Determining How Far an Adult Rare Disease Patient Needs to Travel for a Definitive Diagnosis: A Cross-Sectional Examination of the 2018 National Rare Disease Survey in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey on Rare Disease Patients
2.2. Measurement of Accessibility to Definitive Diagnosis
2.3. Impact Factors of Accessibility
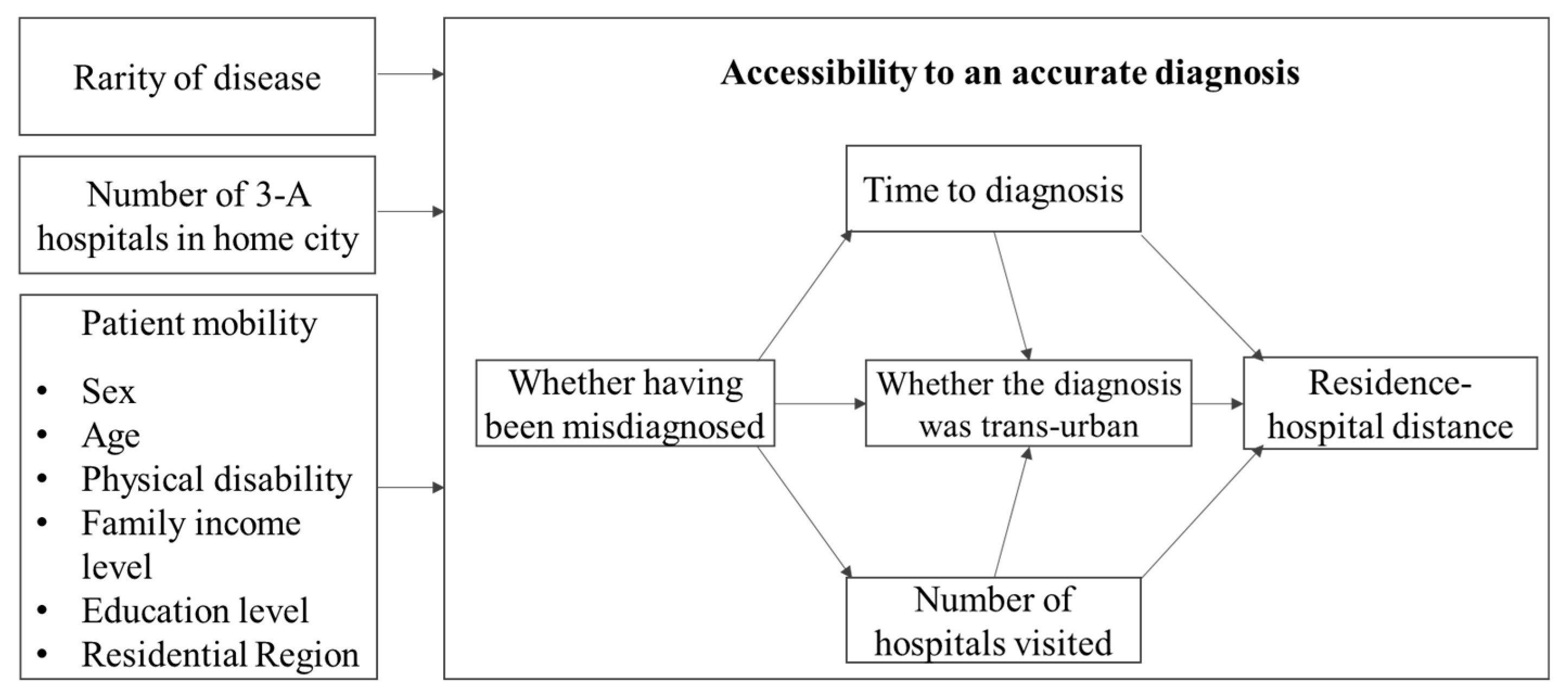
2.4. Conceptual Framework of Structural Equation Model
2.5. Ethical Approval
3. Results
3.1. Description Analysis
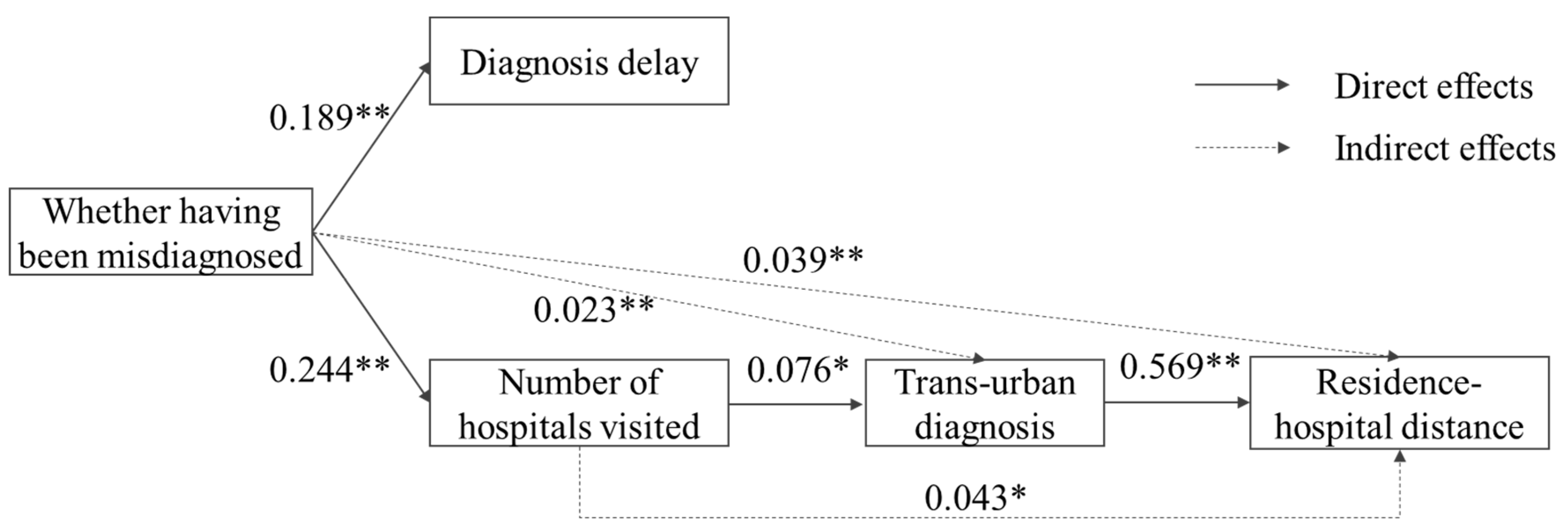
3.2. Results of SEM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Disease | Prevalence | Data Source of Prevalence | Frequencies in This Study Sample | |
|---|---|---|---|---|
| Extremely rare (Prevalence < 1/100,000) | ||||
| 1 | Acute transverse myelitis | 1/1,000,000~1/250,000 | orpha.net | 3 |
| 2 | Atypical hemolytic uremic syndrome, aHUS | 1–9/1,000,000 | orpha.net | 1 |
| 3 | Bartter syndrome | 1/1,000,000 | orpha.net | 1 |
| 4 | Erythrokeratoderma | 200 cases reported | orpha.net | 1 |
| 5 | Growth hormone deficiency | 1–9/1,000,000 | Stanley T. (2012). Diagnosis of growth hormone deficiency in childhood. Current opinion in endocrinology, diabetes, and obesity, 19(1), 47–52. doi:10.1097/MED.0b013e32834ec952 | 1 |
| 6 | Lymphangioleio-myomatosis | 1–9/1,000,000 | orpha.net | 1 |
| 7 | Massive osteolysis/Gorham-Stout disease | 300 cases reported | orpha.net | 1 |
| 8 | Mitochondrial encephalopathy | 1–9/1,000,000 | orpha.net | 2 |
| 9 | Niemann-Pick disease | <1/1,000,000 | orpha.net | 2 |
| 10 | Peutz–Jeghers syndrome | 1–9/1,000,000 | orpha.net | 68 |
| Rare (1/10,000<Prevalence < 1/100,000) | ||||
| 11 | Acromegaly | 1–9/100,000 | orpha.net | 85 |
| 12 | Albinism | 1/10,000–1/20,000 | Mártinez-García, M. and Montoliu, L. (2013), Albinism in Europe. J Dermatol, 40: 319-324, doi:10.1111/1346-8138.12170 | 9 |
| 13 | Amyotrophic lateral sclerosis | 1–9/100,000 | orpha.net | 3 |
| 14 | Anti-neutrophil cytoplasmic antibody-associated vasculitis | 4.6–18.4/100,000 | Watts, R., Mahr, A., Mohammad, A., Gatenby, P., Basu, N., and Flores-Suárez, L. (2015). Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrology Dialysis Transplantation, 30 (Suppl1), I14-I22. | 2 |
| 15 | Behcet disease | 1–9/100,000 | orpha.net | 147 |
| 16 | Congenital Adrenal Hyperplasia | 1–9/100,000 | orpha.net | 3 |
