | The risk of having a male with cryptorchidism significantly increased in women who smoked during pregnancy (pooled crude OR 1.18, 95% CI: 1.12–1.24; I2 = 30%, p = 0.10). |
| 26 | Veisani (2019) [3] | 16 | Effect of smoking cessation on low birth weight (LBW) and standardized mean differences between smoking cessation intervention and control groups | Incidence of LBW was decreased in the intervention group. The effect of smoking cessation on LBW was OR 0.65, (95% CI: 0.42–0.88; I2 = 80.7%; p ≤ 001). |
| Study Number | Author/Date | Included Studies | Outcomes Measured | Overall Results |
|---|---|---|---|---|
| Effects of exposure to second-hand smoke (SHS) / environmental tobacco smoke (ETS) | ||||
| 1 | Salmasi (2010) [42] | 76 | Primary outcome: perinatal mortality. Secondary outcomes were birthweight, gestational age at delivery, preterm delivery (< 37 weeks gestation), and low birthweight (LBW, < 2,500 g). | No study examined the primary outcome of perinatal mortality. ETS-exposed infants weighed less [WMD –60 g, 95% CI –80 to –39 g; I2 = 100%; p < 0.00001], with increased risk of low birthweight (LBW, < 2,500 g; RR 1.16; 95% CI 0.99–1.36; I2 = 39%, p = 0.11), although the duration of gestation and preterm delivery were similar (WMD 0.02 weeks, 95% CI = –0.09 to 0.12 weeks; I2 = 60%, p = 0.0007 and RR 1.07; 95% CI 0.93–1.22). ETS-exposed infants had longer lengths (WMD = 1.75 cm; 95% CI 1.37–2.12 cm), increased risks of congenital anomalies (OR 1.17; 95% CI 1.03–1.34) and a trend towards smaller head circumferences (WMD = –0.11 cm; 95% CI –0.22 to 0.01 cm). |
| 2 | Leonardi-Bee (2011) [48] | 19 | Spontaneous abortion, perinatal and neonatal death, stillbirth, and congenital malformations. | No evidence of a statistically significant effect of SHS exposure on the risk of spontaneous abortion (OR: 1.17 [95% CI: 0.88–1.54; I2 = 66%, p = 0.008]. SHS exposure significantly increased the risk of stillbirth (OR: 1.23 [95% CI: 1.09–1.38; I2 = 0%, p = 0.60]; and congenital malformation (OR: 1.13 [95% CI: 1.01–1.26; I2 = 18%, p = 0.30]; 7 studies), SHS had no significant effect on perinatal or neonatal death. |
| 3 | Burke * (2011) [19] | 71 | Wheeze and asthma during 3 different age ranges (≤2 years, 3 to 4 years, 5 to 18 years). | Exposure to postnatal maternal smoking was associated with the strongest effects on the incidence of wheeze, ≤2 years (OR 1.70, 95% CI 1.24–2.35, I² = 0%). Passive household smoking: increased the risk of wheeze in children aged ≤2 years (OR = 1.35, 95% CI = 1.10–1.64, I² = 64.5%, 9 studies). |
| 4 | Jones (2011) [49] | 60 | Lower respiratory infections (LRI), with diagnostic subcategories including bronchiolitis, in infants aged two years and under. | Exposure to smoking by any household member was associated with a statistically significant increase in the odds of LRI for infants <2 years by 1.54 (95% CI 1.40 to 1.69; I2 = 62%, p < 0.00001). Both parents smoking demonstrated a statistically significant increase in the odds of LRI, by 1.62 (95% CI 1.38 to 1.89; I2 = 65%, p = 0.0004) Maternal smoking after birth was associated with a statistically significant increase in odds of LRI, by 1.58 (95% CI 1.45 to 1.73; I2 = 57%, p < 0.0001. |
| 5 | Tsai (2017) [45] | 16 | Children’s health outcomes. | ETS may affect infant birth weight, children’s neurodevelopment, and development of allergies |
| 6 | Suzuki (2019) [50] | 8 | Initiation of breastfeeding. Exclusive or partial breastfeeding was measured as prevalence or duration. Discontinuation of breastfeeding 6 months after birth. | There was a significant increased risk of discontinuation of any breastfeeding before six months for women who were exposed to SHS during pregnancy (pooled OR = 1.07 [95% CI: 1.01–1.14; I2 = 34%) |
| 7 | Suzuki (2019) [53] | 7 | Depressive symptoms during pregnancy and postpartum in pregnant women exposed to SHS | Depressive symptoms at any time during pregnancy and postpartum significantly increased (OR = 1.77 [95% CI = 1.12–2.79]; I2 = 28%, n = 4103, two studies). Increased odds of antenatal suicidal ideation in SHS exposed women (OR = 1.75 [95% CI = 1.14–2.70; I2 = 51%, n = 2670, two studies). |
| 8 | Sabbagh (2015) [51] | 15 | Non syndromic orofacial clefts (NSOFC) in offspring of women exposed to SHS | There was a significant relationship between passive maternal smoking and NSOFC. (OR: 2.11, 95% CI: 1.54 to 2.89; I2 = 91%, p < 0.00001). |
