Chemical Reduction of Nitrate by Zero-Valent Iron: Shrinking-Core versus Surface Kinetics Models
Abstract
1. Introduction
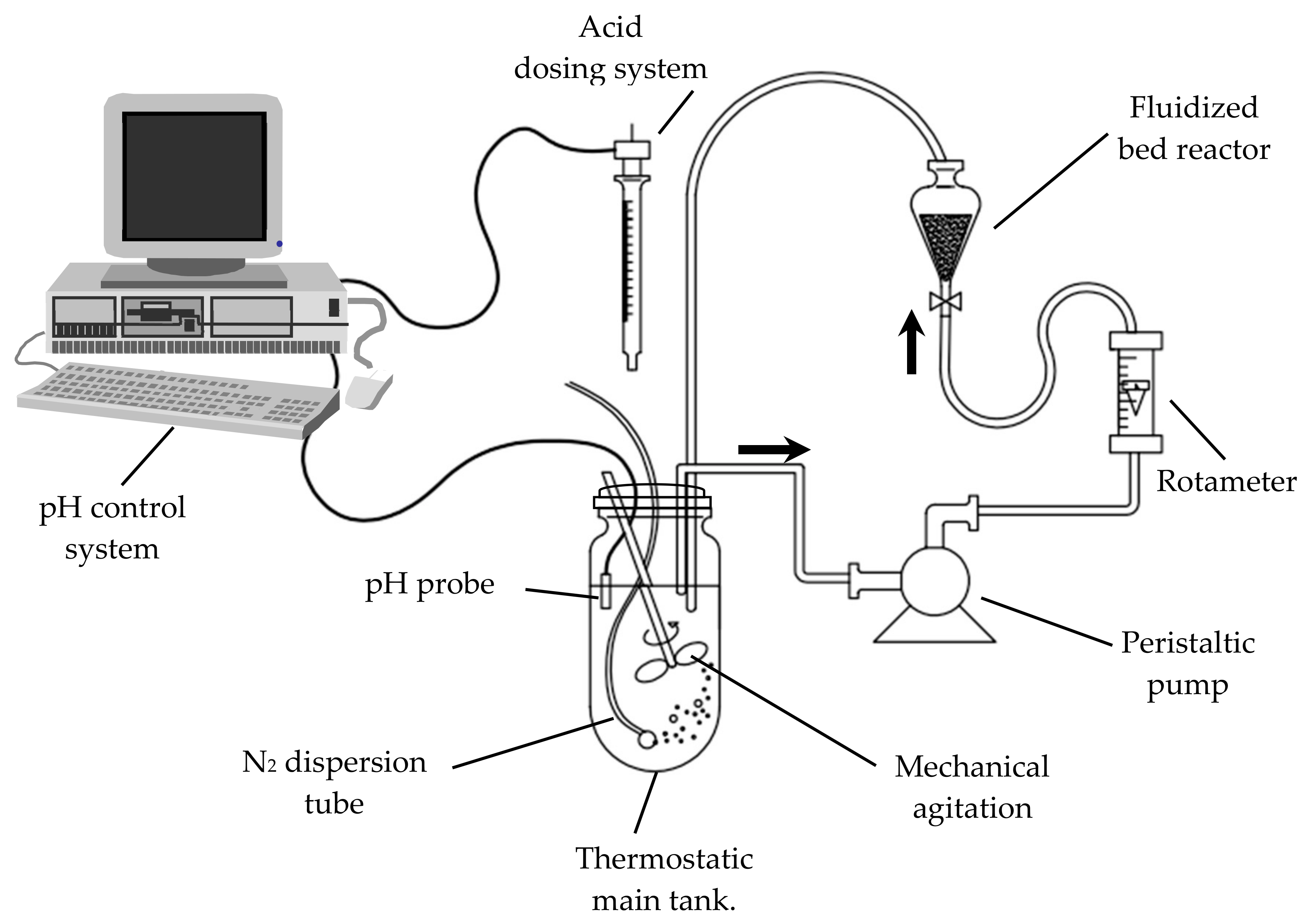
2. Materials and Methods
- A Heidolph RZR1 (Heidolph Instruments GmbH & CO. KG, Schwabach, Germany) motor connected to a stirring shaft with a two-blade propeller provides mechanical agitation ;
- The dissolved oxygen concentration was maintained at zero by means of a continuous nitrogen current bubbled through the aqueous phase;
- A pH control system of our own design [8] allowed us to maintain the pH value within ±0.05 the target value, by the addition of the necessary volume of sulfuric acid solution. This device also made it possible to track the amount of acid added versus time.