| 17 | Crohn’s disease | 1.2–21.2/100,000 | Prideaux, L., Kamm, M. A., De Cruz, P. P., Chan, F. K., and Ng, S. C. (2012). Inflammatory bowel disease in Asia: a systematic review. Journal of gastroenterology and hepatology, 27(8), 1266-1280. | 1 |
| 18 | Duchenne Muscular Dystrophy | 1–9/100,000 | orpha.net | 20 |
| 19 | Eisenmenger’s syndrome | 1–9/1,000,000 | orpha.net | 1 |
| 20 | Epidermolysis bullosa | 1–9/1,000,000 | orpha.net | 5 |
| 21 | Fabry disease | 1/100,000 | Branton, M. H., Schiffmann, R., Sabnis, etc. (2002). Natural history of Fabry renal disease: influence of α-galactosidase A activity and genetic mutations on clinical course. Medicine, 81(2), 122-138. | 68 |
| 22 | Gaucher disease | 1–9/100,000 | orpha.net | 20 |
| 23 | Glycogen storage disease due to acid maltase deficiency | 1–9/100,000 | orpha.net | 63 |
| 24 | Glycogen storage disease II | 1–9/100,000 | orpha.net | 1 |
| 25 | Granulomatosis with Polyangiitis | 1–9/100,000 | orpha.net | 7 |
| 26 | Hemophilia | 1–9/100,000 | orpha.net | 5 |
| 27 | Hepatolenticular degeneration/Wilson disease | 1–9/100,000 | orpha.net | 6 |
| 28 | Huntington’s disease | 1–9/100,000 | orpha.net | 19 |
| 29 | Ichthyosis | average of subtypes | orpha.net | 1 |
| 30 | Idiopathic Hypogonadotropic Hypogonadism | 1/4000–1/10,000 in males, and 2 to 5 times less frequent in females | Silveira, L. G., and Latronico, A. C. (2013). Approach to the Patient With Hypogonadotropic Hypogonadism. The Journal of Clinical Endocrinology and Metabolism, 98(5), 1781-1788. | 5 |
| 31 | Immunologic thrombocytopenic purpura | 5/100,000 | Fogarty, P. F., and Segal, J. B. (2007). The epidemiology of immune thrombocytopenic purpura. Current opinion in hematology, 14(5), 515-519. | 1 |
| 32 | Kallmann Syndrome, KS | 1–9/100,000 | orpha.net | 30 |
| 33 | Mucopolysaccharidosis | 1–9/100,000 | orpha.net | 15 |
| 34 | Multiple Sclerosis | 1–2/100,000 | Cheng, Q., Cheng, X.-J., and Jiang, G.-X. (2009). Multiple sclerosis in China—history and future. Multiple Sclerosis, 15(6), 655-660. | 53 |
| 35 | Myasthenia Gravis | 1–9/100,000 | orpha.net | 4 |
| 36 | Myelodysplastic syndromes | 1–9/100,000 | orpha.net | 1 |
| 37 | Neuromyelitis optica | 1–9/100,000 | orpha.net | 43 |
| 38 | Prader-Willi syndrome | 1–9/100,000 | orpha.net | 3 |
| 39 | Pseudomyxoma peritonei | 1–9/100,000 | orpha.net | 61 |
| 40 | Pulmonary hypertension | 1–9/100,000 | orpha.net | 50 |
| 41 | Sclerosis | 5/100,000 | Tullman, M. (2013). Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. The American Journal of Managed Care, 19 (2 Suppl), S15-S20. | 1 |