| 9 | Silvestri * (2015) [26] | 43 | Asthma or wheezing in offspring who are exposed to smoke after birth | Association between postnatal maternal smoking and wheezing in the past 12 months had an effect size of 1.21 (95% CI: 1.13–1.31; I2 = 47.0%, p = 0.067). The pooled estimate of the effect of postnatal exposure to parental smoking was very similar to that of exposure to maternal smoking: OR: 1.30 (95% CI: 1.13–1.51; I2 = 71.1%, p = 0.002). |
| 10 | Cui (2016) [52] | 24 | Preterm birth in offspring of women exposed to SHS during pregnancy | Overall, the SORs of preterm birth for women who were ever exposed to passive smoking versus women who had never been exposed to passive smoking at any place and at home were 1.20 (95%CI = 1.07–1.34, I2 = 36.1%) and 1.16 (95%CI = 1.04–1.30, I2 = 4.4%), respectively. |
| 11 | Meng * (2018) [36] | 23 | Neural tube defects (NTDs) | The pooled OR for the risk of NTDs 1.898 (95% CI 1.557–2.313; I2 = 50.5%) with passive smoking. |
| Effects of smokeless tobacco products exposure | ||||
| 1 | Ratsch (2014) [54] | 21 | (1) Birth outcome (live/stillbirth), (2) foetal distress, neonatal apnoea, early neonatal death and neurobehavioural assessment, (3) gender ratio, (4) gestational age and (5) anthropometric measures. | Many studies lacked sufficient power to estimate precise risks. However, there were indications that maternal smokeless tobacco use increases rates of stillbirth, low birth weight and alters the male: female live birth ratio. |
| 2 | Inamdar (2015) [44] | 9 Observational studies (16 reports) | Adverse health outcomes in newborns including LBW, preterm, stillbirth and SGA, | Significant associations with ST use were seen in for LBW, preterm, stillbirth and SGA. Heterogeneity between results was moderate for LBW (I2 = 44%) and stillbirth (I2 = 52%), and high for preterm (I2 = 87%) and SGA (I2 = 65%). Meta-analysis was not undertaken. |
| 3 | Suliankatchi (2016) [43] | 2 | Low birth weight, pre-term birth and still birth in offspring of women who use ST during pregnancy | Pooled odds ratio was significant for all three outcomes: low birth weight (OR 1.88, 95 % CI 1.38–2.54; I2 = 38 %), preterm birth (OR 1.39, 95 % CI 1.01–1.91; I2 = 0%) and stillbirth (OR 2.85, 95 % CI 1.62–5.01; I2 = 0%). |
| Effects of water pipe smoking | ||||
| 1 | El-Zaatari (2015) [46] | 49 | Obstetrical and perinatal outcomes | Water pipe smoking (WPS) has been associated with obstetric and perinatal complications including low birthweight (LBW), infant mortality, low APGAR scores, and pulmonary complications at birth. Three studies reported an overall 2.12 times odds of LBW in association with WPS. |
| 2 | Akl (2011) [55] | 3 | Pregnancy outcomes (low birth weight) and infertility | Water pipe tobacco smoking was associated with low birth weight (OR = 2.12; 95% CI 1.08–4.18; I2 = 0%, p = 0.55) and infertility (OR = 2.5; 95% CI 1.0–6.3). |
| Study Number | Author/Date | Included Studies | Interventions | Outcomes | Results |
|---|---|---|---|---|---|
| Interventions directed at smoking cessation | |||||
| 1 | Akerman (2015) [56] | Three trials of any study type and design evaluating any treatment for smoking in pregnant women undergoing opioid medication-assisted treatment | One trial used contingency management (incentive-based treatment), two trials used brief behavioral interventions | Daily self-reported cigarette use in the pregnant methadone-maintained women, carbon monoxide and cotinine levels | Contingency management/ incentive based treatment, was the most promising intervention: 31% of participants achieved abstinence within the 12-week study period, compared to 0% in a non-contingent behavior incentive group and a group receiving usual care. Two studies of brief behavioral interventions resulted in reductions in smoking but not cessation. |