3. Results and Discussion
3.1. Kinetics of the Corrosion of Iron in Acid
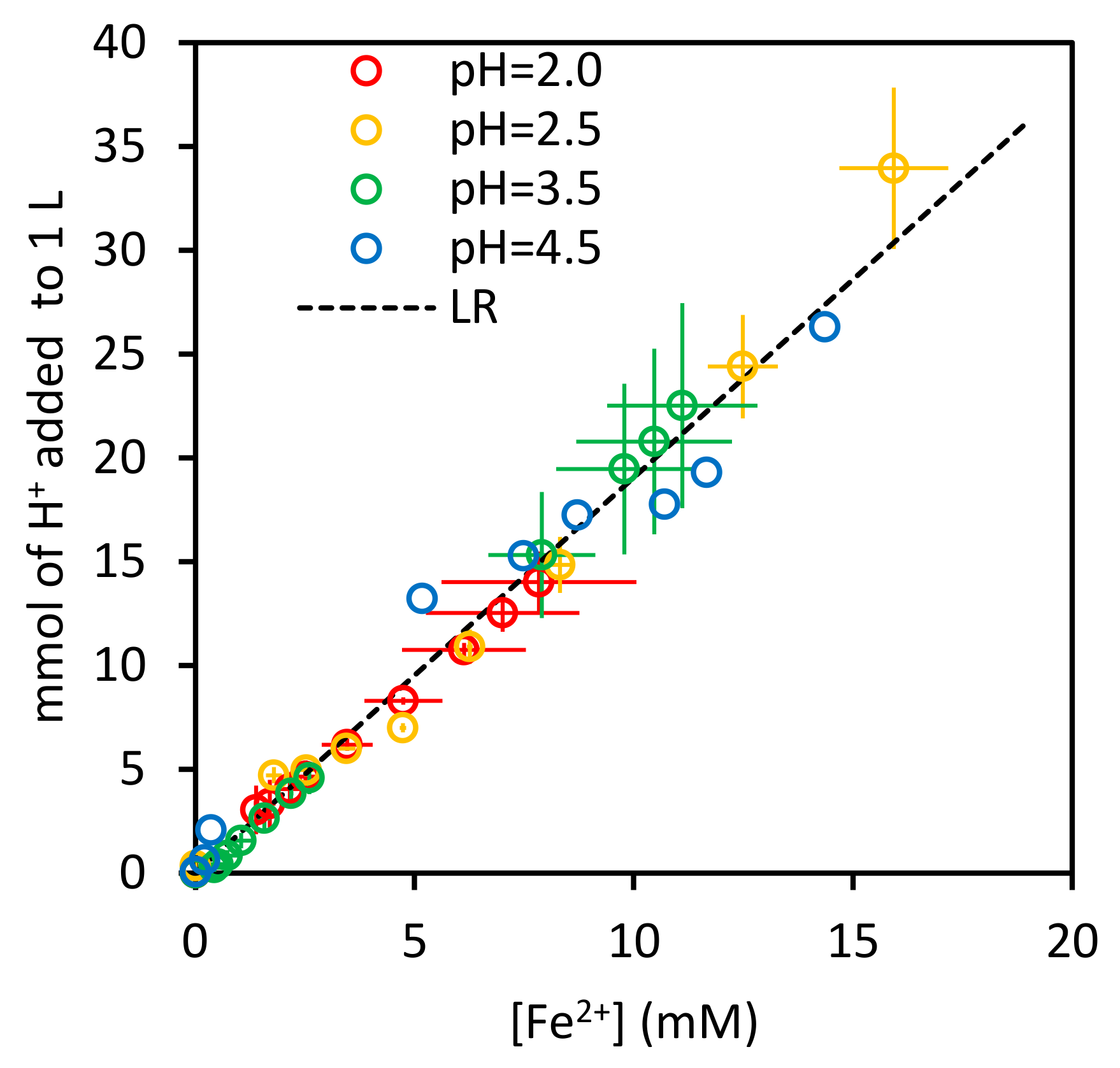
3.1.1. Stoichiometry
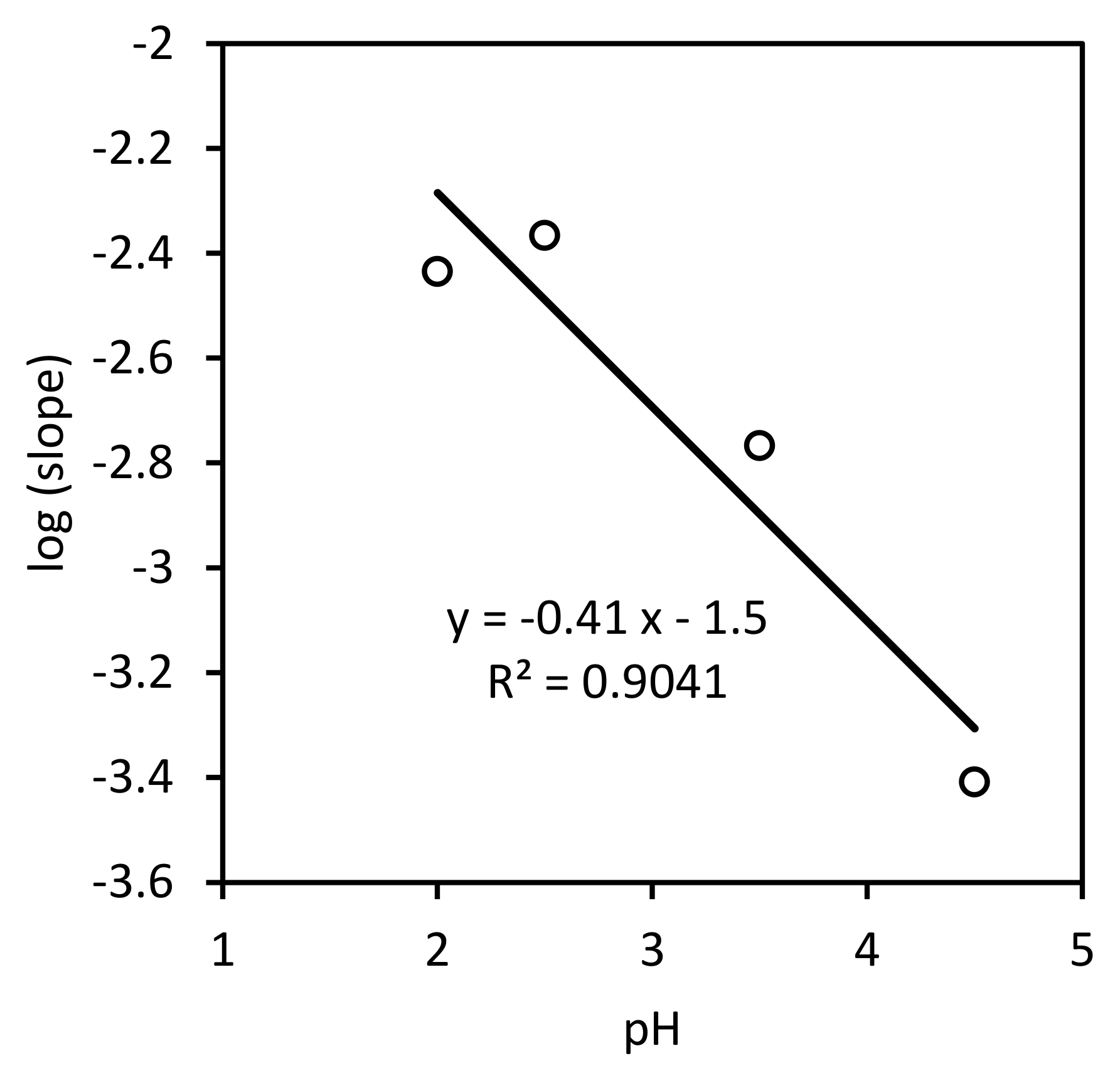
3.1.2. Empirical Rate Equation of nth Order
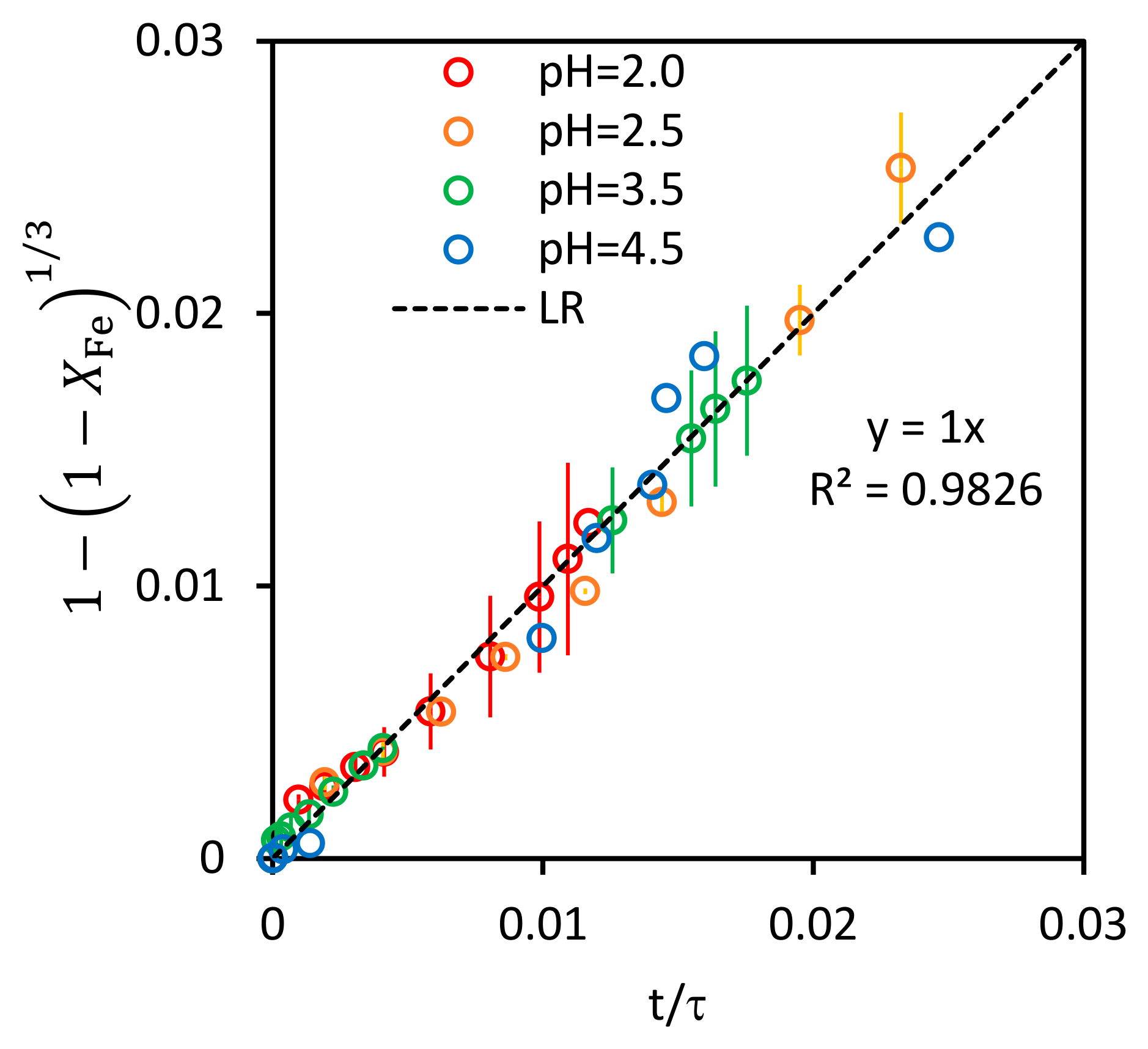
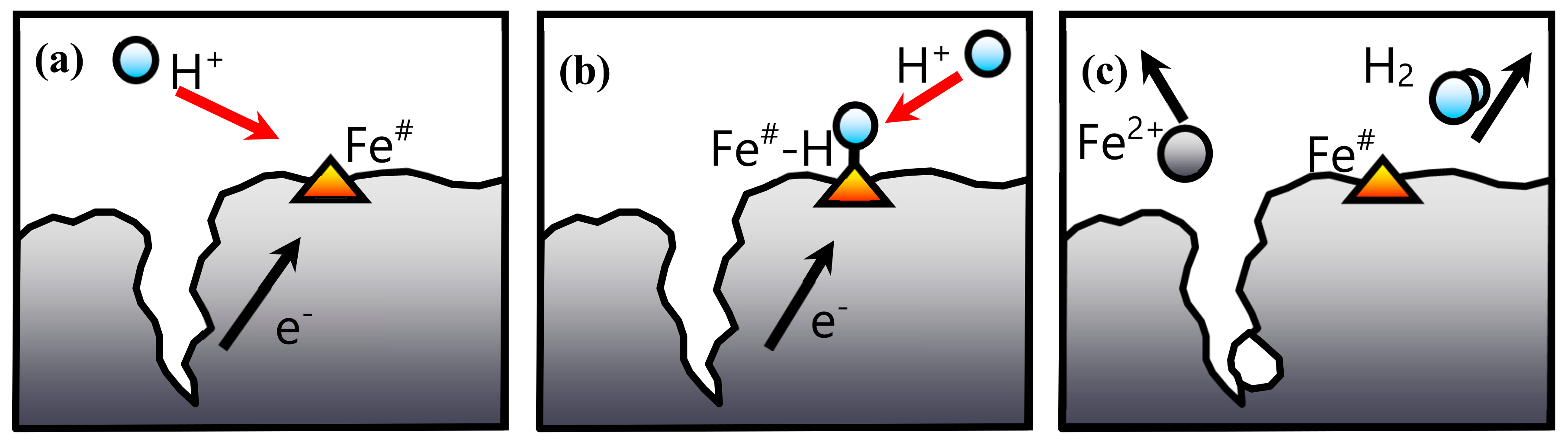
3.1.3. Shrinking Unreacted-Core Model (SCM)
3.1.4. Surface Kinetics Model (SKM)
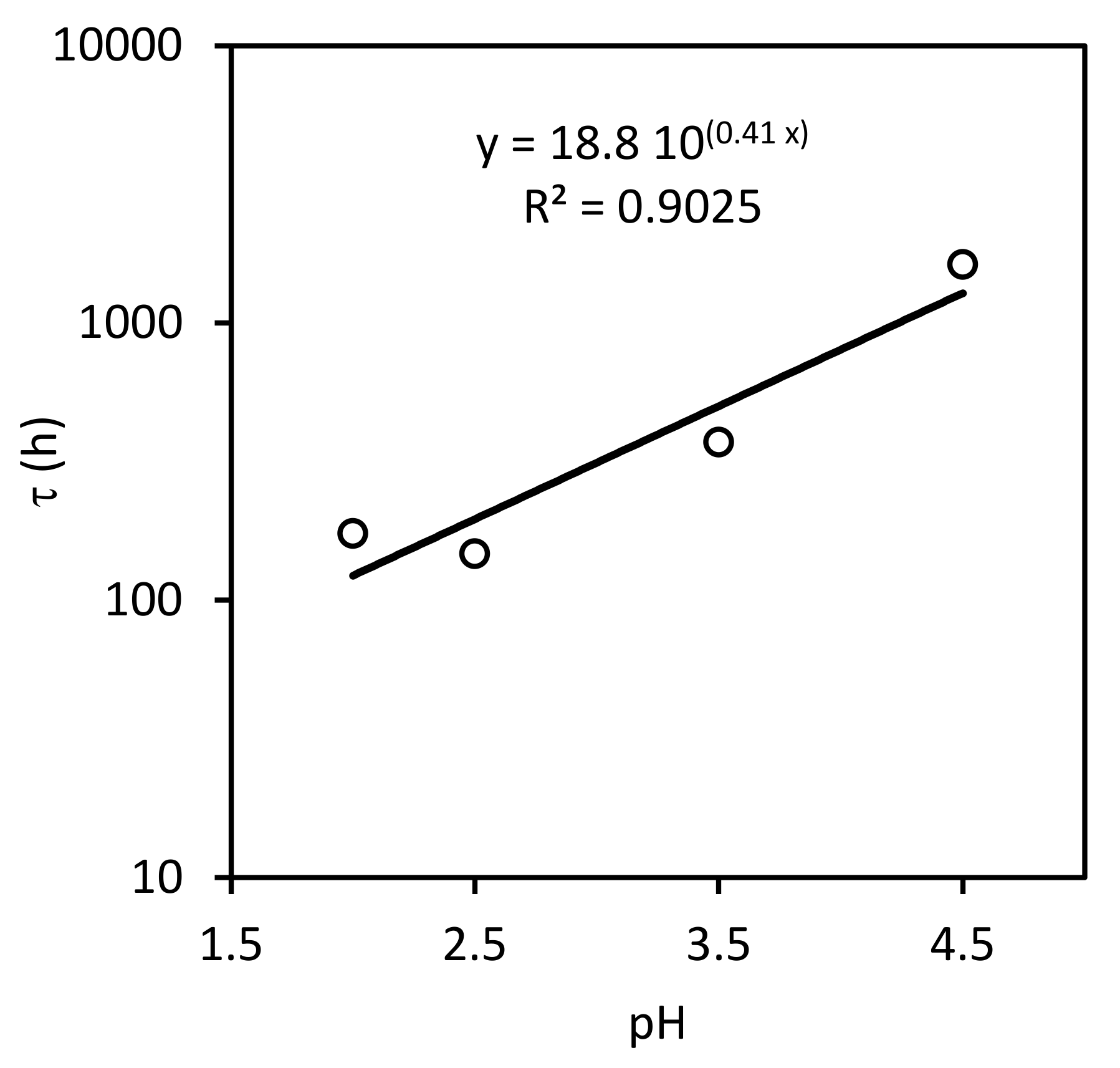
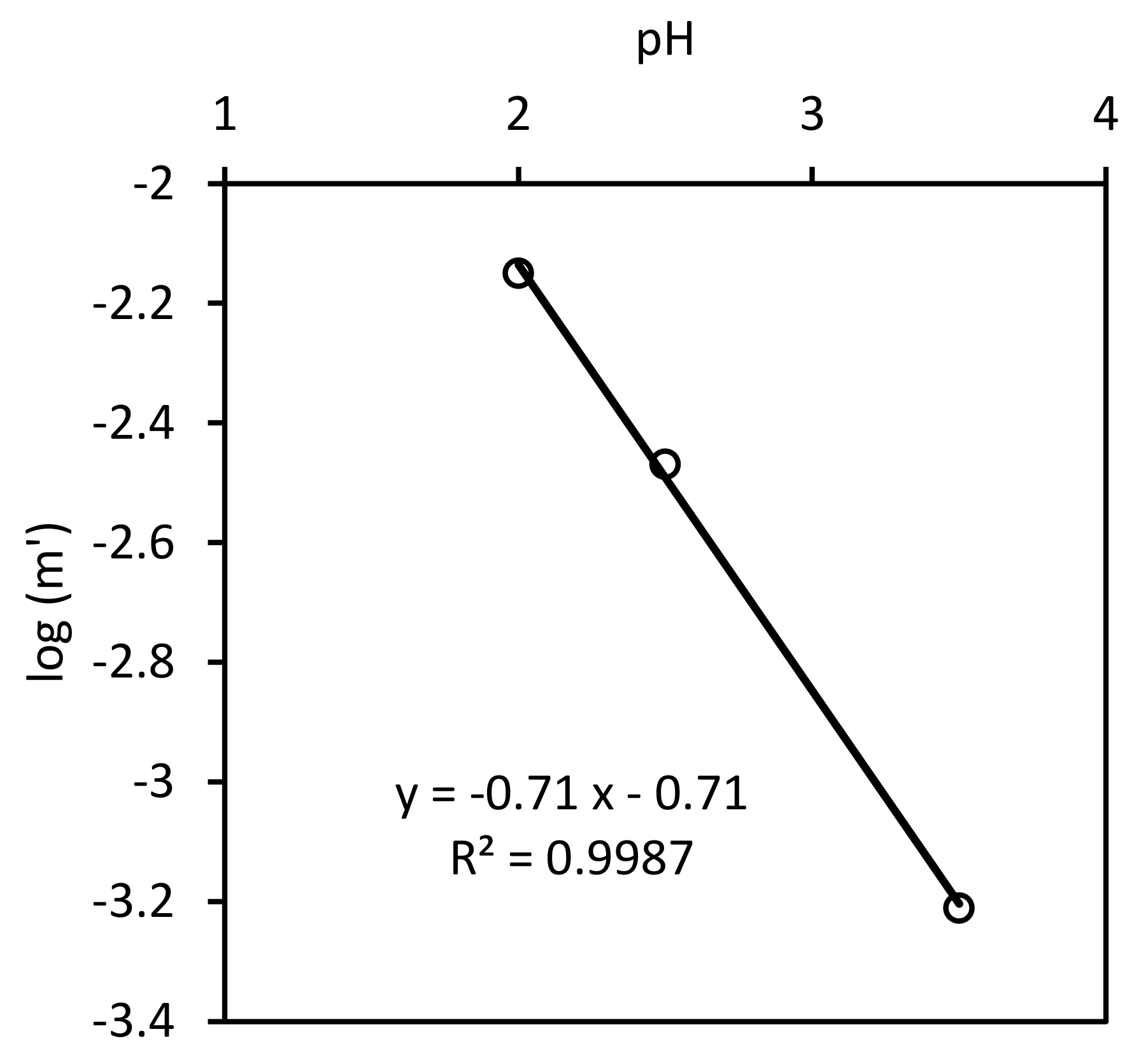
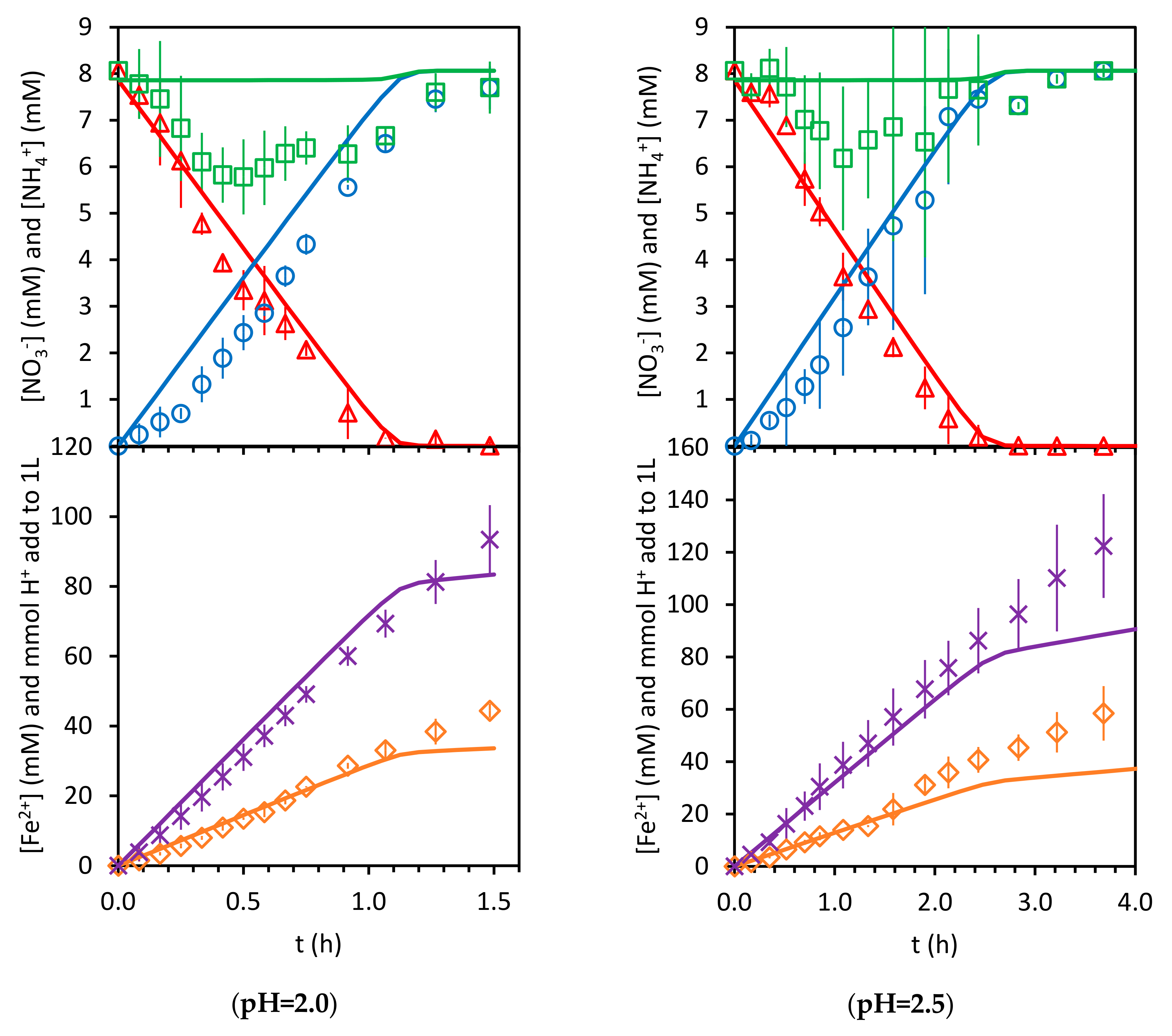
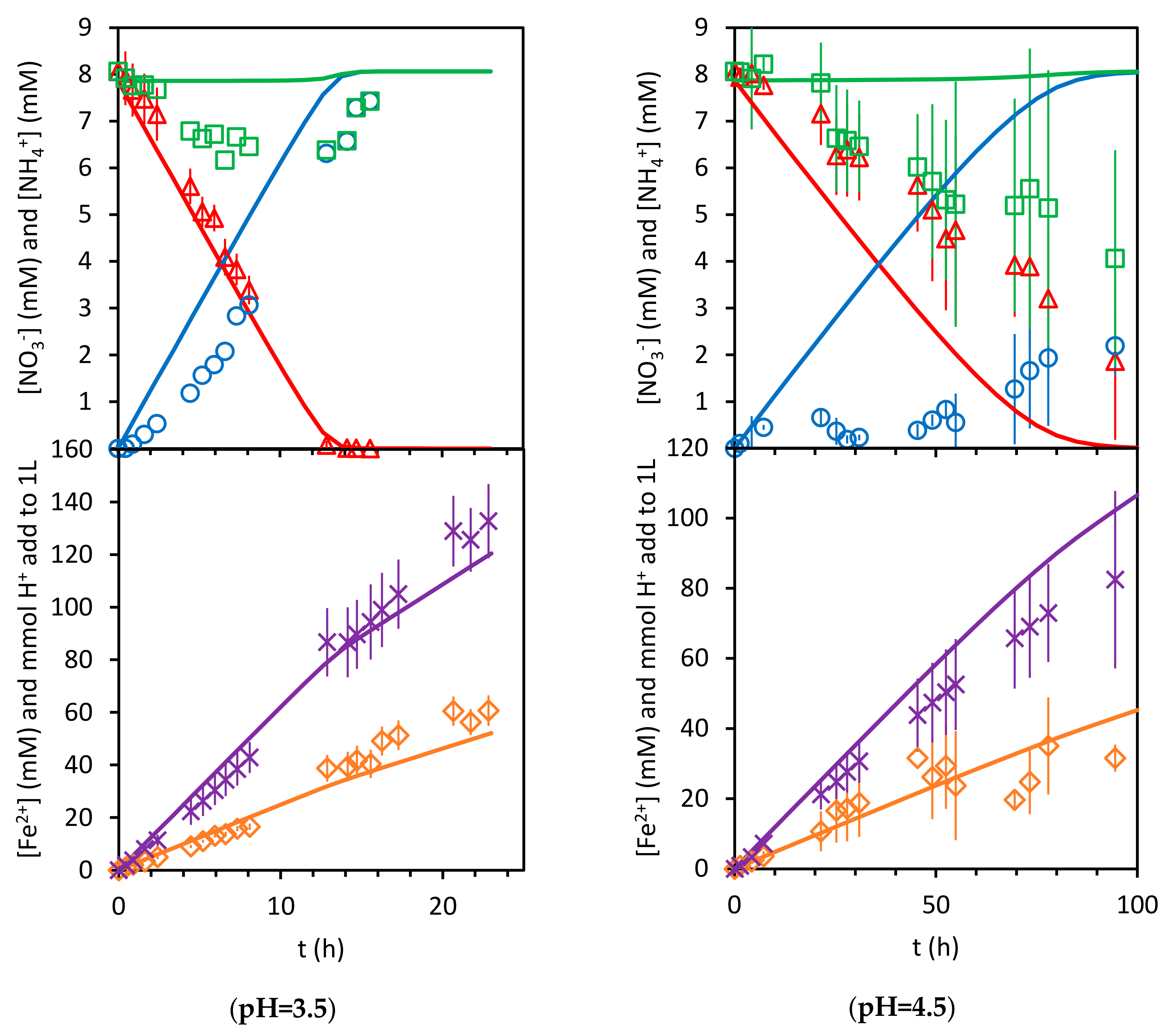
3.2. Kinetics of Nitrate Reduction
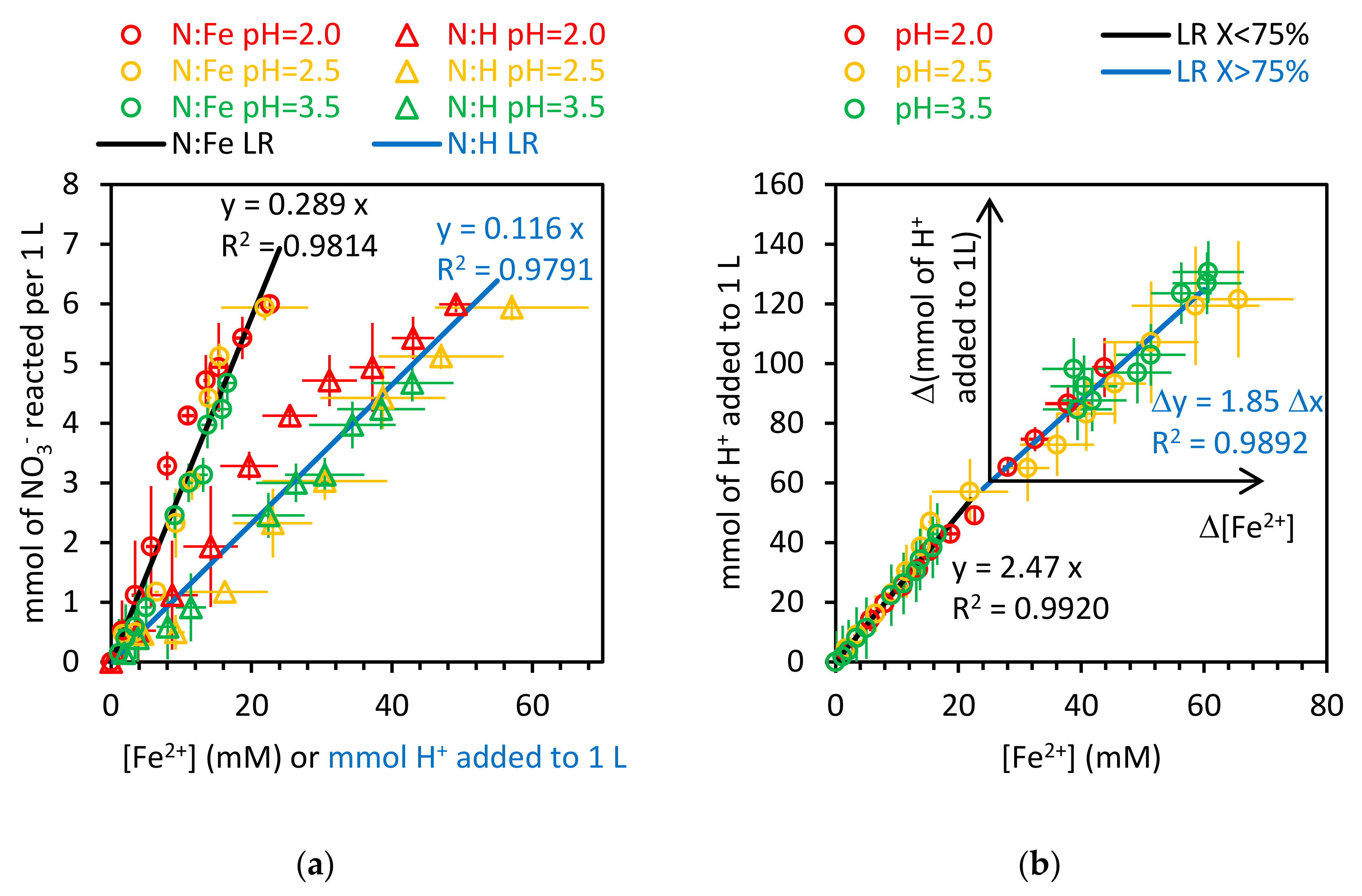
3.2.1. Stoichiometry
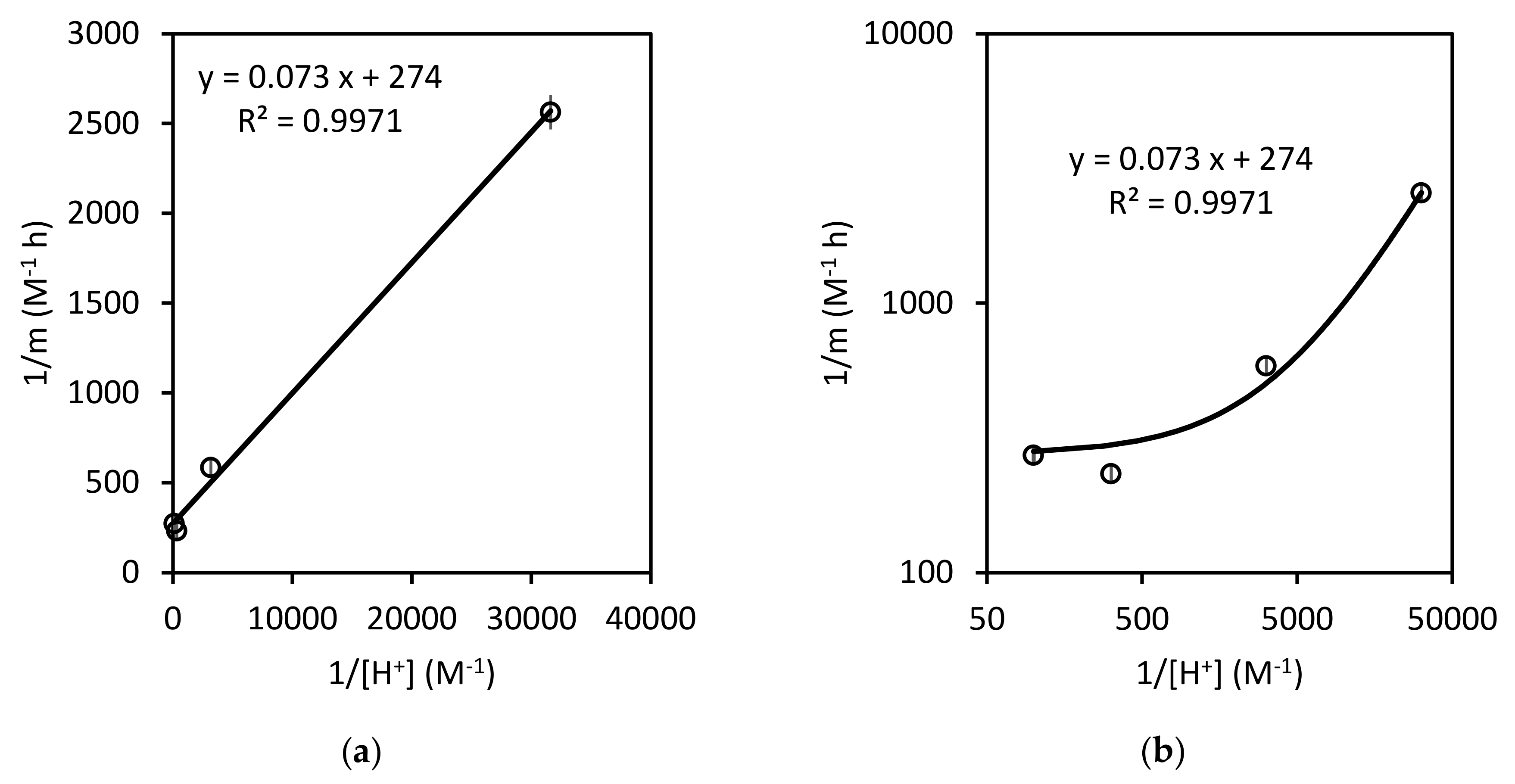
