Irradiation with Polychromatic Incoherent Low Energy Radiation of Human Peripheral Blood Mononuclear Cells In Vitro: Effects on Cytokine Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
- Age 20 to 45 (mean age of 32.7 ± 8.1, M:F = 9:1),
- Hematologic parameters within the range of physiological values,
- Leukocytes ranging from 6000/μL to 9000/μL,
- Lymphocytes ranging from 1800/μL to 2600/μL,
- Monocytes ranging from 270/μL to 420/μL.
- History of UV exposure for at least 6 months before the study,
- History of photosensitivity,
- History of acute or chronic inflammatory diseases,
- History of auto-immune, cardiovascular, metabolic, endocrine, and oncological diseases,
- Oral intake of medications (e.g., steroidal and/or non-steroidal anti-inflammatory agents, photosensitizing agents),
- Treatment with hormone therapy,
- Treatment for cancer (chemotherapy, immunotherapy, biological therapy, radiation),
- Pregnancy.
2.2. PILER Source
2.3. Cells
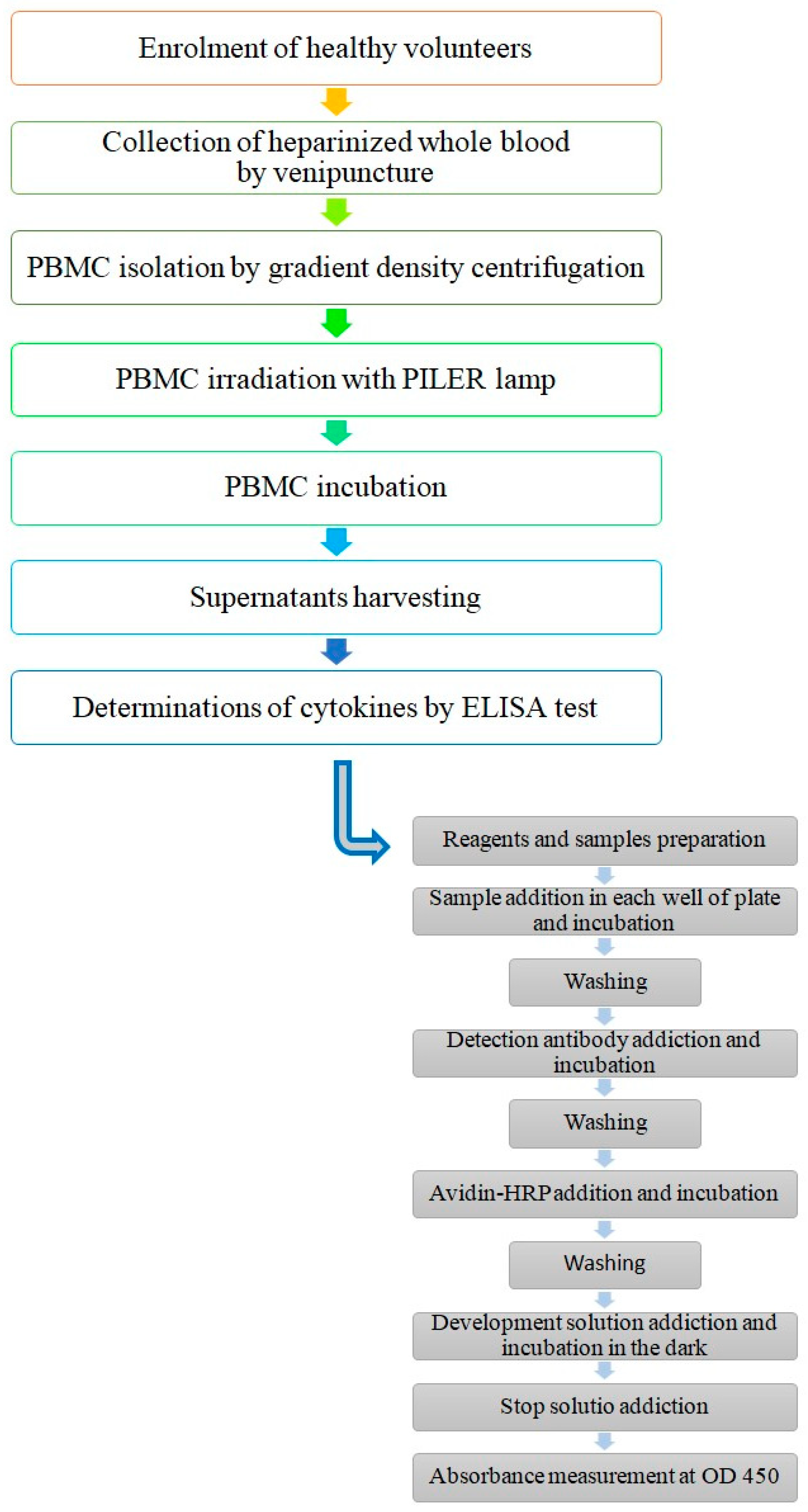
2.3.1. PBMC Collection and Preparation
2.3.2. PBMC Treatment
2.4. Determination of Cytokines by ELISA
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gambicler, T.; Terras, S.; Kreuter, A. Treatment regimens, protocols, dosage, and indications for UVA1 phototherapy: Facts and controversies. Clin. Dermatol. 2013, 31, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Totonchy, M.B.; Chiu, M.W. UV-based therapy. Dermatol. Clin. 2014, 32, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Mussttaf, R.A.; Jenkins, D.F.L.; Jha, A.N. Assessing the impact of low level laser therapy (LLLT) on biological systems: A review. Int. J. Radiat. Biol. 2019, 95, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Selting, W.; Hamblin, M.R. Review of light parameters and photobiomodulation efficacy: Dive into complexity. J. Biomed. Opt. 2018, 23, 1–17. [Google Scholar] [CrossRef]
- Ablon, G. Phototherapy with Light Emitting Diodes: Treating a Broad Range of Medical and Aesthetic Conditions in Dermatology. J. Clin. Aesthetic Dermatol. 2018, 11, 21–27. [Google Scholar]