| 2 | Filion (2011) [57] | Eight RCTs conducted in pregnant women in which the effect of counselling could be isolated. Trials reported biochemically validated abstinence at 6 or 12 months after the target quit date. | Counselling, including minimal clinical intervention, individual counselling, group counselling or telephone counselling | Abstinence at 6 months. Measures were biochemically validated using expired carbon monoxide or salivary cotinine. | The proportion of women that remained abstinent at the end of follow-up was modest, 4 to 24% among those randomized to counselling and from 2 to 21% among control women. The absolute difference in abstinence reached a maximum of only 4%. Summary estimates are inconclusive because of wide confidence intervals, albeit with little evidence to suggest that counselling is efficacious at promoting abstinence (OR 1.08, 95% CI 0.84–1.40; I2 = 0%) |
| 3 | Myung (2012) [58] | Seven (five RCTs, one quasi-RCT and one prospective study | Pharmacotherapy (NRT and Bupropion) | Smoking cessation (assessed by both self-report and biochemical verification) | In a fixed-effects meta-analysis of all seven studies based on the longest follow-up data available, pharmacotherapy had a significant effect on smoking cessation (relative risk RR = 1.80; 95% CI = 1.32–2.44; I2 = 41.5%). The abstinence rate at late pregnancy in the intervention ranged from 7% to 22.6% (mean abstinence rate 13.0%; 95% CI 10.9–15.2%; Cochrane’s Q = 0.062). Effect was strongest for midterm (12–24 weeks) follow-up (RR 1.65, 95% CI 1.20–2.28; I2 = 46.7%) and least for long term (>24 weeks) follow-up studies (RR 1.34, 95% CI 0.90–1.99 I2 = 0%). |
| 4 | Hemsing (2012) [59] | Nine interventional studies. | Interventions to enhance partner support for pregnant/postpartum women’s smoking reduction or cessation and cessation treatments for the partners themselves. For example, quit smoking counselling/resources to pregnant women and/or their partners, a mass media campaign, biofeedback interventions, and providing information booklets aimed at facilitating partner support. | Smoking cessation of a pregnant women and/or partner | Very few intervention studies demonstrated significant results in either encouraging partners to support smoking cessation during pregnancy and postpartum or in improving the partner’s smoking cessation. Overall, there is limited evidence for the efficacy of encouraging partners to support smoking cessation during pregnancy and postpartum. |
| 5 | Chamberlain (2017) [60] | A total of 102 randomized controlled trials, cluster-randomized trials, and quasi-randomized controlled trials of psychosocial smoking cessation interventions during pregnancy | Psychosocial interventions: counselling, health education, feedback, incentives, social support, exercise and dissemination | smoking abstinence | High quality evidence that counselling increased smoking cessation in late pregnancy compared with usual care (RR = 1.44, 95% CI = 1.19–1.73; I2 = 49%) and less intensive interventions (RR = 1.25, 95% CI 1.07–1.47; I2 = 28%). High-quality evidence suggests incentive-based interventions are effective when compared with an alternative (non-contingent incentive) intervention (RR 2.36, 95% CI = 1.36–4.09; I2 = 0%). High-quality evidence suggests the effect is unclear in social support interventions provided by peers (RR 1.42, 95% CI 0.98–2.07). High quality evidence from pooled results demonstrated that women who received psychosocial interventions had a reduction in adverse birth outcomes. |
| 6 | Passey (2013) [61] | Two interventional studies with control group | Culturally tailored interventions for Indigenous populations. These used face-to-face counselling, structured follow-up, family involvement and nicotine replacement therapy (NRT). | Smoking cessation among pregnant Indigenous women | Both studies found no treatment effect. The systematic review found that there is currently no evidence for interventions that are effective in supporting pregnant Aboriginal and Torres Strait Islander women to quit smoking. |
| 7 | Coleman (2015) [64] | Nine RCTs on the efficacy of pharmacotherapies for smoking cessation in pregnancy | Pharmacotherapy (Nicotine Replacement Therapy (NRT) or Bupropion) | Primary efficacy outcome was smoking cessation in later pregnancy (in all but one trial, at or around delivery); safety included 11 outcomes (principally birth outcomes) related to neonatal and infant well-being | Compared to placebo and non-placebo controls, there was a difference in smoking rates observed in later pregnancy favoring use of NRT (risk ratio (RR) 1.41, 95% CI = 1.03 to 1.93; I2 = 18%). In the one trial of bupropion (Stotts 2015), two (out of five) placebo group participants had validated smoking cessation, but no bupropion group participants reported abstinence. |
