Analysis of Biomaterials as Green Coagulants to Control Suspended Solids for Surface Water Treatment
Abstract
1. Introduction
- To evaluate the use of different materials in order to reduce the suspended particles in water, namely: diatomaceous earth, calcium lactate and lactic acid.
- To propose the materials that best reduce the turbidity in water.
- To optimize the process of coagulation-flocculation by means of an incomplete factorial design.
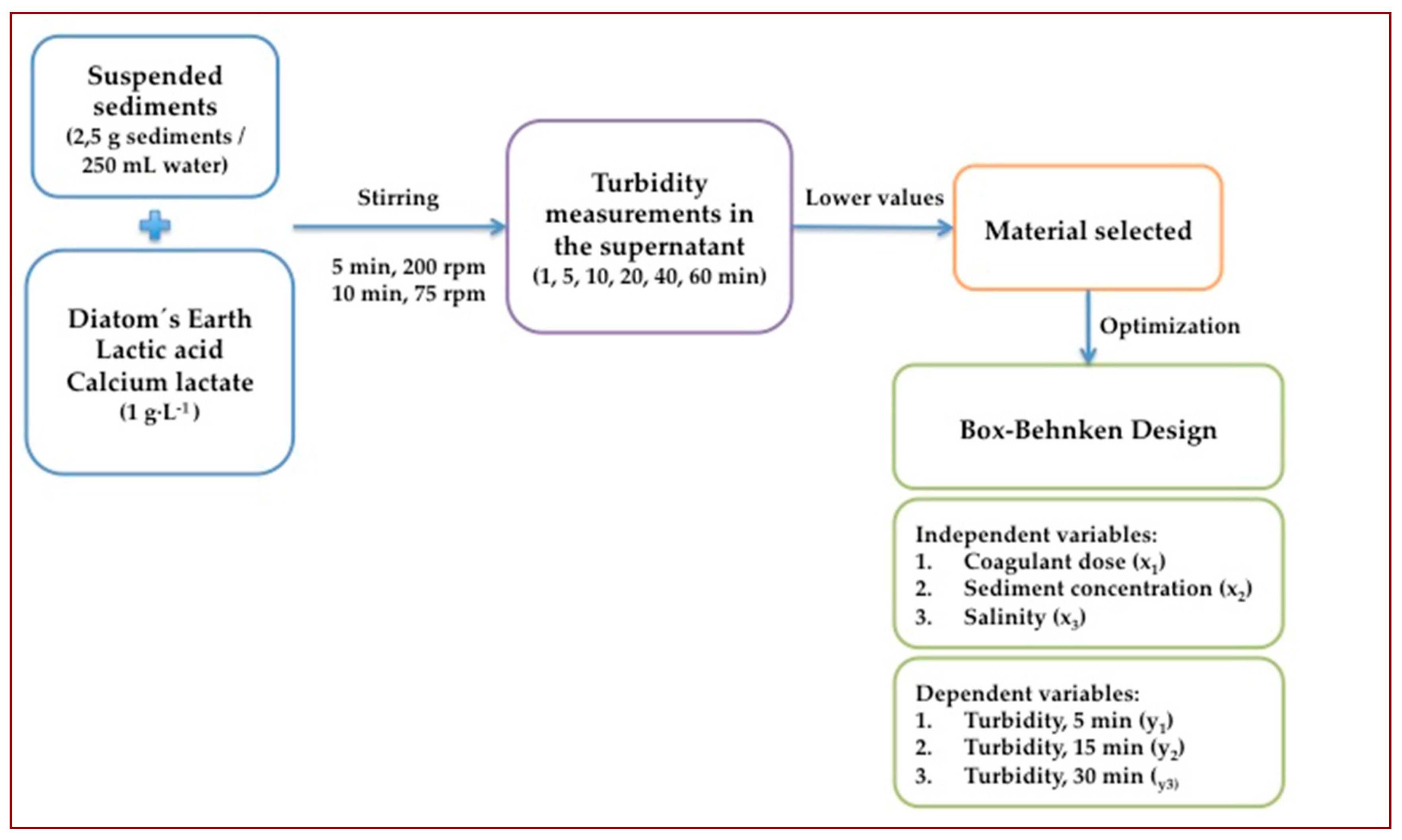
2. Materials and Methods
2.1. Materials
2.2. Coagulation-Flocculation Experiments
2.3. Box–Behnken Experimental Design
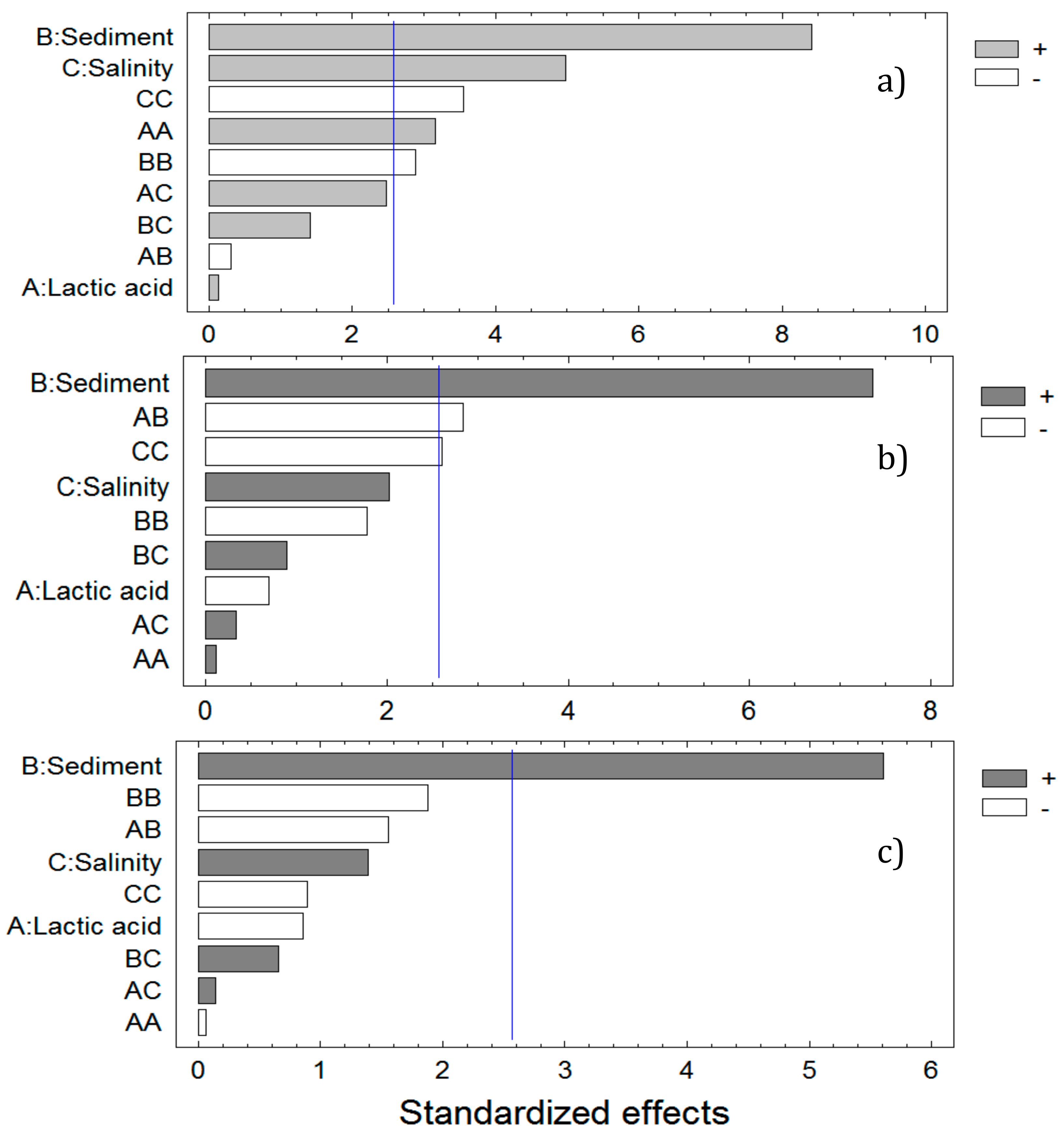
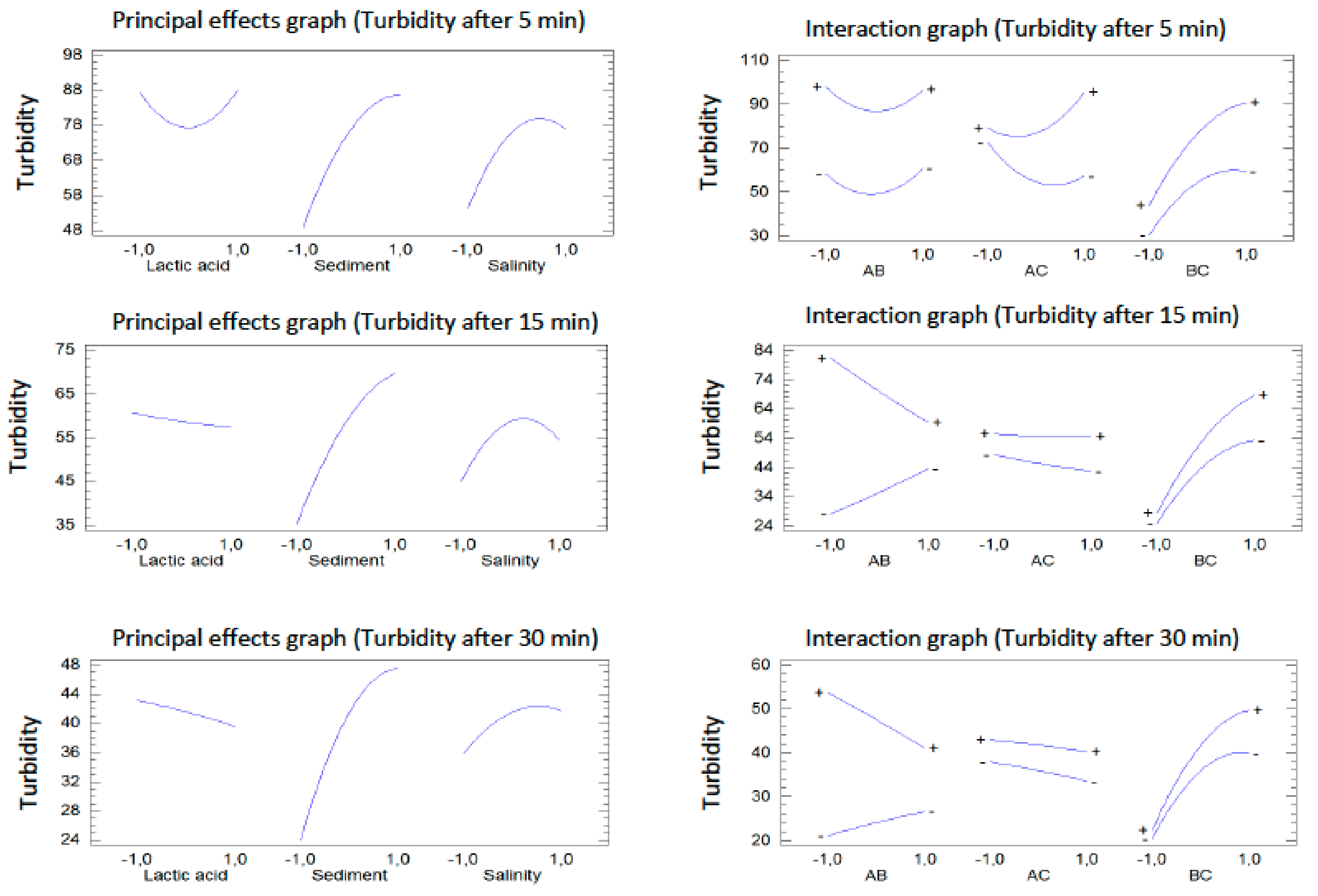
3. Results and Discussion
3.1. Evaluation of Three Coagulant-Floculants
3.2. Model Statistical Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Lima, C.A.I.; Guapyassú, A.M.S.; Pedrosa, S. Distribution of colored dissolved organic matter and disolved organic carbon in a watershed. In Proceedings of the HydroEco, Río Imbe–Lagoa de Cima, RJ, Brazil, 2009. [Google Scholar]
- Devesa-Rey, R.; Iglesias, M.L.; Pérez-Moreira, R.; Díaz-Fierros, F.; Barral, M.T. Application of the Weng’s ratio for the identification of Zn, Cu, and Pb contamination in soils and sediments. J. Soils Sediments 2013, 13, 932–942. [Google Scholar] [CrossRef]
- Reynolds, T.D.; Richards, P.A. Unit Operations and Processes in Environmental Engineering, 2nd ed.; PWS Publications: Boston, MA, USA, 1995. [Google Scholar]
- Ghernaout, D.; Ghernaout, B. Sweep flocculation as a second form of charge neutralisation—A review. Desalin. Water Treat. 2012, 44, 15–28. [Google Scholar] [CrossRef]
- World Health Organization. Health Criteria and other supporting information. In WHO/SDE/WSH/03.04/53. Guidelines for Drinking-Water Quality, 2nd ed.; WHO: Geneva, Switzerland, 1998; Volume 2. [Google Scholar]
- Nacheva, P.M.; Bustillos, L.T.; Camperos, E.R.; Armenta, S.L.; Vigueros, L.C. Characterization and coagulation–flocculation treatability of Mexico City wastewater applying ferric chloride and polymers. Water Sci. Technol. 1996, 34, 235–247. [Google Scholar] [CrossRef]
