A Meta-Regression Analysis of Utility Weights for Breast Cancer: The Power of Patients’ Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Extraction
2.3. Statistical Analysis
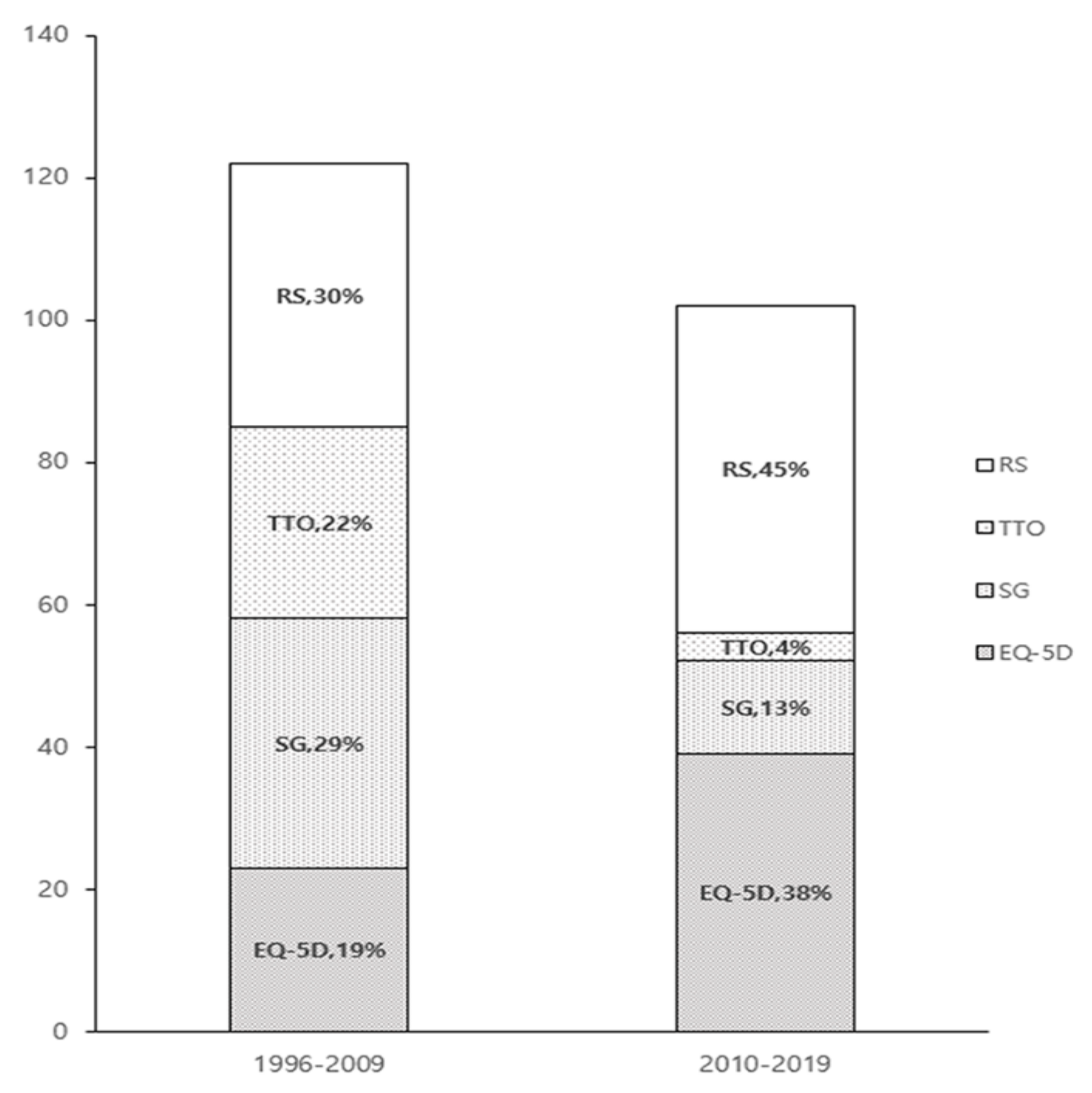
3. Results
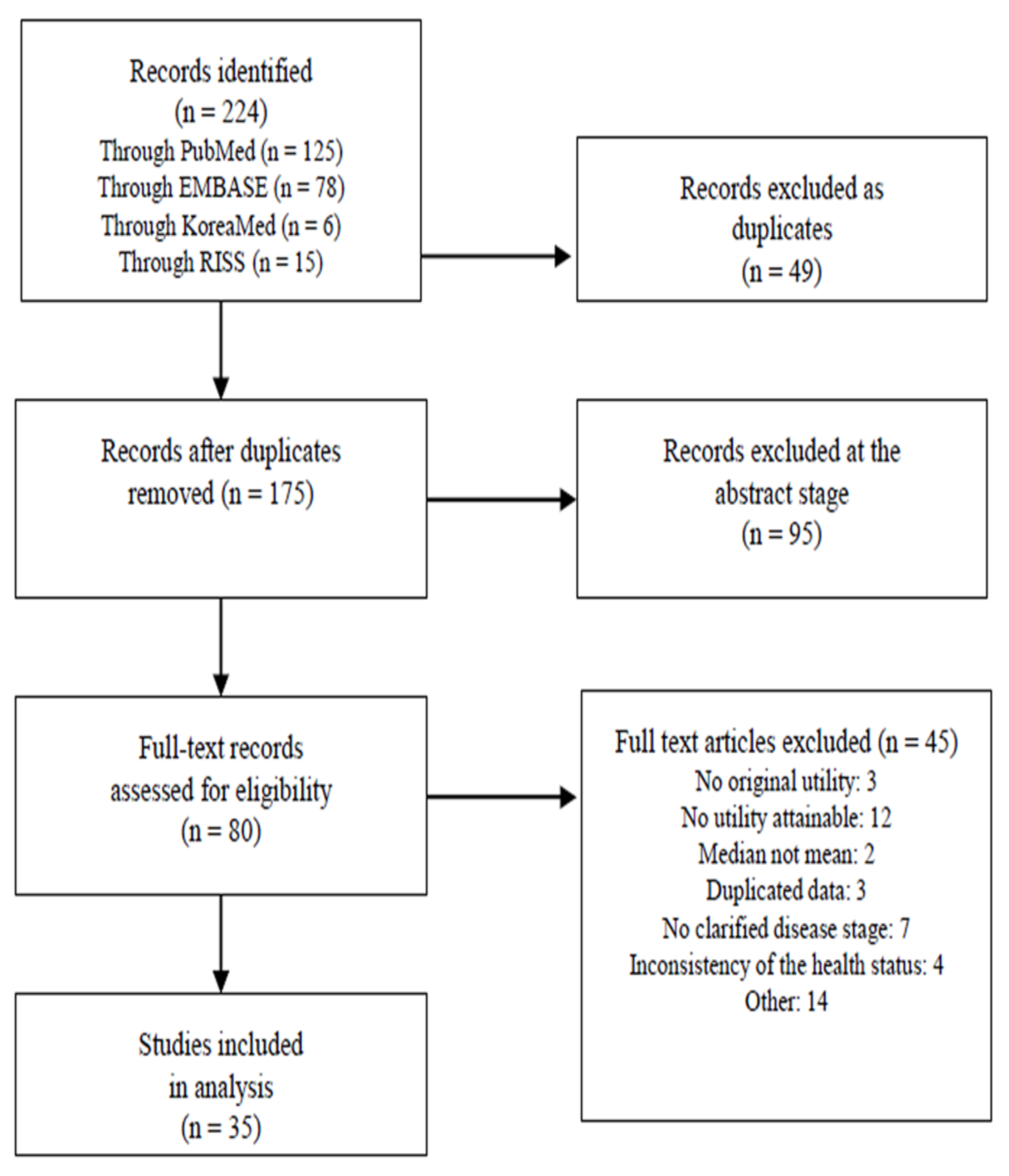
3.1. Literature Review
3.2. Study Characteristics
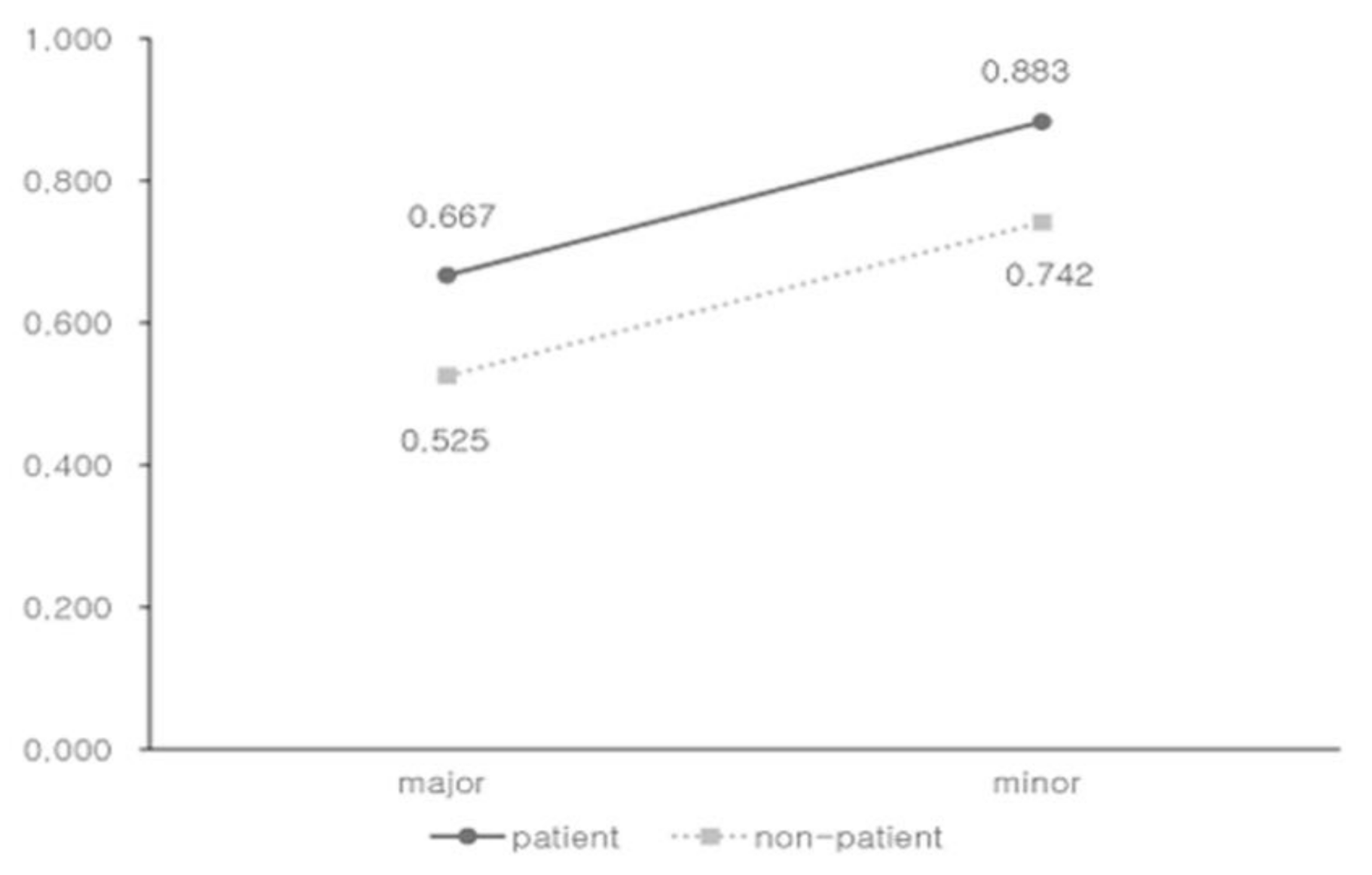
3.3. Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B. Quality Evaluation of the Included Studies
| Reference | Sample Size | Respondent Selection and Recruitment | Inclusion/ Exclusion Criteria | Response Rates to the Instrument Used | Loss to Follow-Up | Missing Data | Any Other Problems with the Study |
| Grann (1998) | 54 | not reported | Yes | not repoted | No follow-up | not repoted | small samples for generalization |
| Hürny (1998) | 83 | Yes | Yes | 100% | No follow-up | not reported | population (patient) from various countries and cultures |
| Jansen (1998) | 70 | Yes | Yes | 36% | No follow-up | 7% | small samples for generalization |
| Grann (1999) | 135 | Yes | Yes | not reported | No follow-up | not reported | representative of the health states |
| Chie (2000) | 979 | Yes | Yes | not reported | No follow-up | not reported | low response rate |
| Jansen (2000) | 41 | Yes | Yes | 76% | 24% | not reported | small sample for generalization |
| Jansen (2000) | 70 | Yes | Yes | 64% | 22% | not reported | small sample for generalization |
| Polsky (2002) | 784 | Yes | Yes | 68% | not reported | 5% | selection bias for generalization |
| Jansen (2004) | 448 | Yes | Yes | 62% | No follow-up | 10% | selection bias for generalization |
| Conner-Spady (2005) | 52 | Yes | Yes | 92% | 14% | not reported | small sample for generalization |
| Lloyd (2006) | 106 | Yes | not reported | not reported | No follow-up | 6% | health state validation unclear |
| Milne (2006) | 50 | Yes | Yes | not reported | No follow-up | No | small sample for generalization |
| Schleinitz (2006) | 156 | Yes | Yes | 78% | No follow-up | not reported | language difference |
| Lidgren (2007) | 345 | Yes | Yes | 96% | No follow-up | 6% | selection bias |
| Mansel (2007) | not reported | not reported | Yes | not reported | not reported | not reported | lack of information |
| Buyukdamgaci-Alogan (2008) | 30 | Yes | Yes | not reported | No follow-up | not reported | small sample for generalization |
| Kimman (2009) | 192 | Yes | Yes | 87% | no follow-up | not reported | small subgroup analysis for generalization |
| Freedman (2010) | 1050 | Yes | Yes | not reported | 54% | not reported | representative of the health states |
| Haines (2010) | 89 | Yes | Yes | 76% | 18% | not reported | low response and follow-up rate |
| Kimman (2011) | 299 | Yes | Yes | not reported | 12% | 1% | selection bias |
| Cheng (2012) | 152 | Yes | Yes | not reported | No follow-up | not reported | large amount of missing data |
| Kim (2012) | 199 | Yes | Yes | 100% | no follow-up | no exist | selection bias for generalization |
| Shih (2012) | 20 | Yes | Yes | 61% | No follow-up | no exist | small sample for generalization |
| Frederix (2013) | 200 | Yes | Yes | not reported | No follow-up | No exist | representative of the health states |