| 8 | Jones (2015) [47] | A total of 18 studies of interventions delivered to pregnant women, which reported any relevant economic evaluation metric. | Any interventions or combination of interventions, both real and hypothetical (an intervention with an assumed quit rate for economic modelling), aimed at encouraging pregnant smokers to quit. Interventions included counselling, self-help materials, NRT, financial incentives and physical activity. | Clinical or economic outcomes considered relevant to the mother and/or child (e.g., smoking status at end of pregnancy, low birth weight (LBW) (birth weight < 2500 g) births averted, sudden infant deaths (SIDs) averted, and quality adjusted life years (QALYs). | Seventeen studies identified that within-pregnancy interventions are cost-effective, with only one trial reporting that usual care was better than the experimental intervention (motivational interviewing) |
| 9 | Washio (2016) [62] | Nine controlled studies of predominantly racial/ethnic-minority pregnant smokers | Most studies provided some form of brief smoking cessation counselling, with two adding incentives and one adding pharmacotherapy. | Biochemically-verified smoking abstinence with breath, saliva, or urine samples and/or self-reported smoking abstinence. Birth outcomes were also reported. | Treatment effects on the smoking outcomes were not consistently significant among the reviewed studies. Three studies provided biochemically-verified outcomes, showing high postpartum relapse rates. Reduction in smoking during pregnancy was reported in three studies defined as a fifty percent decrease in cotinine levels from baseline to the end of pregnancy or as decrease in the number of cigarettes smoked per day during pregnancy. Not all reports showed significant smoking reduction. |
| 10 | Arden-Close (2017) [63] | A total of 14 studies (overall); 2 studies for smoking in pregnancy | Couple based counselling interventions for smoking cessation in pregnant women | smoking cessation | A non-randomized intervention study (Øien et al., 2008) of three min of advice given to expectant couples by a health care professional during an antenatal appointment did not influence smoking cessation six weeks post-birth. Similarly, an RCT of a couple-based intervention (six counselling calls; three during pregnancy, three post-partum) supplemented by a booklet and video did not increase smoking cessation at 12 months post-partum relative to usual care (McBride et al., 2004). |
| Interventions directed at other tobacco products use or second-hand smoke exposure | |||||
| 11 | Duckworth (2012) [65] | A total of 5 original research reports of smoking cessation interventions for partners of pregnant or postpartum women through 12 months after delivery | Interventions included telephone support, couple support and communication, nicotine patches, and various modes of cessation education. | Quit rates of partners of women who are pregnant | Four of the studies yielded significantly reduced post-intervention smoking rates among the partners. One intervention had no effect on the partners’ smoking. |
| 12 | Tong (2015) [66] | A total of 5 randomized controlled trials which met the inclusion criteria: non-smoking pregnant women exposed to SHS, clinical interventions that intended to reduce SHS, a control group and outcomes included reduction in SHS or quit rates among partners | Four of the studies involved psychosocial interventions delivered to pregnant women within the antenatal care setting, and the fifth study involved psychosocial intervention plus medication to partners of pregnant women. | Pregnant women’s exposure to second-hand smoke (SHS) and quit rates among partners of pregnant women | Results from all five studies showed positive findings based on study-defined outcome measures. Four of the studies showed reduced exposure in pregnant women and one study reported 7- and 30-day abstinence in partners of pregnant women. |
| 13 | Dherani (2017) [67] | Six clinical trials. Participants were men encouraged to change their smoking behaviors by their pregnant wife/partner. | Behavior change interventions (BCI) to reduce SHS at home, compared to no intervention or usual care. | Self-reported or objectively assessed (nicotine/cotinine/ CO levels or clinical measures) SHS exposure of the pregnant woman at home; smoking behavior of the man, or awareness/knowledge of the risks of SHS. | The BCI administered showed a low to moderate success in achieving the selected outcomes. |