- Rytwo, G.; Rettig, A.; Gonen, Y. Organo-sepiolite particles for efficient pretreatment of organic wastewater: Application to winery effluents. Appl. Clay Sci. 2011, 51, 390–394. [Google Scholar] [CrossRef]
- Villani, M.; Onesto, V.; Coluccio, M.; Valpapuram, I.; Majewska, R.; Alabastri, A.; Battista, E.; Schirato, A.; Calestani, D.; Coppedé, N.; et al. Transforming diatomaceous earth into sensing devices by surface modification with gold nanoparticles. Micro Nano Eng. 2019, 2, 29–34. [Google Scholar] [CrossRef]
- Marthi, R.; Smith, Y.R. Selective recovery of lithium from the Great Salt Lake using lithium manganese oxide-diatomaceous earth composite. Hydrometallurgy 2019, 186, 115–125. [Google Scholar] [CrossRef]
- Lyngsie, G.; Katika, K.; Fabricius, I.L.; Hansen, H.C.B.; Borggaard, O.K. Phosphate removal by iron oxide-coated diatomite: Laboratory test of a new method for cleaning drainage water. Chemosphere 2019, 222, 884–890. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Fernández, N.; Cruz, J.M.; Moldes, A. Optimization of the dose of calcium lactate as a new coagulant for the coagulation–flocculation of suspended particles in water. Desalination 2011, 280, 63–71. [Google Scholar] [CrossRef]
- Ibrahim, A.; Zahrim, A.Y.; Chong, S.H.; Ng, S.W. Estimation of Melanoidin concentration in palm oil mill effluent ponding system and its treatment using Calcium Lactate. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 12078. [Google Scholar] [CrossRef]
- Zahrim, A.; Nasimah, A.; Hilal, N. Coagulation/flocculation of lignin aqueous solution in single stage mixing tank system: Modeling and optimization by response surface methodology. J. Environ. Chem. Eng. 2015, 3, 2145–2154. [Google Scholar] [CrossRef]
- Moldes, A.B.; Torrado, A.M.; Barral, M.T.; Domínguez, J.M.; Menduiña, A.B.M. Evaluation of Biosurfactant Production from Various Agricultural Residues byLactobacillus pentosus. J. Agric. Food Chem. 2007, 55, 4481–4486. [Google Scholar] [CrossRef] [PubMed]