| Moro-Valdezate (2013) | 364 | Yes | Yes | 66% | 34% | Not reported | lack of baseline HRQOL measurements prior to treatment |
| Farkkila (2014) | 27 | Yes | Yes | not reported | No follow-up | not reported | small sample for generalization |
| Matter-Walstra (2014) | 92 | Yes | Yes | not reported | No follow-up | No exist | language difference |
| Tan (2014) | 64 | Yes | Yes | 68% | No follow-up | not reported | small sample |
| Kim (2015) | 827 | Yes | Yes | 83% | No follow-up | 21% | generalizability (one hospitals selected) |
| Kim (2015) | 299 | Yes | Yes | 90% | No follow-up | not reported | representative of the health states |
| Luo (2015) | 269 | Yes | Yes | 94% | 6% | 1% | Selection bias |
| Pickard (2016) | 50 | Yes | Yes | not reported | No follow-up | No exist | small sample |
| Kim (2017) | 509 | Yes | Yes | not reported | No follow-up | not reported | selection bias |
| Rautalin (2018) | 840 | Yes | Yes | 59% | No follow-up | No exist | low response rate |
| Li (2019) | 608 | Yes | Yes | not reported | No follow-up | 2% | selection bias |
References
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. A Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Global Health Data Exchange. Retrieved from GBD Compare 2019. Available online: http://vizhub.healthdata.org/gbd-compare/ (accessed on 30 November 2019).
- Kang, S.Y.; Kim, Y.S.; Kim, Z.; Kim, H.Y.; Kim, H.J.; Park, S.; Bae, S.Y.; Yoon, K.H.; Lee, S.B.; Lee, S.K.; et al. Breast cancer statistics in Korea in 2017: Data from a breast cancer registry. J. Breast Cancer 2020, 23, 115–128. [Google Scholar] [CrossRef]
- Pleis, J.R.; Ward, W.B.; Lucas, J.W. Summary health statistics for U.S. adults: National health interview survey, 2009. Vital Health Stat. 2010, 10, 1–207. [Google Scholar]
- Korean Breast Cancer Society. Breast Cancer in Korea, in Korean Breast Cancer Sociery; Seoul, Korea, 2014. Available online: http://www.kbcs.or.kr/sub02/sub04.html/ (accessed on 30 November 2019).
- Korean Ministry of Health Welfare & Family Affairs. Annual Report of Cancer Incidence, Cancer Prevalence and Survival in Korea; Korean Ministry of Health Welfare & Family Affairs: Sejong, Korea, 2011.
- American Cancer Society. Breast Cancer Survival Rates; American Cancer Society: Bedford, NH, USA, 2018. [Google Scholar]
- Hopwood, P. The impact of age and clinical factors on quality of life in early breast cancer: An analysis of 2208 women recruited to the UK START Trial (Standardisation of Breast Radiotherapy Trial). Breast 2007, 16, 241–251. [Google Scholar] [CrossRef]
- Byar, K.L.; Berger, A.M.; Bakken, S.L.; Cetak, M.A. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol. Nurs. Forum 2006, 33, E18–E26. [Google Scholar] [CrossRef]
- Brown, D.S.; Trogdon, J.G.; Ekwueme, D.U.; Chamiec-Case, L.; Guy Jr, G.P.; Tangka, F.K.; Li, C.; Trivers, K.F.; Rodriguez, J.L. Health state utility impact of breast cancer in U.S. women aged 18–44 years. Am. J. Prev. Med. 2016, 50, 255–261. [Google Scholar] [CrossRef]