| Study Number | Author/Date | Included Papers | Population/Outcomes Assessed | Results |
|---|---|---|---|---|
| 1 | Baxter (2010) [68] | 23; 10 qualitative, 10 quantitative (cross sectional data (surveys)) and 3 narrative. | All women who smoke who are planning a pregnancy, are pregnant, or have an infant aged less than 12 months. The review examined factors underpinning the delivery of interventions to this population from the perspective of staff, users, and potential service users. | Key themes included: 1. Whether or not a health professional mentions smoking 2. The content of advice and information provided 3. The manner of communication 4. Use of service protocols 5. Follow-up discussion 6. Staff confidence in their skills 7. The impact of time and resource constraints 8. Staff perceptions of ineffectiveness 9. Differences between professionals 10. Obstacles to accessing interventions. |
| 2 | Ingall (2010) [69] | 7. Only qualitative studies that collected data during the postpartum stage about changes made to smoking behavior during pregnancy. | Women (15 years or over) who had attempted to quit smoking during pregnancy. | Women’s awareness about health risks to the foetus was not sufficient motivation to quit. Barriers to quitting included: willpower, role and meaning of smoking, issues with cessation provision, changes in relationship interactions, understanding of facts, changes in smell and taste and influence of family and friends. Cessation service provision by health professionals was viewed negatively by women. |
| 3 | Okoli (2010) [70] | 28 quantitative articles on assessments of and interventions addressing health care providers’ (HCP) delivery of care among pregnant girls and women. | HCP with pregnant clients | Although > 50% of health care practitioners are likely to ask women about their smoking status and advise pregnant smokers to quit, <50% assess readiness to change, assist in smoking cessation, or arrange for follow-up appointments/referrals. Important provider-specific, patient-specific, and system/organizational barriers were found to hinder the provision of smoking cessation care by the health care practitioner. |
| 4 | Schneider (2010) [82] | 19 | Characteristics of pregnant women who quit and who continue to smoke during pregnancy | Predictors of smoking during pregnancy were: a partner who smokes, a large number of children, a high rate of tobacco consumption and deficiencies in prenatal care. |
| 5 | Gould (2013) [71] | 7; 4 qualitative (focus groups) and 3 quantitative (questionnaires) | Aboriginal and Torres Strait Islander women. Outcomes assessed were experiences of smoking, experiences of environmental tobacco smoke (ETS), knowledge of health effects of smoking and ETS, beliefs about and attitudes to the health effects of smoking and ETS, knowledge about cessation, beliefs and attitudes about cessation, strategies for cessation, and influences on and barriers to cessation. | A total of eleven third-order constructs operating on the levels of self, family, and social networks, the wider Aboriginal community and broader external influences. Highlighted are social norms and stressors within the Aboriginal community perpetuating tobacco use; insufficient knowledge of smoking harms; inadequate saliency of antismoking messages; and lack of awareness and use of pharmacotherapy. Indigenous health workers have a challenging role, not yet fulfilling its potential. Pregnancy is an opportunity to encourage positive change where a sense of a “protector role” is expressed. |
| 6 | Crane (2013) [72] | 31 | Strength of relationship between smoking and intimate partner violence (IPV) among pregnant women | Women who have experienced IPV are at greater risk of smoking than those who have not. Subsequent moderator analyses indicated that the association is moderately stronger among pregnant compared to non-pregnant victims. The strength of the victimization-smoking relationship did not differ by relationship type or ethnicity. |