- Devesa-Rey, R.; Moldes, A.B.; Diaz-Fierros, F.; Barral, M.T.; Menduiña, A.B.M. Toxicity of Anllóns River Sediment Extracts Using Microtox and the Zucconi Phytotoxicity Test. Bull. Environ. Contam. Toxicol. 2008, 80, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Devesa-Rey, R.; Moldes, A.B.; Diaz-Fierros, F.; Barral, M.T.; Menduiña, A.B.M. Study of phytopigments in river bed sediments: Effects of the organic matter, nutrients and metal composition. Environ. Monit. Assess. 2008, 153, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Behnken, D.W. Simplex-sum designs—A class of 2nd order rotatable designs derivable of those of 1st order. Ann. Math. Stat. 1960, 31, 838–864. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.; Ferreira, H.; Matos, G.; David, J.; Brandão, G.; Da Silva, E.G.P.; Portugal, L.; Dos Reis, P.; Souza, A.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Latorre-Sánchez, M.; Unger, P.; Schneider, R.; Lozano, C.C.; Venus, J. Assessing the organic fraction of municipal solid wastes for the production of lactic acid. Biochem. Eng. J. 2019, 150. [Google Scholar] [CrossRef]
- Morrone, B. Residual Biomass Resources: An Invaluable Reservoir of Energy and Matter. In Innovations in Sustainable Energy and Cleaner Environment. Green Energy and Technology; Gupta, A., De, A., Aggarwal, S., Kushari, A., Runchal, A., Eds.; Springer: Singapore, 2020. [Google Scholar]
- González-Leos, A.; De Tamaulipas, U.A.; Bustos-Vázquez, M.; Rodríguez-Castillejos, G.C.; Durán, L.V.R.; Ángel, A.D. KINETICS OF LACTIC ACID FERMENTATION FROM SUGARCANE BAGASSE BY Lactobacillus pentosus. Rev. Mex. Ing. Química 2019, 19, 377–386. [Google Scholar]
- Soltanian, S.; Aghbashlo, M.; Farzad, S.; Tabatabaei, M.; Mandegari, M.; Gorgens, J. Exergoeconomic analysis of lactic acid and power cogeneration from sugarcane residues through a biorefinery approach. Renew. Energy 2019, 143, 872–889. [Google Scholar] [CrossRef]
- Chandra, V.; Mohapatra, P.K.; Nestmann, F. Effect of Flow Depth, Ions, and Salinity on Suspended Sediment Concentration. J. Hydraul. Eng. 2012, 138, 348–352. [Google Scholar] [CrossRef]