| 42 | Spinal muscular atrophy | 1–9/100,000 | orpha.net | 22 |
| 43 | Spinocerebellar ataxias | 1–9/100,000 | orpha.net | 10 |
| 44 | Systemic Vasculitis | 1–2/100,000 | Lane, S., Watts, E., and Scott, R. (2005). Epidemiology of systemic vasculitis. Current Rheumatology Reports, 7(4), 270-275. | 2 |
| 45 | Takayasu arteritis | 1–9/100,000 | orpha.net | 1 |
| Somewhat rare (Prevalence >1/10,000) | ||||
| 46 | Charcot–Marie–Tooth disease | 1–5/10,000 | orpha.net | 4 |
| 47 | Fuchs’ syndrome | 3.7–9.2% in patients over 50 years of age | Pilger, Daniel, Brockmann, Claudia, Maier, Anna-Karina B., and Bertelmann, Eckart. (2019). Predictive Factors for Clinical Outcomes after Primary Descemet’s Membrane Endothelial Keratoplasty for Fuchs’ Endothelial Dystrophy. Current Eye Research, 44(2), 147-153. | 1 |
| 48 | Hyperammonemia | 1–5/10,000 | orpha.net | 1 |
| 49 | Isolated spina bifida | 1–5/10,000 | orpha.net | 2 |
| 50 | Keratoconus | 5.4/10,000 | Gokhale N. S. (2013). Epidemiology of keratoconus. Indian journal of ophthalmology, 61(8), 382–383, doi:10.4103/0301-4738.116054 | 1 |
| 51 | Klinefelter syndrome | 1/1000 | Wattendorf D.J., Muenke M. (2005) Klinefelter syndrome. Am Fam Physician. 72(11):2259-62. | 1 |
| 52 | Lysosomal storage diseases | 1/5000 | Fuller M., Meikle P.J., Hopwood J.J. Epidemiology of lysosomal storage diseases: an overview. Chapter 2. Oxford: Oxford Pharmagenesis, 2006. | 1 |
| 53 | Marfan Syndrome | 1–5/10,000 | orpha.net | 2 |
| 54 | Neurofibromatosis | 1–5/10,000 | orpha.net | 32 |
| 55 | Osteogenesis imperfecta | 1–5/10,000 | orpha.net | 37 |
| 56 | Pemphigus vulgaris | 1–5/10,000 | orpha.net | 1 |
| 57 | Primary adrenal insufficiency/Addison’s disease | 1–5/10,000 | orpha.net | 2 |
| 58 | Stargardt disease | 1–5/10,000 | orpha.net | 1 |
| 59 | Systemic lupus erythematosus | 1–5/10,000 | orpha.net | 1 |
| 60 | Systemic sclerosis | 1–5/10,000 | orpha.net | 36 |
| 61 | Tuberous sclerosis complex | 1–5/10,000 | orpha.net | 33 |
| 62 | Turner syndrome | 1–5/10,000 | orpha.net | 1 |
| 63 | Uveitis | 1–5/10,000 | orpha.net | 4 |
References
- Schieppati, A.; Henter, J.-I.; Daina, E.; Aperia, A. Why rare diseases are an important medical and social issue. Lancet 2008, 371, 2039–2041. [Google Scholar] [CrossRef]