- Jagdeo, J.; Austin, E.; Mamalis, A.; Wong, C.; Ho, D.; Siegel, D.M. Light-emitting diodes in dermatology: A systematic review of randomized controlled trials. Lasers Surg. Med. 2018, 50, 613–628. [Google Scholar] [CrossRef]
- Feehan, J.; Burrows, S.P.; Cornelius, L.; Cook, A.M.; Mikkelsen, K.; Apostolopoulos, V.; Husaric, M.; Kiatos, D. Therapeutic applications of polarized light: Tissue healing and immunomodulatory effects. Maturitas 2018, 116, 11–17. [Google Scholar] [CrossRef]
- Mihaylova, M.; Ruseva, Z.; Filkova, S. The effect of polarized polychromatic non-coherent light (Bioptron) therapy on patients with lower back pain. Scr. Sci. Salut. Publicae 2017, 3, 23–27. [Google Scholar] [CrossRef][Green Version]
- Michos, I.; Talias, M.; Lamnisos, D.; Dimopoulos, C.; Stasinopoulos, D. Tendinopathy: The Role of Polarised Polychromatic Non-Coherent Light Commonly called Bioptron Light. J. Prev. Infect. Control 2016, 2, 1–4. [Google Scholar] [CrossRef][Green Version]
- Raeissadat, S.A.; Rayegani, S.M.; Rezaei, S.; Sedighipour, L.; Bahrami, M.H.; Eliaspour, D.; Karimzadeh, A. The effect of polarized polychromatic noncoherent light (bioptron) therapy on patients with carpal tunnel syndrome. J. Lasers Med. Sci. 2014, 5, 39–46. [Google Scholar]
- Lim, J.H.; Lee, J.; Lee, I.S.; Kim, Y.J.; Song, E.Y.; Choi, Y.S.; Yun, Y.M. The Effects of Daily Irradiation with Polychromatic Visible Polarized Light on Human Lymphocyte Populations. Photomed. Laser Surg. 2008, 26, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Faraone, V.; Denaro, L.; Ruello, E.; Scarmato, A.; Vermiglio, G.; Ruggeri, P. Phototreatment of radiation-induced dermal injuries. Acta Med. Mediterr. 2008, 24, 99–104. [Google Scholar]