| 7 | Flemming (2013) [73] | N = 29 (26 studies) | Pregnant women who were smokers prior to pregnancy and who attempted to quit or continued to smoke during pregnancy. A synthesis of women’s experiences influencing their smoking behavior in pregnancy, including attempts to quit, used meta-ethnography. | Four lines of argument were identified to trace the journeys made by women who were smokers at the start of their pregnancy namely: 1) being a smoker, 2) being a pregnant smoker, 3) quitting and trying to quit smoking, and 4) continuing to smoke. Important themes were: the embeddedness of smoking in women’s lives, questioned only because of pregnancy; quitting for pregnancy rather than for good; quitting had significant costs for the woman and cutting down was a positive alternative; the role of partners and the influence of relationship dynamics on women’s smoking habits |
| 8 | Bottorf (2014) [74] | 40 (39 quantitative and 1 qualitative) | Adolescents aged 19 and under who used alcohol and tobacco during pregnancy and the postpartum period. Outcomes included identifying trends and predictors of alcohol and tobacco use, prior to, during and following pregnancy | Six predictors of tobacco use were: degree of nicotine dependence; number of cigarettes smoked in the last month; alcohol intake pre- pregnancy; religiosity; maternal encouragement to quit; and compatibility of peer and parent attitudes. Tobacco use was significantly related to alcohol use; pregnant adolescents who continued to smoke into the third trimester had more friends who smoked, did not live with a parent, engaged in binge drinking in the first trimester, experienced earlier age of first intercourse and were white. Psychological factors predicting higher levels of smoking included: previous childhood physical or sexual abuse, intention to control weight using cigarettes, depression and anxiety. |
| 9 | Flemming (2015) [75] | 42 (38 studies) | Facilitators and barriers to quitting smoking among pregnant women, the majority from disadvantaged groups | Four factors acted both as barriers and facilitators to women’s ability to quit smoking in pregnancy and postpartum: psychological well-being, relationships with significant others, changing connections with her baby through and after pregnancy; appraisal of the risk of smoking. |
| 10 | Graham (2014) [76] | 29 (26 studies) | Exploration of pregnant women’s perceptions and experiences of cutting down. | Cutting down was both a method of quitting and, for persistent smokers, a method of harm reduction. While pregnant women were aware that official advice was to quit abruptly, cutting down was seen as a positive behavior change in often difficult domestic circumstances, and one that health professionals condoned. |
| 11 | Rhodes-Keefe (2015) [77] | 4 | Relationship between smoking status, rurality, and depression in the pregnant population. | Smoking has been associated with depression in the rural pregnant population. Depression and limited supports promote continued smoking. Rural women do not necessarily identify themselves as depressed. The role of rurality in depression in pregnant smokers is uncertain.” |
| 12 | Bauld (2017) [78] | 65 (55 studies) | Pregnant women, their partners and health providers. The perceived barriers to, and facilitators of, smoking cessation in pregnancy and the identification of potential new/modified interventions. | Themes central to cessation in pregnancy at an individual level: perception of risk to baby, self-efficacy, influence of close relationships and smoking as a way of coping with stress. Interpersonal level: partners’ emotional and practical support, willingness to change smoking behavior and role of smoking within relationships were important. Important across the review and the interviews of HPs were: education to enhance knowledge and confidence in delivering information about smoking in pregnancy and the centrality of the client relationship, and protection of which could be a factor in downplaying risks. |