- Chandra, V.; Mohapatra, P.; Hillebrand, G.; Nestmann, F. Effect of Salinity on Suspended Cohesive Sediments. Int. J. Civ. Eng. 2010, 1, 307–315. [Google Scholar]
- Shadrin, N.V.; Belyakov, V.P.; Bazhora, A.I.; Anufriieva, E.V. Does salinity affect body proportions and “size/mass” ratios of highly halotolerant Baeotedipes noctivagus larvae (Diptera, Chironomidae). Oceanol. Hydrobiol. Stud. 2019, 48, 305–315. [Google Scholar] [CrossRef]
- Khiari, R.; Dridi-Dhaouadi, S.; Aguir, C.; Mhenni, M.F. Experimental evaluation of eco-friendly flocculants prepared from date palm rachis. J. Environ. Sci. 2010, 22, 1539–1543. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Paradelo, R.; Diaz-Fierros, F.; Barral, M.T. Fractionation and Bioavailability of Arsenic in the Bed Sediments of the Anllóns River (NW Spain). Water Air Soil Pollut. 2008, 195, 189–199. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Iglesias, M.L.; Diaz-Fierros, F.; Barral, M.T. Total Phosphorous Distribution and Bioavailability in the Bed Sediments of an Atlantic Basin (Galicia, NW Spain): Spatial Distribution and Vertical Profiles. Water Air Soil Pollut. 2008, 200, 341–352. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Diaz-Fierros, F.; Barral, M.T. Trace metals in river bed sediments: An assessment of their partitioning and bioavailability by using multivariate exploratory analysis. J. Environ. Manag. 2010, 91, 2471–2477. [Google Scholar] [CrossRef]
- Jin, W.; Chang, J.; Wang, Y.; Bai, T. Long-term water-sediment multi-objectives regulation of cascade reservoirs: A case study in the Upper Yellow River, China. J. Hydrol. 2019, 577. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Gu, X.; Guo, Z.; Song, J.; Zhu, D.; Liu, Y.; Liu, Y.; Xue, G.; Li, X.; et al. Stabilizing lactate production through repeated batch fermentation of food waste and waste activated sludge. Bioresour. Technol. 2020, 300. [Google Scholar] [CrossRef]