- Wickenheisser, V.A.; Zywot, E.M.; Rabjohns, E.M.; Lee, H.H.; Lawrence, D.S.; Tarrant, T.K. Laser Light Therapy in Inflammatory, Musculoskeletal, and Autoimmune Disease. Curr. Allergy Asthma Rep. 2019, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wolf, P. How It Works. The Immunology Underlying Phototherapy. Dermatol. Clin. 2020, 38, 37–53. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Martins, D.F.; Turnes, B.L.; Cidral-Filho, F.J.; Bobinski, F.; Rosas, R.F.; Danielski, L.G.; Petronilho, F.; Santos, A.R. Light-emitting diode therapy reduces persistent inflammatory pain: Role of interleukin 10 and antioxidant enzymes. Neuroscience 2016, 324, 485–495. [Google Scholar] [CrossRef]
- Zhevago, N.A.; Zimin, A.A.; Glazanova, T.V.; Davydova, N.I.; Bychkova, N.V.; Chubukina, Z.V.; Buinyakova, A.I.; Ballyuzek, M.F.; Samoilova, K.A. Polychromatic light (480–3400 nm) similar to the terrestrial solar spectrum without its UV component in post-surgical immunorehabilitation of breast cancer patients. J. Photochem. Photobiol. 2017, 166, 44–51. [Google Scholar] [CrossRef]
- Zhevago, N.A.; Samoĭlova, K.A.; Calderhead, R.G. Polychromatic light similar to the terrestrial solar spectrum without its UV component stimulates DNA synthesis in human peripheral blood lymphocytes in vivo and in vitro. Photochem. Photobiol. 2006, 82, 1301–1308. [Google Scholar] [CrossRef]
- Zhevago, N.A.; Samoĭlova, K.A. Pro- and anti-inflammatory cytokine content in human peripheral blood after its transcutaneous (in vivo) and direct (in vitro) irradiation with polychromatic visible and infrared light. Photomed. Laser Surg. 2006, 24, 129–139. [Google Scholar] [CrossRef]
- Zhevago, N.A.; Samoĭlova, K.A. Modulation of proliferation of peripheral blood lymphocytes after irradiation of volunteers with polychromatic visible and infrared light. Tsitologiia 2004, 46, 567–577. [Google Scholar]
- Samoilova, K.A.; Obolenskaya, K.D.; Vologdina, A.V.; Snopov, S.A.; Shevchenko, E.V. Single skin exposure to visible polarized light induces rapid modification of entire circulating blood. I. Improvement of rheologic and immune parameters. In Effects of Low-Power Light on Biological Systems IV; International Society for Optics and Photonics: Stockholm, Sweden, 1998; Volume 3569, pp. 90–103. [Google Scholar] [CrossRef]