- Dawkins, H.J.; Draghia-Akli, R.; Lasko, P.; Lau, L.P.; Jonker, A.H.; Cutillo, C.M.; Rath, A.; Boycott, K.M.; Baynam, G.; Lochmüller, H. Progress in rare diseases research 2010–2016: An IRDiRC perspective. Clin. Transl. Sci. 2018, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Sada, D.; Nguyen, M.T. Diagnosis of rare diseases under focus: Impacts for Canadian patients. J. Commun. Genet. 2018, 9, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Zurynski, Y.; Frith, K.; Leonard, H.; Elliott, E. Rare childhood diseases: How should we respond? Arch. Dis. Child. 2008, 93, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- EURORDIS. Survey of the Delay in Diagnosis for 8 Rare Diseases in Europe (‘EurordisCare2’); EURORDIS: Paris, France, 2006. [Google Scholar]
- Austin, C.P.; Cutillo, C.M.; Lau, L.P.L.; Jonker, A.H.; Rath, A.; Julkowska, D.; Thomson, D.; Terry, S.F.; de Montleau, B.; Ardigò, D.; et al. Future of Rare Diseases Research 2017–2027: An IRDiRC Perspective. Clin. Transl. Sci. 2018, 11, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Boycott, K.M.; Hartley, T.; Biesecker, L.G.; Gibbs, R.A.; Innes, A.M.; Riess, O.; Belmont, J.; Dunwoodie, S.L.; Jojic, N.; Lassmann, T.; et al. A Diagnosis for All Rare Genetic Diseases: The Horizon and the Next Frontiers. Cell 2019, 177, 32–37. [Google Scholar] [CrossRef] [PubMed]
- EURORDIS. The Voice of 12,000 Patients—Experiences and Expectations of Rare Disease Patients on Diagnosis and Care in Europe; EURORDIS: Paris, France, 2009. [Google Scholar]
- Rosenmöller, M.; McKee, M.; Baeten, R. Patient Mobility in the European Union: Learning from Experience; World Health Organization: Copenhagen, Denmark, 2006. [Google Scholar]
- Glinos, I.A.; Baeten, R.; Helble, M.; Maarse, H. A typology of cross-border patient mobility. Health Place 2010, 16, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Lunt, N.; Mannion, R. Patient mobility in the global marketplace: A multidisciplinary perspective. Int. J. Health Policy Manag. 2014, 2, 155. [Google Scholar] [CrossRef] [PubMed]
- Bellia, V.; Battaglia, S.; Catalano, F.; Scichilone, N.; Incalzi, R.A.; Imperiale, C.; Rengo, F. Aging and disability affect misdiagnosis of COPD in elderly asthmatics: The SARA study. Chest 2003, 123, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Storla, D.G.; Yimer, S.; Bjune, G.A. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- China Daily. Rare Disease Medicine Debuts in China. Available online: http://www.chinadaily.com.cn/a/201904/28/WS5cc556c5a3104842260b8e4d.html (accessed on 28 February 2020).