- Ao, T.; Luo, Y.; Chen, Y.; Cao, Q.; Liu, X.; Li, D. Towards zero waste: A valorization route of washing separation and liquid hot water consecutive pretreatment to achieve solid vinasse based biorefinery. J. Clean. Prod. 2020, 248. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.; Barral, M.T.; Cruz, J.M.; Moldes, A. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Latorre-Sánchez, M.; Lozano, C.C.; Venus, J. Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J. Clean. Prod. 2020, 247. [Google Scholar] [CrossRef]
- Alexandri, M.; Venus, J. Feedstock flexibility in sustainable chemistry: Bridging sectors still not sufficiently familiar with each other—Showcases of ongoing and emerging initiatives. Curr. Opin. Green Sustain. Chem. 2017, 8, 24–29. [Google Scholar] [CrossRef]
- Ferreira, S.L.; Junior, M.M.S.; Felix, C.S.; Da Silva, D.L.; Santos, A.S.; Neto, J.H.S.; De Souza, C.T.; Junior, R.A.C.; Souza, A.S. Multivariate optimization techniques in food analysis—A review. Food Chem. 2019, 273, 3–8. [Google Scholar] [CrossRef]
- Veljković, V.; Veličković, A.V.; Avramović, J.M.; Stamenković, O. Modeling of biodiesel production: Performance comparison of Box–Behnken, face central composite and full factorial design. Chin. J. Chem. Eng. 2019, 27, 1690–1698. [Google Scholar] [CrossRef]




| Parameter | Value | Parameter (mg·kg−1) | Value |
|---|---|---|---|
| Lithology | Gabbros | Cr | 284.8 |
| Textural classification | Sandy | Fe | 7.8 |
| Total Organic Carbon (TOC) (%) | 10.60 | Cu | 68.3 |
| Nitrogen (%) | 0.84 | Zn | 287.3 |
| Total Phosphorus (mg·kg−1) | 2,324 | Total chlorophyll | 114.8 |
| Available Phosphorus (mg·kg−1) | 877 | ||
| C/N ratio | 13 | ||
| N/P ratio | 36 |
| Independent Variables | |||
|---|---|---|---|
| Variable | Nomenclature | Units | Range of Variation |
| Lactic acid concentration | [Lactic] | g·L−1 | 0.5−2.5 |
| Sediment concentration | [Sed] | % | 2.5−7.5 |
| Salinity | [S] | g·L−1 | 0−33 |
| Adimensional coded variables | |||
| Variable | Nomenclature | Units | Range of variation |
| Dimensionless lactic acid concentration | x1 | ([Lactic]-3)/1.5 | (-1, 1) |
| Dimensionless sediment concentration | x2 | ([Sed]-10)/5 | (-1, 1) |
| Dimensionless salinity | x3 | ([S]-49)/24.5 | (-1, 1) |