- Kubasova, T.; Horváth, M.; Kocsis, K.; Fenyö, M. Effect of visible light on some cellular and immune parameters. Immunol. Cell Biol. 1995, 73, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Böyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 1968, 21 (Suppl. 97), 77–89. [Google Scholar]
- Lilliefors Test—MATLAB Lillietest. Available online: https://www.mathworks.com/help/stats/lillitest.html (accessed on 4 November 2019).
- Kruskal-Wallis—MATLAB Kruskalwallis. Available online: https://www.mathworks.com/help/stats/kruskalwallis.html (accessed on 4 November 2019).
- Multiple Comparison Test—MATLAB Multicompare. Available online: https://www.mathworks.com/help/stats/multicompare.html (accessed on 4 November 2019).
- Saravia, J.; Chapman, N.M.; Chi, H. Helper T cell differentiation. Cell. Mol Immunol 2019, 16, 634–643. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 2014, 26, 192–197. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Gadina, M.; Siegel, R.M. Cytokines and cytokine receptors. In Clinical Immunology, Priciples and Practice, 5th ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Elsevier: London, UK, 2019; pp. 127–155. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Schmidt-Weber, C.B.; Blaser, K. Regulation and role of transforming growth factor-β in immune tolerance induction and inflammation. Curr. Opin. Immunol. 2004, 16, 709–716. [Google Scholar] [CrossRef]
| Cytokines | Time of Culture | Unirradiated PBMCs | 6 min Exposure Time | 12 min Exposure Time |
|---|---|---|---|---|
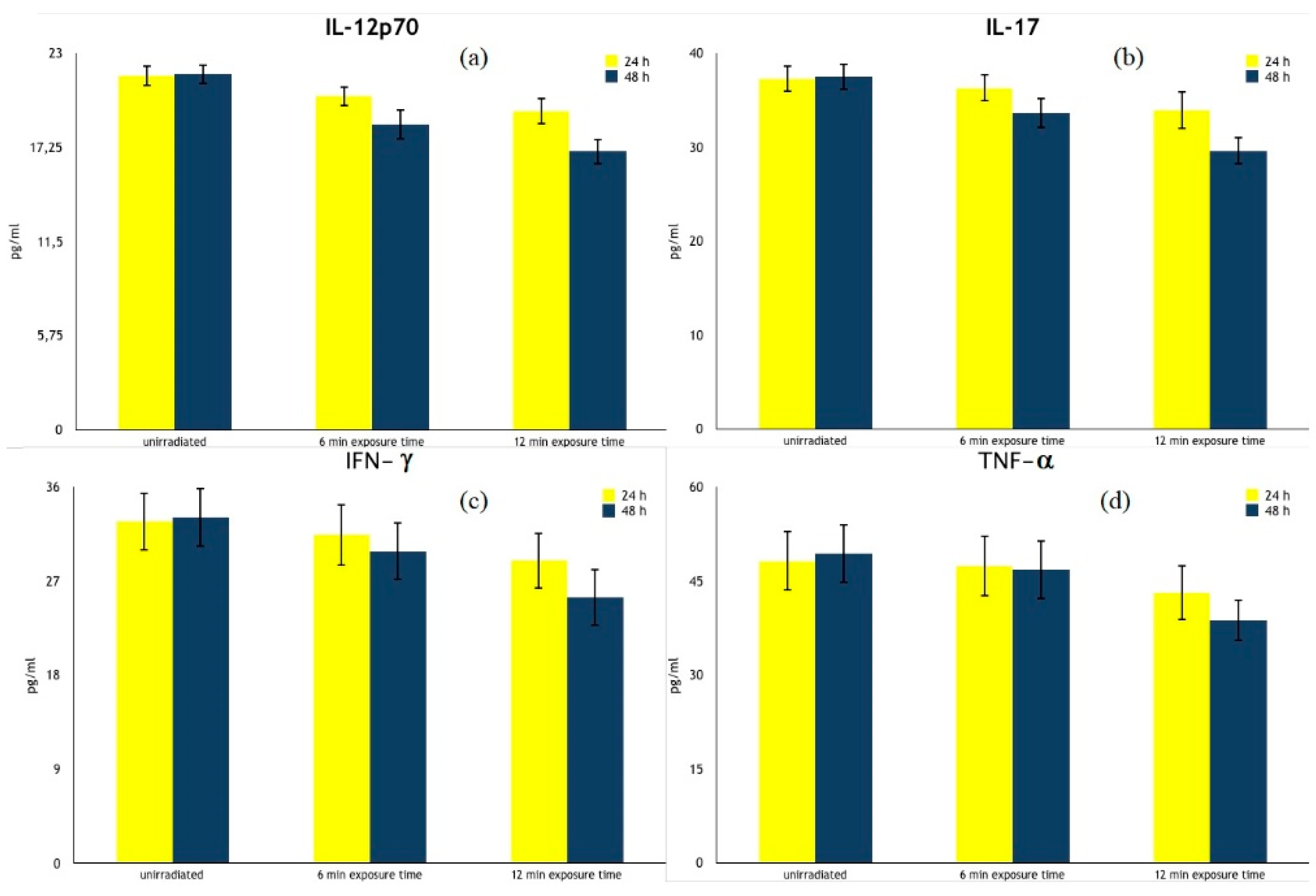
| IL-12p70 (pg/mL) | 24 h | 21.60 ± 0.66 | 20.36 ± 0.63 | 19.45 ± 0.80 2 |
| 48 h | 21.71 ± 0.60 | 18.65 ± 0.94 2 | 17.00 ± 0.78 2 | |
| IL-17 (pg/mL) | 24 h | 37.26 ± 1.45 | 36.27 ± 1.47 | 33.94 ± 2.03 2 |
| 48 h | 37.50 ± 1.42 | 33.59 ± 1.622 | 29.60 ± 1.45 2 | |
| IFN-γ (pg/mL) | 24 h | 32.70 ± 2.80 | 31.43 ± 2.99 | 28.97 ± 2.70 1 |
| 48 h | 33.11 ± 2.81 | 29.85 ± 2.80 1 | 25.46 ± 2.72 2,3 | |
| TNF-α (pg/mL) | 24 h | 48.21 ± 4.78 | 47.38 ± 4.83 | 43.10 ± 4.38 1 |