- Han, J.; Cui, Y.; Zhou, X. Rare diseases research in China: Opportunities, challenges, and solutions. Intract. Rare Dis. Res. 2012, 1, 10–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, D.; Wang, Y. Challenges of rare diseases in China. Lancet 2016, 387, 1906. [Google Scholar] [CrossRef]
- Yu, M.; He, S.; Wu, D.; Zhu, H.; Webster, C. Examining the Multi-Scalar Unevenness of High-Quality Healthcare Resources Distribution in China. Int. J. Environ. Res. Public Health 2019, 16, 2813. [Google Scholar] [CrossRef] [PubMed]
- Shire. Rare Disease Impact Report: Insights from Patients and the Medical Community; Shire Human Genetic Therapies: Lexington, MA, USA, 2013. [Google Scholar]
- Andersen, R.M. National health surveys and the behavioral model of health services use. Med. Care 2008, 46, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.M. Revisiting the behavioral model and access to medical care: Does it matter? J. Health Soc. Behav. 1995, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.R.; Liu, G.G. China’s Healthcare System and Reform; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Penchansky, R.; Thomas, J.W. The concept of access: Definition and relationship to consumer satisfaction. Med. Care 1981, 19, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Frenk, J. The concept and measurement of accessibility. In Health Services Research: An Anthology; White, K.L., Frenck, J., Ordonez, C., Paganimi, J.M., Starfiled, B., Eds.; Pan American Health Organization: Washington DC, USA, 1992; pp. 842–855. [Google Scholar]
- Neal, R.; Allgar, V. Sociodemographic factors and delays in the diagnosis of six cancers: Analysis of data from the ‘National Survey of NHS Patients: Cancer’. Br. J. Cancer 2005, 92, 1971. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Eq. Model. Multidisc. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Illness Challenge Foundation. China’s Rare Disease Report 2018; Illness Challenge Foundation: Beijing, China, 2018. [Google Scholar]