- Weinstein, M.C.; Torrance, G.; McGuire, A. QALYs: The basics. Value Health 2009, 12, S5–S9. [Google Scholar] [CrossRef]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programme, 3nd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Light, D.W.; Kantarjian, K. Market spiral pricing of cancer drugs. Cancer 2013, 119, 3900–3902. [Google Scholar] [CrossRef]
- Frederix, G.W.; Quadri, N.; Hovels, A.M.; van de Wetering, F.T.; Tamminga, H.; Schellens, J.H.; Lloyd, A.J. Utility and work productivity data for economic evaluation of breast cancer therapies in the Netherlands and Sweden. Clin. Ther. 2013, 35, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Tengs, T.O.; Lin, T.H. A meta-analysis of utility estimates for HIV/AIDS. Med. Decis. Mak. 2002, 22, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Sturza, J. A review and meta-analysis of utility values for lung cancer. Med. Decis. Mak. 2010, 30, 685–693. [Google Scholar] [CrossRef]
- Djalalov, S.; Rabeneck, L.; Tomlinson, G.; Bremner, K.E.; Hilsden, R.; Hoch, J.S. A review and meta-analysis of colorectal cancer utilities. Med. Decis. Mak. 2014, 3, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Bae, E.Y.; Lim, S.H. Sourcing quality-of-life weights obtained from previous studies: Theory and reality in Korea. Cent. Outcomes Res. 2014, 7, 141–150. [Google Scholar] [CrossRef]
- Boyd, N.F.; Sutherland, H.J.; Heasman, K.Z.; Tritchler, D.L.; Cummings, B.J. Whose Utilities for Decision Analysis? Med Decis. Mak. 1990, 10, 58–67. [Google Scholar] [CrossRef]
- Nord, E.; Daniels, N.; Kamlet, M. QALYs: Some challenges. Value Health 2009, 12, S10–S15. [Google Scholar] [CrossRef]
- King, J.T.; Styn, M.A.; Tsevat, J.; Roberts, M.S. “Perfect health” versus “disease free”: The impact of anchor point choice on the measurement of preferences and the calculation of disease-specific disutilities. Med. Decis. Mak. 2003, 23, 212–225. [Google Scholar] [CrossRef]
- Eunethta. European Network for Health Technology Assessment (EUnetHTA), Methods for Health Economic Evaluations-A Guideline Based on Current Practices in Europe; Eunethta: Diemen, The Netherlands, 2015. [Google Scholar]
- Drummond, M. Transferability of economic evaluations across jurisdictions: ISPOR good research practices task force report. Value Health 2009, 12, 409–418. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Controlling the risk of spurious findings from meta-regression. Stat. Med. 2004, 23, 1663–1682. [Google Scholar] [CrossRef]
- Peasgood, T.; Brazier, J. Is meta-analysis for utility values appropriate given the potential impact different elicitation methods have on values? Pharmacoeconomics 2015, 33, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Jeffe, D.B.; Pérez, M.; Cole, E.F.; Liu, Y.; Schootman, M. The effects of surgery type and chemotherapy on early-stage breast cancer patients’ quality of life over 2-year follow-up. Ann. Surg. Oncol. 2016, 23, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.A.; Pinheiro, L.C.; Reeder-Hayes, K.E.; Walker, J.S.; Corbie-Smith, G.; Fashaw, S.A.; Woods-Giscombe, C.; Wheeler, S.B. To be young, black, and living with breast cancer: A systematic review of health-related quality of life in young black breast cancer survivors. Breast Cancer Res. Treat. 2016, 160, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Van der Kloot, W.A.; Ucchida, Y.; Inoue, K.; Kobayashi, K.; Yamaoka, K.; Nortier, H.W.; Kaptein, A.A. The effects of illness beliefs and chemotherapy impact on quality of life in Japanese and Dutch patients with breast or lung cancer. Chin. Clin. Oncol. 2016, 5, 3. [Google Scholar] [PubMed]