| 48 h | 49.29 ± 4.72 | 46.73 ± 4.69 | 38.72 ± 3.32 2,4 |
| Cytokines | Time of Culture | Unirradiated PBMCs | 6 min Exposure Time | 12 min Exposure Time |
|---|---|---|---|---|
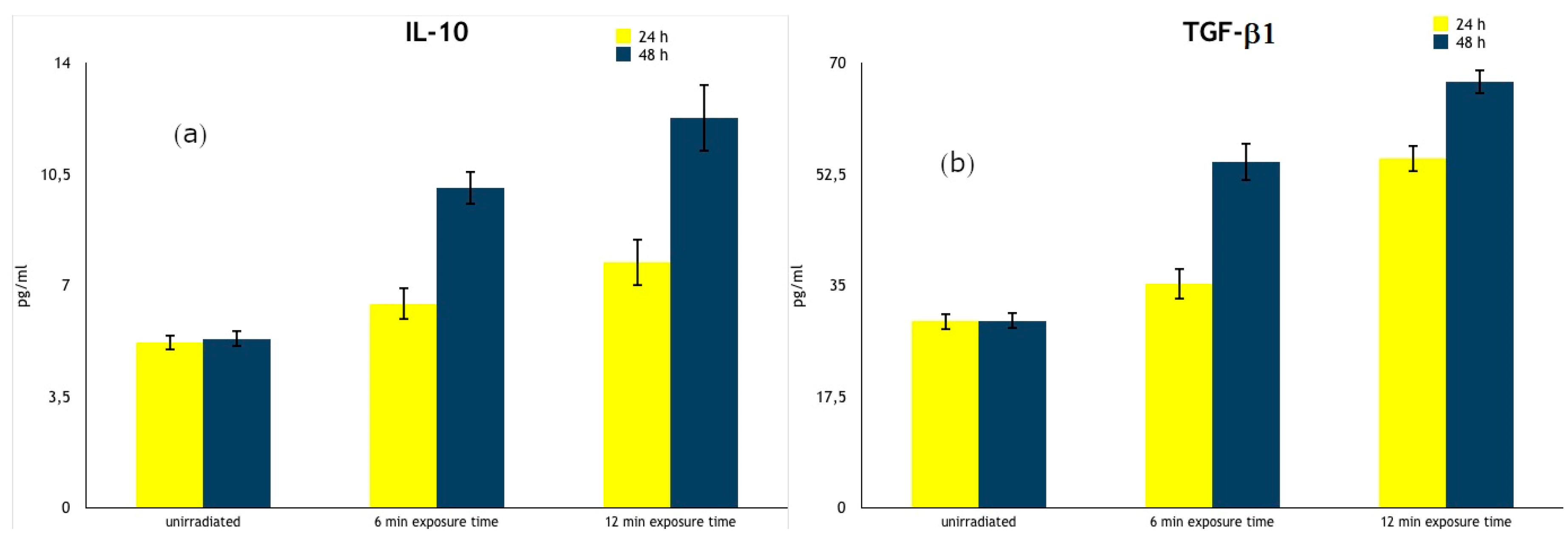
| IL-10 (pg/mL) | 24 h | 5.20 ± 0.25 | 6.42 ± 0.52 1 | 7.72 ± 0.73 2 |
| 48 h | 5.32 ± 0.27 | 10.06 ± 0.52 2 | 12.26 ± 1.07 2 | |
| TGF-β1 (pg/mL) | 24 h | 29.33 ± 1.35 | 35.27 ± 2.46 | 54.93 ± 2.19 2,3 |
| 48 h | 29.41 ± 1.37 | 54.37 ± 3.02 2 | 66.97 ± 1.93 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmeri, F.M.; Denaro, L.; Ruello, E.; Acri, G.; Gurgone, S.; Sansotta, C.; Testagrossa, B. Irradiation with Polychromatic Incoherent Low Energy Radiation of Human Peripheral Blood Mononuclear Cells In Vitro: Effects on Cytokine Production. Int. J. Environ. Res. Public Health 2020, 17, 1233. https://doi.org/10.3390/ijerph17041233
Salmeri FM, Denaro L, Ruello E, Acri G, Gurgone S, Sansotta C, Testagrossa B. Irradiation with Polychromatic Incoherent Low Energy Radiation of Human Peripheral Blood Mononuclear Cells In Vitro: Effects on Cytokine Production. International Journal of Environmental Research and Public Health. 2020; 17(4):1233. https://doi.org/10.3390/ijerph17041233
Chicago/Turabian StyleSalmeri, Francesca Maria, Lucia Denaro, Elisa Ruello, Giuseppe Acri, Sergio Gurgone, Carlo Sansotta, and Barbara Testagrossa. 2020. "Irradiation with Polychromatic Incoherent Low Energy Radiation of Human Peripheral Blood Mononuclear Cells In Vitro: Effects on Cytokine Production" International Journal of Environmental Research and Public Health 17, no. 4: 1233. https://doi.org/10.3390/ijerph17041233
APA StyleSalmeri, F. M., Denaro, L., Ruello, E., Acri, G., Gurgone, S., Sansotta, C., & Testagrossa, B. (2020). Irradiation with Polychromatic Incoherent Low Energy Radiation of Human Peripheral Blood Mononuclear Cells In Vitro: Effects on Cytokine Production. International Journal of Environmental Research and Public Health, 17(4), 1233. https://doi.org/10.3390/ijerph17041233