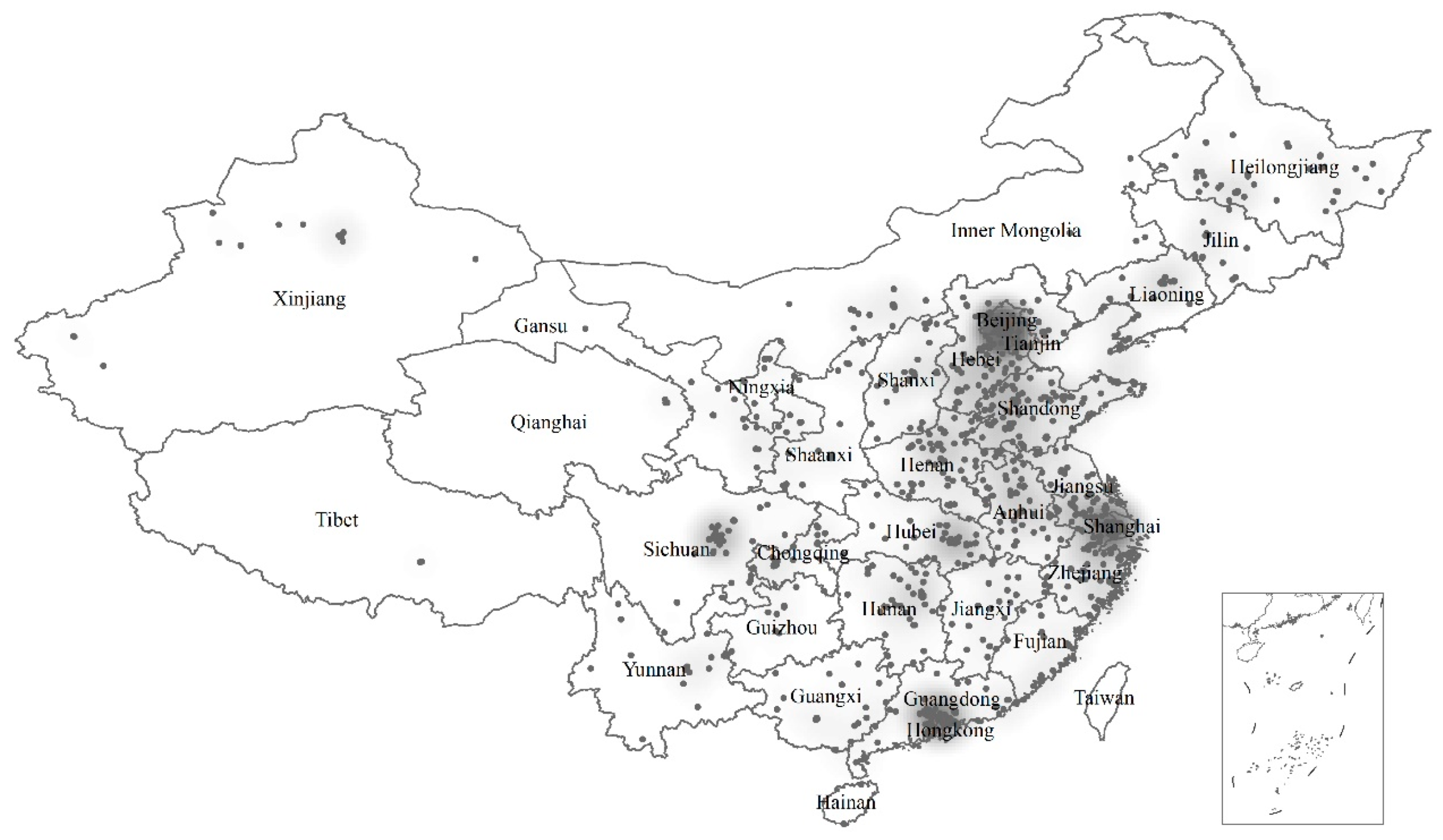
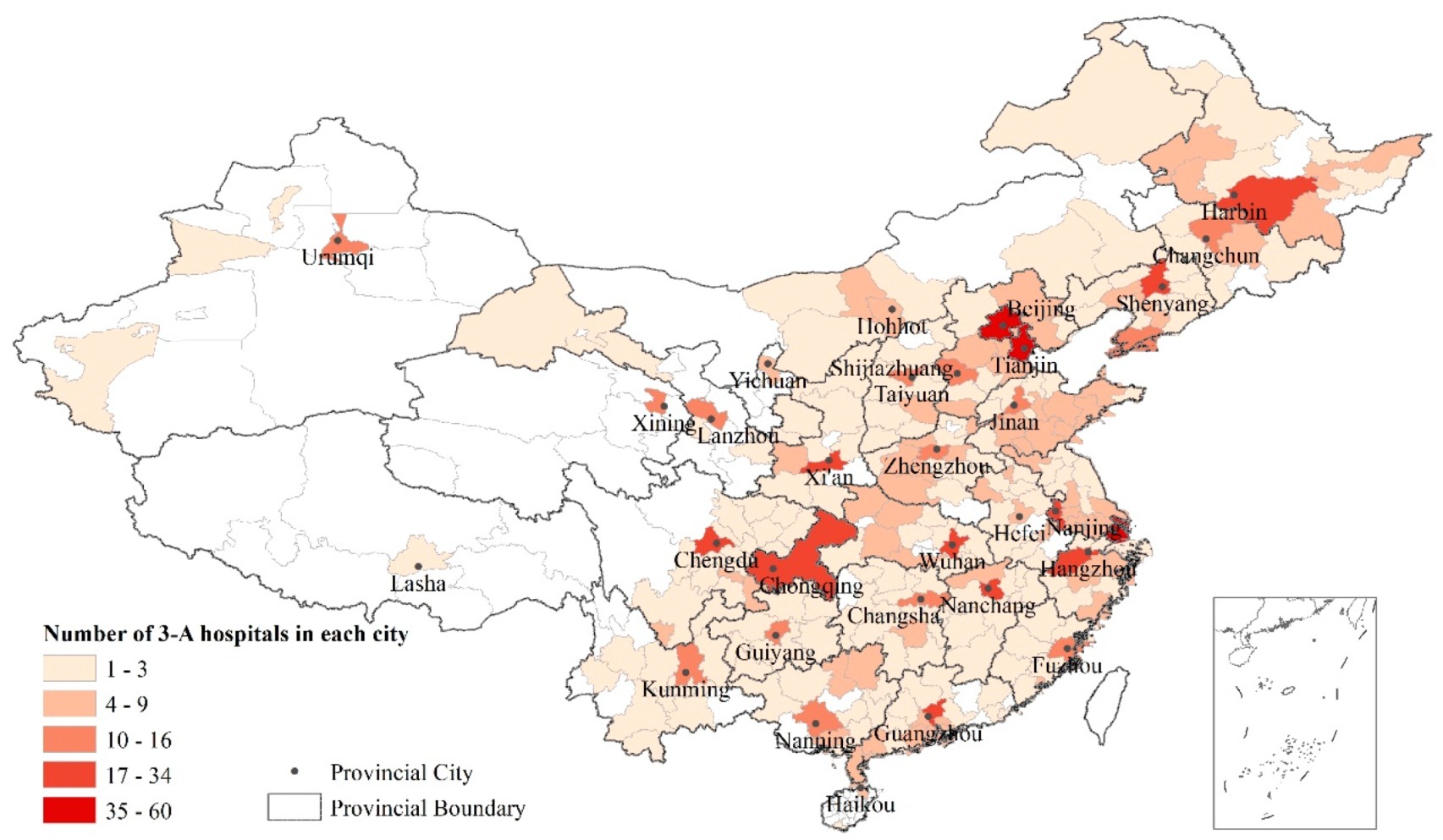

| Indicators | Mean | S.D. | Median | Range |
|---|---|---|---|---|
| Proportion of patients having been misdiagnosed | 72.97% | - | - | - |
| Diagnosis delay (years) | 4.30 | 5.75 | 2 | 1–44 1 |
| Number of hospitals visited prior diagnosis | 2.97 | 4.71 | 2 | 1–80 |
| Proportion of trans-urban diagnosis | 67.13% | - | - | - |
| Residence–hospital distance (km) | 374.41 | 502.91 | 170.51 | 0–3728.18 |
| Residence–hospital distance of trans-urban diagnosis (km) | 562.72 | 523.63 | 383.19 | 23.30–3728.18 |
| Mean (S.D.) | |
|---|---|
| Sex | |
| Male | 46.63% |
| Female | 53.47% |
| Age | 35.51 (11.13) |
| Frequency of using assistive device (Degree of physical disability) | |
| Always | 8.32% |
| Usually | 7.62% |
| Sometimes | 10.10% |
| Occasionally | 18.32% |
| No need | 55.64% |
| Family income level in local city | |
| Low | 23.76% |
| Relatively low | 40.00% |
| Mid-level | 32.67% |
| Relatively high | 3.37% |
| High | 0.20% |
| Years of education | 12.05 (4.12) |
| Residential region | |
| East China | 49.50% |
| Central China | 22.87% |
| West China | 27.63% |
| Number of 3-A hospitals in local city | 12.60 (16.76) |
| Rarity of disease | |
| Extremely rare | 8.51% |
| Rare | 75.74% |
| Somewhat rare | 15.75% |
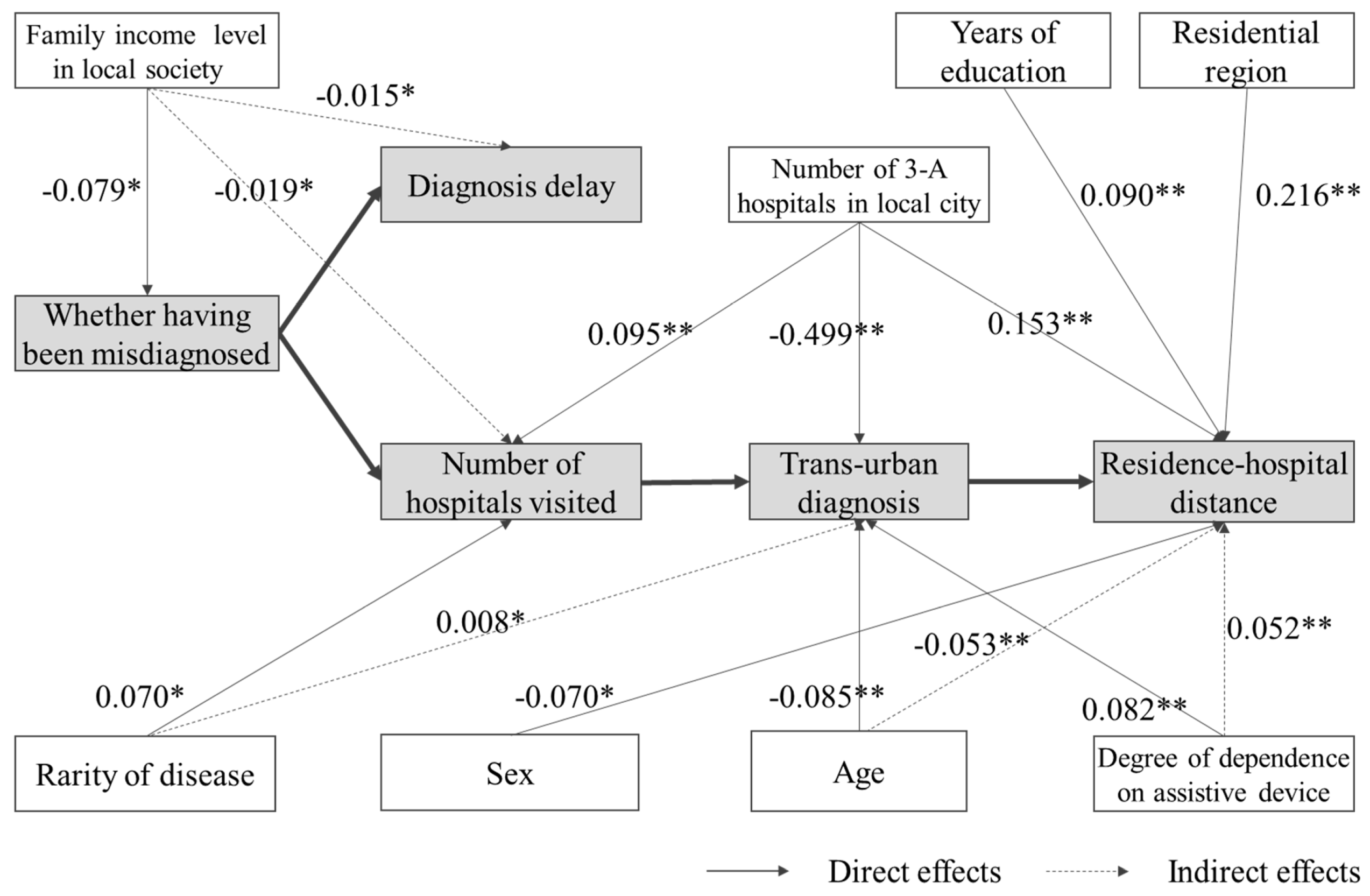
| Whether Having Been Misdiagnosed | Diagnosis Delay | Number of Hospitals Visited | Whether Diagnosis Was Trans-Urban | Residence–Hospital Distance | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct | Indirect | Total | Direct | Indirect | Total | Direct | Indirect | Total | Direct | Indirect | Total | Direct | Indirect | Total | |
| Whether having been misdiagnosed (Not = 0) | - | - | - | 2.444 ** (0.189) | - | 2.444 ** (0.189) | 2.591 ** (0.244) | - | 2.591 ** (0.244) | 0.023 (0.022) | 0.024 * (0.023) | 0.047 (0.045) | - | 44.564 * (0.039) | 44.564 * (0.039) |
| Diagnosis delay | - | - | - | - | - | - | - | - | - | 0.002 (0.024) | - | 0.002 (0.024) | 0.114 (0.001) | 1.173 (0.013) | 1.286 (0.015) |