- Freedman, G.M.; Li, T.; Anderson, P.R.; Nicolaou, N.; Konski, A. Health states of women after conservative surgery and radiation for breast cancer. Breast Cancer Res. Treat. 2010, 121, 519–526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cappelli, M.; Surh, L.; Humphreys, L.; Verma, S.; Logan, D.; Hunter, A.; Allanson, J. Measuring women’s preferences for breast cancer treatments and BRCA1/BRCA2 testing. Qual. Life Res. 2001, 10, 595–607. [Google Scholar] [CrossRef]
- Roine, E.; Blomqvist, C.; Kellokumpu-Lehtinen, P.L.; Sintonen, H.; Saarto, T. Health-related quality of life in breast cancer patients after adjuvant treatments. Breast J. 2016, 22, 473–475. [Google Scholar] [CrossRef]
- Howard-Anderson, J.; Ganz, P.A.; Bower, J.E.; Stanton, A.L. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 386–405. [Google Scholar] [CrossRef]
- Avis, N.E.; Crawford, S.; Manuel, J. Quality of life among younger women with breast cancer. J. Clin. Oncol. 2005, 23, 3322–3330. [Google Scholar] [CrossRef]
- Trogdon, J.G.; Ekwueme, D.U.; Chamiec-Case, L.; Guy, G.P. Breast cancer in young women: Health State Utility Impacts by Race/Ethnicity. Am. J. Prev. Med. 2016, 50, 262–269. [Google Scholar] [CrossRef][Green Version]
- Schleinitz, M.D.; DePalo, D.; Blume, J.; Stein, M. Can differences in breast cancer utilities explain disparities in breast cancer care? J. Gen. Intern. Med. 2006, 21, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H. Cancer Facts and Figures 215 of Korea, 1st ed.; Minister for Health and Welfare President of National Cancer Center: Sejong, Korea, 2015.
- Coons, S.J.; Rao, S.; Keininger, D.L.; Hays, R.D. A comparative review of generic quality-of-life instruments. Pharmacoeconomics 2000, 17, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Peasgood, T.; Ward, S.E.; Brazier, J. Health-state utility values in breast cancer. Expert Rev. Pharm. Outcomes Res. 2010, 10, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Tengs, T.O.; Lin, T.H. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics 2003, 21, 191–200. [Google Scholar] [CrossRef]
- Brazier, J.E.; Yang, Y.; Tsuchiya, A.; Rowen, D.L. A review of studies mapping (or cross walking) non-preference based measures of health to generic preference-based measures. Eur. J. Health Econ. 2010, 11, 215–225. [Google Scholar] [CrossRef]
- Longworth, L.; Rowen, D. Mapping to obtain EQ-5D utility values for use in NICE health technology assessments. Value Health 2013, 16, 202–210. [Google Scholar] [CrossRef]
- Gradishar, W.; Salerno, K.E. NCCN Guidelines for patients, breast cancer: Early stage. J. Natl. Compr. Cancer Netw. 2016, 14, 641–644. [Google Scholar] [CrossRef]
- Canadian Cancer Society, Stages of Breast Cancer. Available online: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2018-EN.pdf?la=en/ (accessed on 30 November 2019).