3.2.2. Surface Kinetics Model (SKM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gillham, R.W.; O’Hannesin, S.F. Enhanced Degradation of Halogenated Aliphatics by Zero-Valent Iron. Groundwater 1994, 32, 958–967. [Google Scholar] [CrossRef]
- O’Hannesin, S.F.; Gillham, R.W. Long-Term Performance of an In Situ “Iron Wall” for Remediation of VOCs. Groundwater 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Warner, S.D. Two Decades of Application of Permeable Reactive Barriers to Groundwater Remediation. In Permeable Reactive Barrier: Sustainable Groundwater Remediation; Naidu, R., Volker, B., Eds.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4822-2448-1. [Google Scholar]
- Araújo, R.; Castro, A.C.M.; Santos Baptista, J.; Fiúza, A. Nanosized iron based permeable reactive barriers for nitrate removal-Systematic review. Phys. Chem. Earth Parts A/B/C 2016, 94, 29–34. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Reduction of nitrate by zero valent iron (ZVI)-based materials: A review. Sci. Total Environ. 2019, 671, 388–403. [Google Scholar] [CrossRef]
- Grau-Martínez, A.; Torrentó, C.; Carrey, R.; Soler, A.; Otero, N. Isotopic evidence of nitrate degradation by a zero-valent iron permeable reactive barrier: Batch experiments and a field scale study. J. Hydrol. 2019, 570, 69–79. [Google Scholar] [CrossRef]
- Scherer, M.M.; Johnson, K.M.; Westall, J.C.; Tratnyek, P.G. Mass Transport Effects on the Kinetics of Nitrobenzene Reduction by Iron Metal. Environ. Sci. Technol. 