| Number of hospitals visited | - | - | - | - | - | - | - | - | - | 0.008 * (0.076) | - | 0.008 * (0.076) | 5.977 (0.056) | 4.602 * (0.043) | 10.579 * (0.099) |
| Whether diagnosis was trans-urban (Diagnosed in local city = 0) | - | - | - | - | - | - | - | - | - | - | - | - | 610.639 ** (0.569) | - | 610.639 ** (0.569) |
| Rarity of disease (Somewhat rare = 0) | 0.017 (0.019) | - | 0.017 (0.019) | 0.752 (0.064) | 0.042 (0.004) | 0.794 (0.067) | 0.680 * (0.070) | 0.044 (0.005) | 0.724 * (0.075) | 0.032 (0.033) | 0.007 * (0.008) | 0.039 (0.041) | 41.481 (0.040) | 28.312 (0.028) | 69.793 * (0.068) |
| Number of 3-A hospitals in local city | −0.002 (−0.062) | - | −0.002 (−0.062) | 0.017 (0.050) | −0.004 (−0.012) | 0.013 (0.038) | 0.027 ** (0.095) | −0.004 (−0.015) | 0.023 ** (0.080) | −0.014 ** (−0.499) | 0.000 (0.006) | −0.014 ** (−0.494) | 4.596 ** (0.153) | −8.288 ** (−0.276) | −3.692 ** (−0.123) |
| Sex (Male = 0) | −0.029 (−0.033) | - | −0.029 (−0.033) | −0.617 (−0.054) | −0.072 (−0.006) | −0.688 (−0.060) | −0.142 (−0.015) | −0.076 (−0.008) | −0.218 (−0.023) | −0.032 (−0.034) | −0.004 (−0.004) | −0.036 (−0.038) | −46.752 (−0.046) | −23.215 (−0.023) | −69.967 * (−0.070) |
| Age | −0.001 (−0.026) | - | −0.001 (−0.026) | −0.015 (−0.028) | −0.003 (−0.005) | −0.017 (−0.033) | −0.015 (−0.035) | −0.003 (−0.006) | −0.018 (−0.042) | −0.004 ** (−0.085) | 0.000 (−0.005) | −0.004 ** (−0.089) | 1.081 (0.024) | −2.400 ** (−0.053) | −1.319 (−0.029) |
| Frequency of using assistive device (No need = 0) | −0.003 (−0.010) | - | 0.003 (0.010) | 0.113 (0.026) | 0.008 (0.002) | 0.121 (0.027) | 0.186 (0.051) | 0.008 (0.002) | 0.194 (0.054) | 0.029 ** (0.082) | 0.002 (0.005) | 0.031 ** (0.087) | 5.490 (0.014) | 20.150 ** (0.052) | 25.640 * (0.067) |
| Family income level in local society (Low = 0) | −0.042 * (−0.079) | - | −0.042 * (−0.079) | 0.055 (0.008) | −0.102 * (−0.015) | −0.047 (−0.007) | −0.132 (−0.023) | −0.108 * (−0.019) | −0.240 (−0.042) | −0.019 (−0.035) | −0.003 (−0.005) | −0.022 (−0.040) | −12.240 (−0.020) | −15.027 (−0.025) | −27.267 (−0.045) |