- Jansen, S.J.T.; Kievit, J.; Nooij, M.A.; Stiggelbout, A.M. Stability of patients’ preferences for chemotherapy: The impact of experience. Med. Decis. Mak. 2001, 21, 295–306. [Google Scholar] [CrossRef]
- Stiggelbout, A.M.; de Vogel-Voogt, E. Health state utilities: A framework for studying the gap between the imagined and the real. Value Health 2008, 11, 76–87. [Google Scholar] [CrossRef]
- Wenzel, L.B.; Fairclough, D.L.; Brady, M.J.; Cella, D.; Garrett, K.M.; Kluhsman, B.C.; Crane, L.A.; Marcus, A.C. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer 1999, 86, 1768–1774. [Google Scholar] [CrossRef]
- NHS Foundation Trust. The menopause; NHS Foundation Trust: London, UK, 2014. [Google Scholar]
- Raudenbush, S.W.; Bryk, A.S. Hierarchical Linear Models: Applications and Data Aanalysis Methods., 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2002. [Google Scholar]
- Frost, C.; Clarke, R.; Beacon, H. Use of hierarchical models for meta-analysis: Experience in the metabolic ward studies of diet and blood cholesterol. Stat. Med. 1999, 18, 1657–1676. [Google Scholar] [CrossRef]
- Teckle, P.; Peacock, S.; McTaggart-Cowan, H.; Van der Hoek, K.; Chia, S.; Melosky, B.; Gelmon, K. The ability of cancer-specific and generic preference-based instruments to discriminate across clinical and self-reported measures of cancer severities. Health Qual Life Outcomes 2011, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Wengstrom, Y.; Eriksen, C.; Sandelin, K. Breast surgeons performing immediate breast reconstruction with implants-assessment of resource-use and patient-reported outcome measures. Breast 2012, 21, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kim, S.Y.; Kim, Y.; Oh, J.; Kim, H.J.; Jo, D.Y.; Kwon, T.G.; Park, J.H. Comparison of health-related quality of life between cancer survivors treated in designated cancer centers and the general public in Korea. Jpn. J. Clin. Oncol. 2014, 44, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Stratmann-Schoene, D.; Kuehn, T.; Kreienberg, R.; Leidl, R. A preference-based index for the SF-12. Health Econ. 2006, 15, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Hann, D.M.; Jacobsen, P.B.; Martin, S.C.; Kronish, L.E.; Azzarello, L.M.; Fields, K.K. Quality of life following bone marrow transplantation for breast cancer: A comparative study. Bone Marrow Transpl. 1997, 19, 257–264. [Google Scholar] [CrossRef]
- Kimman, M.; Jan, S.; Monaghan, H.; Woodward, M. The relationship between economic characteristics and health-related quality of life in newly diagnosed cancer patients in Southeast Asia: Results from an observational study. Qual. Life Res. 2015, 24, 937–949. [Google Scholar] [CrossRef]
- Ashby, J.; O’Hanlon, M.; Buxton, M.J. The time trade-off technique: How do the valuations of breast cancer patients compare to those of other groups? Qual Life Res. 1994, 3, 257–265. [Google Scholar] [CrossRef]
- Grann, V.R.; Sundararajan, V.; Jacobson, J.S.; Whang, W.; Heitjan, D.F.; Antman, K.H.; Neugut, A.I. Decision analysis of tamoxifen for the prevention of invasive breast cancer. Cancer J. 2000, 6, 169–178. [Google Scholar]
- Shih, V.; Chan, A.; Xie, F.; Ko, Y. Economic evaluation of anastrozole versus tamoxifen for early stage breast cancer in singapore. Value Health Reg. Issues 2012, 1, 46–53. [Google Scholar] [CrossRef]
- Hutton, J.; Brown, T.; Borowitz, M.; Abrams, K.; Rothman, M.; Shakespeare, A. A new decision model for cost-utility comparisons of chemotherapy in recurrent metastatic breast cancer. Pharmacoeconomics 1996, 9, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Conner-Spady, B.; Cumming, C.; Nabholtz, J.M.; Jacobs, P.; Stewart, D. Responsiveness of the EuroQol in breast cancer patients undergoing high dose chemotherapy. Qual. Life Res. 2001, 10, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Hsu, S.H.; Gross, C.P.; Sanft, T.; Davidoff, A.J.; Ma, X.; Yu, J.B. Association between time since cancer diagnosis and health-related quality of life: A population-level analysis. Value Health 2016, 19, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Reeve, B.B.; Smith, A.W.; Clauser, S.B. Associations of cancer and other chronic medical conditions with SF-6D preference-based scores in Medicare beneficiaries. Qual. Life Res. 2014, 23, 385–391. [Google Scholar] [CrossRef][Green Version]
- Lee, H.-J.; Lee, T.-J.; Yang, B.-M.; Min, J. Cost-effectiveness analysis of adjuvant hormonal treatments for women with postmenopausal hormone-receptor positive early breast cancer in the Korean context. J. Breast Cancer 2010, 13, 286–298. [Google Scholar] [CrossRef]
- Stalmeier, P.F. Discrepancies between chained and classic utilities induced by anchoring with occasional adjustments. Med. Decis. Making 2002, 22, 53–64. [Google Scholar] [CrossRef]
- Dranitsaris, G.; Cottrell, W.; Spirovski, B.; Hopkins, S. Economic analysis of albumin-bound paclitaxel for the treatment of metastatic breast cancer. J. Oncol. Pharm. Pract. 2009, 15, 67–78. [Google Scholar] [CrossRef]
- Perez, D.J.; Williams, S.M.; Christensen, E.A.; McGee, R.O.; Campbell, A.V. A longitudinal study of health related quality of life and utility measures in patients with advanced breast cancer. Qual Life Res 2001, 10, 587–593. [Google Scholar] [CrossRef]
- Dranitsaris, G.; Yu, B.; Qing, Z.; King, J.; Zhang, A.; Kaura, S. Nab-paclitaxel or docetaxel as alternatives to solvent-based paclitaxel in metastatic breast cancer (Mbc): A cost utility analysis from a Chinese health care perspective. Value Health 2014, 17, A642. [Google Scholar] [CrossRef][Green Version]