2001, 35, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Maroto, J.M.; García-Herruzo, F.; García-Rubio, A.; Gómez-Lahoz, C.; Vereda-Alonso, C. Kinetics of the chemical reduction of nitrate by zero-valent iron. Chemosphere 2009, 74, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef]
- Zhang, Y.; Douglas, G.B.; Pu, L.; Zhao, Q.; Tang, Y.; Xu, W.; Luo, B.; Hong, W.; Cui, L.; Ye, Z. Zero-valent iron-facilitated reduction of nitrate: Chemical kinetics and reaction pathways. Sci. Total Environ. 2017, 598, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Kurosu, S.; Yamaguchi, R.; Kawase, Y. A phenomenological reaction kinetic model for Cu removal from aqueous solutions by zero-valent iron (ZVI). Chemosphere 2018, 200, 542–553. [Google Scholar] [CrossRef]
- The Interstate Technology & Regulatory Council (ITRC). Permeable Reactive Barrier: Technology Update; The Interstate Technology & Regulatory Council (ITRC): Washington, DC, USA, 2011; p. 234. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999; ISBN 0-471-25424-X. [Google Scholar]
- Evans, U.R. An Introduction to Metallic Corrosion; Metallic Corrosion; St Martin’s Press: New York, NY, USA, 1963. [Google Scholar]
- Lenell, B.A.; Arai, Y. Perrhenate sorption kinetics in zerovalent iron in high pH and nitrate media. J. Hazard. Mater. 2017, 321, 335–343. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villen-Guzman, M.; Paz-Garcia, J.M.; Arhoun, B.; Cerrillo-Gonzalez, M.d.M.; Rodriguez-Maroto, J.M.; Vereda-Alonso, C.; Gomez-Lahoz, C. Chemical Reduction of Nitrate by Zero-Valent Iron: Shrinking-Core versus Surface Kinetics Models. Int. J. Environ. Res. Public Health 2020, 17, 1241. https://doi.org/10.3390/ijerph17041241
Villen-Guzman M, Paz-Garcia JM, Arhoun B, Cerrillo-Gonzalez MdM, Rodriguez-Maroto JM, Vereda-Alonso C, Gomez-Lahoz C. Chemical Reduction of Nitrate by Zero-Valent Iron: Shrinking-Core versus Surface Kinetics Models. International Journal of Environmental Research and Public Health. 2020; 17(4):1241. https://doi.org/10.3390/ijerph17041241
Chicago/Turabian StyleVillen-Guzman, Maria, Juan Manuel Paz-Garcia, Brahim Arhoun, Maria del Mar Cerrillo-Gonzalez, Jose Miguel Rodriguez-Maroto, Carlos Vereda-Alonso, and Cesar Gomez-Lahoz. 2020. "Chemical Reduction of Nitrate by Zero-Valent Iron: Shrinking-Core versus Surface Kinetics Models" International Journal of Environmental Research and Public Health 17, no. 4: 1241. https://doi.org/10.3390/ijerph17041241
APA StyleVillen-Guzman, M., Paz-Garcia, J. M., Arhoun, B., Cerrillo-Gonzalez, M. d. M., Rodriguez-Maroto, J. M., Vereda-Alonso, C., & Gomez-Lahoz, C. (2020). Chemical Reduction of Nitrate by Zero-Valent Iron: Shrinking-Core versus Surface Kinetics Models. International Journal of Environmental Research and Public Health, 17(4), 1241. https://doi.org/10.3390/ijerph17041241