| Years of education | 0.006 (0.051) | - | 0.006 (0.051) | −0.025 (−0.018) | 0.013 (0.010) | −0.011 (−0.008) | −0.080 (−0.070) | 0.014 (0.012) | −0.066 (−0.058) | 0.003 (0.028) | 0.000 (−0.004) | 0.003 (0.024) | 10.976 ** (0.090) | 1.281 (0.011) | 12.257 ** (0.100) |
| Residential region (East China = 0) | 0.010 (0.024) | 0.010 (0.024) | 0.234 (0.041) | 0.025 (0.005) | 0.259 (0.046) | 0.218 (0.047) | 0.027 (0.006) | 0.245 (0.053) | −0.003 (−0.007) | 0.003 (0.006) | −0.001 (−0.002) | 106.771 ** (0.216) | 0.985 (0.022) | 107.756 ** (0.218) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; He, S.; Dong, D. Determining How Far an Adult Rare Disease Patient Needs to Travel for a Definitive Diagnosis: A Cross-Sectional Examination of the 2018 National Rare Disease Survey in China. Int. J. Environ. Res. Public Health 2020, 17, 1757. https://doi.org/10.3390/ijerph17051757
Yan X, He S, Dong D. Determining How Far an Adult Rare Disease Patient Needs to Travel for a Definitive Diagnosis: A Cross-Sectional Examination of the 2018 National Rare Disease Survey in China. International Journal of Environmental Research and Public Health. 2020; 17(5):1757. https://doi.org/10.3390/ijerph17051757
Chicago/Turabian StyleYan, Xiang, Shenjing He, and Dong Dong. 2020. "Determining How Far an Adult Rare Disease Patient Needs to Travel for a Definitive Diagnosis: A Cross-Sectional Examination of the 2018 National Rare Disease Survey in China" International Journal of Environmental Research and Public Health 17, no. 5: 1757. https://doi.org/10.3390/ijerph17051757
APA StyleYan, X., He, S., & Dong, D. (2020). Determining How Far an Adult Rare Disease Patient Needs to Travel for a Definitive Diagnosis: A Cross-Sectional Examination of the 2018 National Rare Disease Survey in China. International Journal of Environmental Research and Public Health, 17(5), 1757. https://doi.org/10.3390/ijerph17051757