- Hershman, D.; Sundararajan, V.; Jacobson, J.S.; Heitjan, D.F.; Neugut, A.I.; Grann, V.R. Outcomes of tamoxifen chemoprevention for breast cancer in very high-risk women: A cost-effectiveness analysis. J. Clin. Oncol. 2002, 20, 9–16. [Google Scholar] [CrossRef]
- Franic, D.M.; Pathak, D.S.; Gafni, A. Quality-adjusted life years was a poor predictor of women’s willingness to pay in acute and chronic conditions: Results of a survey. J. Clin. Epidemiol. 2005, 58, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Takashima, T.; Mukai, H.; Hara, F.; Matsubara, N.; Saito, T.; Takano, T.; Park, Y.; Toyama, T.; Hozumi, Y.; Tsurutani, J.; et al. Taxanes versus S-1 as the first-line chemotherapy for metastatic breast cancer (SELECT BC): An open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2016, 17, 90–98. [Google Scholar] [CrossRef]
- Kim, J.G.; Kwon, L.S. Measurement of quality of life related to health by demographic characteristics of adult patients with cancer using EQ-5D index-focused on the Korea health & nutrition examination survey. J. Digit. Converg. 2013, 11, 281–291. [Google Scholar]
- Ha, E.H. The influence of health related quality of life on depressive symptoms of breast cancer patients. Korean J. Woman Psychol. 2011, 16, 499–515. [Google Scholar]
- Kim, M.S. Study on Health-Related Quality of Life and Needs of Breast Cancer Patients; Yonsei University Grduation School: Seoul, Korea, 2005. [Google Scholar]
- Lee, H.S. The Social Network Analysis for Research of the Cancer Patients’ Health-Related Quality of Life; Chungang University Graduate School: Seoul, Korea, 2014. [Google Scholar]
- Cho, S.M. Factors Affecting Symptom Clusters in Patients with Breast Cancer Receiving Chemotherapy; Yonsei University Graduate School: Seoul, Korea, 2013. [Google Scholar]
- Kim, S.I.; Lee, Y.; Son, Y.; Jun, S.Y.; Yun, S.; Bae, H.S.; Lim, M.C.; Jung, S.Y.; Joo, J.; Lee, E.S. Assessment of breast bancer patients’ knowledge and decisional conflict regarding tamoxifen use. J. Korean Med. Sci. 2015, 30, 1604–1611. [Google Scholar] [CrossRef]
- Tan, X.-Y.; Aung, M.-M.; Ngai, M.-I.; Xie, F.; Ko, Y. Assessment of preference for hormonal treatment–related health states among patients with breast cancer. Value Health Reg. Issues 2014, 3, 27–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.; Wang, M.; Liu, L.; Chen, G. Which approach is better in eliciting health state utilities from breast cancer patients? Evidence from mainland China. Eur. J. Cancer Care 2019, 28, e12965. [Google Scholar] [CrossRef]
- Mansel, R.; Locker, G.; Fallowfield, L.; Benedict, A.; Jones, D. Cost-effectiveness analysis of anastrozole vs tamoxifen in adjuvant therapy for early stage breast cancer in the United Kingdom: The 5-year completed treatment analysis of the ATAC (“Arimidex”, Tamoxifen alone or in combination) trial. Br. J. Cancer 2007, 97, 152–161. [Google Scholar] [CrossRef]
- Cheng, T.F.; Wang, J.D.; Uen, W.C. Cost-utility analysis of adjuvant goserelin (Zoladex) and adjuvant chemotherapy in premenopausal women with breast cancer. BMC Cancer 2012, 12, 33. [Google Scholar] [CrossRef]
- Grann, V.R.; Panageas, K.S.; Whang, W.; Antman, K.H.; Neugut, A.I. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J. Clin. Oncol. 1998, 16, 979–985. [Google Scholar] [CrossRef]
- Buyukdamgaci-Alogan, G.; Elele, T.; Hayran, M.; Erman, M.; Kilickap, S. A decision-analytic model for early stage breast cancer: Lumpectomy vs mastectomy. Neoplasma 2008, 55, 222–228. [Google Scholar] [PubMed]
- Shih, V.; Chan, A.; Xie, F.; Ko, Y.E. Health State Utility Assessment for Breast Cancer. Value Health Reg. Issues 2012, 1, 93–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kimman, M.L.; Dirksen, C.D.; Voogd, A.C.; Falger, P.; Gijsen, B.C.; Thuring, M.; Lenssen, A.; Van der Ent, F.; Verkeyn, J.; Haekens, C.; et al. Economic evaluation of four follow-up strategies after curative treatment for breast cancer: Results of an RCT. Eur. J. Cancer 2011, 47, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.J.; Stiggelbout, A.M.; Nooij, M.A.; Kievit, J. The effect of individually assessed preference weights on the relationship between holistic utilities and nonpreference-based assessment. Qual. Life Res. 2000, 9, 541–557. [Google Scholar] [CrossRef]
- Moro-Valdezate, D.; Peiro, S.; Buch-Villa, E.; Caballero-Garate, A.; Morales-Monsalve, M.D.; Martinez-Agullo, A.; Checa-Ayet, F.; Ortega-Serrano, J. Evolution of health-related quality of life in breast cancer patients during the first year of follow-up. J. Breast Cancer 2013, 16, 104–111. [Google Scholar] [CrossRef][Green Version]
- Lidgren, M.; Wilking, N.; Jonsson, B.; Rehnberg, C. Health related quality of life in different states of breast cancer. Qual. Life Res. 2007, 16, 1073–1081. [Google Scholar] [CrossRef]
- Lloyd, A.; Nafees, B.; Narewska, J.; Dewilde, S.; Watkins, J. Health state utilities for metastatic breast cancer. Br. J. Cancer 2006, 95, 683–690. [Google Scholar] [CrossRef]
- Farkkila, N.; Torvinen, S.; Roine, R.P.; Sintonen, H.; Hanninen, J.; Taari, K.; Saarto, T. Health-related quality of life among breast, prostate, and colorectal cancer patients with end-stage disease. Qual. Life Res. 2014, 23, 1387–1394. [Google Scholar] [CrossRef]
- Jansen, S.J.; Otten, W.; Van de Velde, C.J.; Nortier, J.W.; Stiggelbout, A.M. The impact of the perception of treatment choice on satisfaction with treatment, experienced chemotherapy burden and current quality of life. Br. J. Cancer 2004, 91, 56–61. [Google Scholar] [CrossRef][Green Version]
- Conner-Spady, B.L.; Cumming, C.; Nabholtz, J.M.; Jacobs, P.; Stewart, D. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transpl. 2005, 36, 251–259. [Google Scholar] [CrossRef][Green Version]