| 13 | Riaz (2018) [79] | 55 (observational studies and clinical trials) | Predictors of both biochemically validated and non-biochemically validated smoking abstinence in pregnancy | The most frequently observed significant factors associated with cessation were: higher level of education (OR 2.16, 95% CI: 1.80–2.84; I2=93.2%, p < 0.001), higher socio-economic status: (OR 1.97 95% CI: 1.20–3.24; I2 = 82.3%, p = 0.004), overseas maternal birth: (OR 2.00, 95% CI: 1.40–2.84; I2 = 81.9%, p = 0.001), Medicaid coverage or private insurance: (OR 1.54 95% CI: 1.29–1.85; I2 = 41.5%, p = 0.145), living with partner or married: (OR 1.49, 95% CI: 1.38–1.61; I2 = 60.5%, p = 0.019), partner/other members of the household do not smoke: (OR 0.42, 95% CI: 0.35–0.50; I2 = 71.9%, p < 0.001), lower heaviness of smoking index score: (OR 0.45, 95% CI: 0.27–0.77;I2 = 87.5%, p < 0.001) lower baseline cotinine level: (OR 0.78, 95% CI: 0.64–0.94; I2 = 96.6%, p < 0.001), low exposure to second-hand smoking: (OR 0.45, 95% CI: 0.20–1.02; I2 = 95.3%, p < 0.001), not consuming alcohol before and/or during pregnancy: (OR 2.03, 95% CI: 1.47–2.80;I2 = 0.0%, p = 0.950), primiparity: (OR 1.85, 95% CI: 1.68–2.05; I2 = 78.4%, p < 0.001), planned breastfeeding: (OR 1.99 (95% CI: 1.94–2.05;I2 = 0.0%, p = 0.514), perceived adequate pre-natal care: (OR 1.74, 95% CI: 1.38–2.19;I2 = 92.3%, p < 0.001), no depression: (OR 2.65, 95% CI: 1.62–4.30; I2 = 0.0%, p = 0.694) and low stress during pregnancy: (OR 0.58, 95% CI: 0.44–0.77;I2 = 0.0%, p = 0.887) |
| 14 | Small (2018) [80] | 13 | Experiences of smoking during pregnancy for Indigenous women and the smoking cessation needs of Indigenous women during pregnancy. | Being pregnant is a motivator for Indigenous women to quit, try to quit, or cut down on smoking, mainly because they want to protect their children from the harmful effects of maternal smoking during pregnancy, but also because of biological (morning sickness and altered taste and smell for cigarettes) and environmental deterrents (perceived social pressure to quit) to smoking during pregnancy. Barriers to quitting include smoking dependence, being under stress, living in a smoking environment, lacking social support for quitting, rejecting or not knowing the facts about smoking harms, unreceptivity to anti-smoking messages, and boredom. |
| 15 | Harris (2019) [81] | 9 | Facilitators and barriers to smoking cessation amongst Aboriginal and/or Torres Strait Islander women during pregnancy. | Social and familial influences and daily stress have a strong impact on whether a woman feels she can quit smoking during pregnancy. Information and advice regarding potential adverse effects of smoking on the foetus, or lack thereof, from HPs either facilitated cessation of smoking in pregnancy or was a barrier to quitting. A lack of awareness from midwives and doctors on smoking cessation strategies, such as nicotine replacement therapy, was a barrier for women |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gould, G.S.; Havard, A.; Lim, L.L.; The PSANZ Smoking in Pregnancy Expert Group; Kumar, R. Exposure to Tobacco, Environmental Tobacco Smoke and Nicotine in Pregnancy: A Pragmatic Overview of Reviews of Maternal and Child Outcomes, Effectiveness of Interventions and Barriers and Facilitators to Quitting. Int. J. Environ. Res. Public Health 2020, 17, 2034. https://doi.org/10.3390/ijerph17062034
Gould GS, Havard A, Lim LL, The PSANZ Smoking in Pregnancy Expert Group, Kumar R. Exposure to Tobacco, Environmental Tobacco Smoke and Nicotine in Pregnancy: A Pragmatic Overview of Reviews of Maternal and Child Outcomes, Effectiveness of Interventions and Barriers and Facilitators to Quitting. International Journal of Environmental Research and Public Health. 2020; 17(6):2034. https://doi.org/10.3390/ijerph17062034
Chicago/Turabian StyleGould, Gillian S., Alys Havard, Ling Li Lim, The PSANZ Smoking in Pregnancy Expert Group, and Ratika Kumar. 2020. "Exposure to Tobacco, Environmental Tobacco Smoke and Nicotine in Pregnancy: A Pragmatic Overview of Reviews of Maternal and Child Outcomes, Effectiveness of Interventions and Barriers and Facilitators to Quitting" International Journal of Environmental Research and Public Health 17, no. 6: 2034. https://doi.org/10.3390/ijerph17062034
APA StyleGould, G. S., Havard, A., Lim, L. L., The PSANZ Smoking in Pregnancy Expert Group, & Kumar, R. (2020). Exposure to Tobacco, Environmental Tobacco Smoke and Nicotine in Pregnancy: A Pragmatic Overview of Reviews of Maternal and Child Outcomes, Effectiveness of Interventions and Barriers and Facilitators to Quitting. International Journal of Environmental Research and Public Health, 17(6), 2034. https://doi.org/10.3390/ijerph17062034