| Dependent Variables | ||
|---|---|---|
| Variable | Nomenclature | Units |
| Turbidity, 5 min | y1 | NTU |
| Turbidity, 15 min | y2 | NTU |
| Turbidity, 30 min | y3 | NTU |
| Independent | Dependent | |||||
|---|---|---|---|---|---|---|
| Exp. | x1 | x2 | x3 | y1 | y2 | y3 |
| 1 | 0 | -1 | -1 | 31.0 | 29.2 | 23.2 |
| 2 | 0 | 1 | -1 | 62.0 | 51.3 | 37.3 |
| 3 | 0 | -1 | 1 | 41.0 | 29.9 | 24.8 |
| 4 | 0 | 1 | 1 | 90.0 | 63.9 | 46.7 |
| 5 | -1 | -1 | 0 | 56.5 | 28.6 | 22.0 |
| 6 | -1 | 1 | 0 | 94.2 | 88.1 | 60.1 |
| 7 | 1 | -1 | 0 | 64.3 | 36.6 | 20.2 |
| 8 | 1 | 1 | 0 | 98.0 | 58.5 | 39.9 |
| 9 | -1 | 0 | -1 | 73.3 | 43.0 | 34.0 |
| 10 | -1 | 0 | 1 | 83.3 | 53.0 | 39.3 |
| 11 | 1 | 0 | -1 | 53.0 | 45.0 | 37.0 |
| 12 | 1 | 0 | 1 | 94.5 | 59.5 | 44.0 |
| 13 | 0 | 0 | 0 | 84.0 | 61.1 | 40.0 |
| 14 | 0 | 0 | 0 | 79.0 | 56.8 | 43.2 |
| 15 | 0 | 0 | 0 | 69.0 | 58.2 | 41.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devesa-Rey, R.; González-Aller, J.D.; Urréjola, S. Analysis of Biomaterials as Green Coagulants to Control Suspended Solids for Surface Water Treatment. Int. J. Environ. Res. Public Health 2020, 17, 1777. https://doi.org/10.3390/ijerph17051777
Devesa-Rey R, González-Aller JD, Urréjola S. Analysis of Biomaterials as Green Coagulants to Control Suspended Solids for Surface Water Treatment. International Journal of Environmental Research and Public Health. 2020; 17(5):1777. https://doi.org/10.3390/ijerph17051777
Chicago/Turabian StyleDevesa-Rey, Rosa, J.D. González-Aller, and Santiago Urréjola. 2020. "Analysis of Biomaterials as Green Coagulants to Control Suspended Solids for Surface Water Treatment" International Journal of Environmental Research and Public Health 17, no. 5: 1777. https://doi.org/10.3390/ijerph17051777
APA StyleDevesa-Rey, R., González-Aller, J. D., & Urréjola, S. (2020). Analysis of Biomaterials as Green Coagulants to Control Suspended Solids for Surface Water Treatment. International Journal of Environmental Research and Public Health, 17(5), 1777. https://doi.org/10.3390/ijerph17051777