- Luo, N.; Cheung, Y.B.; Ng, R.; Lee, C.F. Mapping and direct valuation: Do they give equivalent EQ-5D-5L index scores? Health Qual. Life Outcomes 2015, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Ko, S.K.; Kang, H.Y. Mapping the cancer-specific EORTC QLQ-C30 and EORTC QLQ-BR23 to the generic EQ-5D in metastatic breast cancer patients. Qual. Life Res. 2012, 21, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Haines, T.P.; Sinnamon, P.; Wetzig, N.G.; Lehman, M.; Walpole, E.; Pratt, T.; Smith., A. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Res. Treat. 2010, 124, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Polsky, D.; Keating, N.L.; Weeks, J.C.; Schulman, K.A. Patient choice of breast cancer treatment: Impact on health state preferences. Med. Care 2002, 40, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.J.; Stiggelbout, A.M.; Wakker, P.P.; Vliet Vlieland, T.P.; Leer, J.W.; Nooy, M.A.; Kievit, J. Patients’ utilities for cancer treatments: A study of the chained procedure for the standard gamble and time tradeoff. Med. Decis. Mak. 1998, 18, 391–399. [Google Scholar] [CrossRef]
- Grann, V.R.; Jacobson, J.S.; Sundararajan, V.; Albert, S.M.; Troxel, A.B.; Neugut, A.I. The quality of life associated with prophylactic treatments for women with BRCA1/2 mutations. Cancer J. Sci. Am. 1999, 5, 283–292. [Google Scholar]
- Kimman, M.L.; Dirksen, C.D.; Lambin, P.; Boersma, L.J. Responsiveness of the EQ-5D in breast cancer patients in their first year after treatment. Health Qual. Life Outcomes 2009, 7, 11. [Google Scholar] [CrossRef]
- Hurny, C.; Van Wegberg, B.; Bacchi, M.; Bernhard, J.; Thurlimann, B.; Real, O.; Perey, L.; Bonnefoi, H.; Coates, A. Subjective health estimations (SHE) in patients with advanced breast cancer: An adapted utility concept for clinical trials. Br. J. Cancer 1998, 77, 985–991. [Google Scholar] [CrossRef][Green Version]
- Jansen, S.J.; Stiggelbout, A.M.; Wakker, P.P.; Nooij, M.A.; Noordijk, E.M.; Kievit, J. Unstable preferences: A shift in valuation or an effect of the elicitation procedure? Med. Decis. Making 2000, 20, 62–71. [Google Scholar] [CrossRef]
- Pickard, A.S.; Jiang, R.; Lin, H.W.; Rosenbloom, S.; Cella, D. Using patient-reported outcomes to compare relative burden of cancer: EQ-5D and functional assessment of cancer therapy-general in eleven types of cancer. Clin. Ther. 2016, 38, 769–777. [Google Scholar] [CrossRef]
- Matter-Walstra, K.; Klingbiel, D.; Szucs, T.; Pestalozzi, B.C.; Schwenkglenks, M. Using the EuroQol EQ-5D in swiss cancer patients, which value set should be applied? Pharmacoeconomics 2014, 32, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Chie, W.C.; Huang, C.S.; Chen, J.H.; Chang, K.J. Utility assessment for different clinical phases of breast cancer in Taiwan. J. Formos. Med. Assoc. 2000, 99, 677–683. [Google Scholar] [PubMed]
- Kim, S.H.; Jo, M.W.; Lee, J.W.; Lee, H.J.; Kim, J.K. Validity and reliability of EQ-5D-3L for breast cancer patients in Korea. Health Qual. Life Outcomes 2015, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.J.; Heaton-Brown, K.H.; Hansen, P.; Thomas, D.; Harvey, V.; Cubitt, A. Quality-of-life valuations of advanced breast cancer by New Zealand women. Pharmacoeconomics 2006, 24, 281–292. [Google Scholar] [CrossRef]
- Rautalin, M.; Färkkilä, N.; Sintonen, H.; Saarto, T.; Taari, K.; Jahkola, T.; Roine, R.P. Health-related quality of life in different states of breast cancer–comparing different instruments. Acta Oncologica 2018, 57, 622–628. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jo, M.-W.; Ock, M.; Lee, H.-J.; Lee, J.-W. Estimation of health state utilities in breast cancer. Patient Prefer. Adherence 2017, 11, 531. [Google Scholar] [CrossRef][Green Version]
- Papaioannou, D.; Brazier, J.; Paisley, S. Systematic searching and selection of health state utility values from the literature. Value Health 2013, 16, 686–695. [Google Scholar] [CrossRef]
- Bleichrodt, H. Characterizing QALYs risk neutrality. J. Risk Uncertain 1997, 15, 107–114. [Google Scholar] [CrossRef]
- Attema, A.E.; Brouwer, W.B.F. On the (not so) constant proportional trade-off in TTO. Qual. Life Res. 2010, 19, 489–497. [Google Scholar] [CrossRef]
- Tosh, J.C.; Longworth, L.J.; George, E. Utility values in national institute for health and clinical excellence (NICE) technology appraisals. Value Health 2011, 14, 102–109. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence. Guide to the Methods of Technology Appraisal. Available online: https://heatinformatics.com/sites/default/files/images-videosFileContent/UK%20NHS_NICE%20HTA%202008.pdf/ (accessed on 30 November 2019).
- Pharmaceutical Benefits Advisory Committee. Guidelines for Preparing Submissions to the Pharmaceutical Benefits Advisory Committee (Version 4.3); Australian Government Department of Health: Canberra, Australia, 2008.
- Bae, S.; Lee, S.; Bae, E.Y.; Jang, S. Korean guidelines for pharmacoeconomic evaluation (second and updated version). Pharmacoeconomics 2013, 31, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Brazier, J.; Deverill, M. A checklist for judging preference-based measures of health related quality of life: Learning from psychometrics. Health Econ. 1999, 8, 41–51. [Google Scholar] [CrossRef]
| Reference | Location | Disease Stage | Assessment Method | Respondent Type | Lower and Upper Bound of the Scale | Age |
|---|---|---|---|---|---|---|
| Grann (1998) | USA | Early-stage/ Late-stage | TTO | Non-patient | Other | Premenopausal |
| Hürny (1998) | Switzerland | Late-stage | TTO | Patient | Death to Perfect health | Not-stated |
| Jansen (1998) | Netherlands | Early-stage | SG/TTO | Patient | Other | Not-stated |
| Grann (1999) | USA | Not-stated | TTO/RS | Patient/ Non-patient | Other | Not-stated/ Premenopausal |
| Chie (2000) | Taiwan | Early-stage/ Late-stage | RS | Non-patient | Death to Perfect health | Not-stated |
| Jansen (2000) | Netherlands | Early-stage | SG/TTO/RS | Patient | Death to Perfect health or Other | Premenopausal |
| Jansen (2000) | Netherlands | Early-stage | SG/TTO/RS | Patient | Death to Perfect health or Other | Not-stated |
| Polsky (2002) | USA | Early-stage | RS | Patient | Other | Postmenopausal |
| Jansen (2004) | Netherlands | Early-stage | EQ-5D/RS | Patient | Death to Perfect health or Other | Not-stated |
| Conner-Spady (2005) | Canada | Late-stage | EQ-5D/RS | Patient | Death to Perfect health or Other | Not-stated |
| Lloyd (2006) | UK | Late-stage | SG | Non-patient | Other | Not-stated |
| Milne (2006) | New Zealand | Late-stage | EQ-5D/TTO/RS | Non-patient | Other | Not-stated |
| Schleinitz (2006) | USA | Early-stage/ Late-stage | SG | Non-patient | Death to Perfect health | Not-stated |
| Lidgren (2007) | Sweden | Early-stage/ Late-stage | EQ-5D/TTO | Patient | Other | Not-stated |
| Mansel (2007) | UK | Early-stage | SG | Non-patient | Death to Perfect health | Postmenopausal |
| Buyukdamgaci-Alogan (2008) | Tukey | Early-stage | SG | Non-patient | Other | Premenopausal |
| Kimman (2009) | Netherlands | Not-stated | EQ-5D/RS | Patient | Other | Not-stated |
| Freedman (2010) | USA | Early-stage | EQ-5D/RS | Patient | Other | Not-stated/Premenopausal/ Postmenopausal |
| Haines (2010) | Australia | Not-stated | EQ-5D/RS | Patient | Other | Not-stated |
| Kimman (2011) | Netherland | Not-stated | EQ-5D | Patient | Other | Not-stated |
| Cheng (2012) | Taiwan | Early-stage | SG | Patient | Other | Not-stated |
| Kim (2012) | Korea | Late-stage | EQ-5D | Patient | Death to Perfect health | Not-stated |
| Shih (2012) | Singapore | Not-stated | RS | Non-patient | Other | Not-stated |
| Frederix (2013) | Netherland | Early-stage/ Late-stage | TTO/RS | Patient | Death to Perfect health | Postmenopausal |
| Moro-Valdezate (2013) | Spain | Not-stated | EQ-5D | Patient | Other | Not-stated |
| Farkkila (2014) | Finland | Late-stage | EQ-5D/RS | Patient | Other | Not-stated |
| Matter-Walstra (2014) | Switzerland | Not-stated | EQ-5D/RS | Patient | Other | Not-stated/Premenopausal/ Postmenopausal |
| Tan (2014) | Singapore | Early-stage/ Late-stage | SG/RS | Patient | Death to Perfect health or Other | Not-stated |
| Kim (2015) | Korea | Not-stated/ Early-stage/ Late-stage | EQ-5D/RS | Patient | Other | Not-stated/Premenopausal/ Postmenopausal |
| Kim (2015) | Korea | Not-stated | RS | Patient | Other | Not-stated |
| Luo (2015) | Singapore | Not-stated | EQ-5D | Patient | Other | Not-stated |
| Pickard (2016) | USA | Late-stage | EQ-5D/RS | Patient | Other | Not-stated |
| Kim (2017) | Korea | Early-stage/ Late-stage | SG/RS | Non-Patient | Other | Not-stated |
| Rautalin (2018) | Finland | Late-stage | EQ-5D/RS | Patient | Other | Not-stated |
| Li (2019) | China | Not-stated | EQ-5D/TTO | Patient | Other | Premenopausal/ Postmenopausal |
| Variable | Number of Utilities (%) | Number of Studies | References | |
|---|---|---|---|---|
| Disease stage | Not-stated | 67 (29.9) | 11 | [78,80,85,86,88,94,96,99,100,104,106] |
| Late-stage | 70 (31.3) | 16 | [16,37,79,83,89,90,91,93,95,101,103,105,106,107,108,109] | |
| Early-stage | 87 (38.8) | 17 | [16,31,37,79,81,82,83,84,87,89,92,97,98,102,105,106,109] | |
| Assessment method | EQ-5D | 62 (27.7) | 17 | [31,80,86,88,89,91,92,93,94,95,96,100,103,104,106,107,108] |
| SG | 48 (21.4) | 10 | [37,79,81,82,84,87,90,98,102,109] | |
| TTO | 31 (13.8) | 10 | [16,80,83,87,89,98,99,101,102,107] | |
| RS | 83 (37.1) | 21 | [16,31,78,79,85,87,91,92,93,96,97,99,100,102,103,104,105,106,107,108,109] | |
| Respondent type | Patient | 153 (68.3) | 26 | [16,31,78,79,80,82,86,87,88,89,91,92,93,94,95,96,97,98,99,100,101,102,103,104,106,108] |
| Non-patient | 71 (31.7) | 10 | [37,81,83,84,85,90,99,105,107,109] | |
| Survey origin | Not-stated | 3 (1.3) | 1 | [83] |
| Scenario | 125 (55.8) | 14 | [16,37,79,81,84,85,90,98,99,100,102,105,107,109] | |
| Own health | 96 (42.9) | 20 | [31,62,78,80,82,86,87,88,89,92,93,94,95,96,97,101,103,104,106,108] | |
| Administration method | Not-stated | 21 (9.4) | 4 | [81,83,84,94] |
| Self | 92 (41.1) | 15 | [31,78,88,89,91,92,93,94,95,99,103,104,105,106,108] | |
| Interview | 111 (49.5) | 18 | [16,37,79,80,82,85,86,87,90,94,96,97,98,100,101,102,107,109] | |
| Low and upper bounds of the scales | Others | 179 (79.9) | 29 | [31,78,79,80,82,83,84,85,86,87,88,89,90,91,92,93,94,96,97,98,99,100,102,103,104,106,107,108,109] |
| Perfect health/ Death | 45 (20.1) | 11 | [16,37,79,81,87,92,93,95,101,102,105] | |
| Age | Not-stated | 166 (74.1) | 28 | [31,37,78,79,82,85,86,88,89,90,91,92,93,94,95,96,98,99,100,101,102,103,104,105,106,107,108,109] |
| <50 | 36 (16.1) | 8 | [31,80,83,84,87,99,104,106] | |
| ≥50 | 22 (9.8) | 7 | [16,31,80,81,97,104,106] | |
| Variables | Coefficient Estimates | 95% CI | p-Value |
|---|---|---|---|
| Intercept | 0.742 | 0.624, 0.859 | <0.001 |
| Disease stage | |||
| Not-stated | −0.039 | −0.115, 0.038 | 0.321 |
| Late-stage | −0.216 | −0.282, −0.150 | <0.001 |
| Early-stage | Reference | Reference | Reference |
| Assessment method | |||
| EQ-5D | −0.065 | −0.140, 0.009 | 0.084 |
| SG | −0.037 | −0.113, 0.038 | 0.329 |
| RS | −0.135 | −0.203, −0.068 | <0.001 |
| TTO | Reference | Reference | Reference |
| Respondent type | |||
| Patient | 0.142 | 0.065, 0.218 | <0.001 |
| Non-Patient | Reference | Reference | Reference |
| Lower and upper bounds of the scales | |||
| Others | 0.014 | −0.051, 0.078 | 0.676 |
| Death to perfect health | Reference | Reference | Reference |
| Age | |||
| Not-stated | 0.003 | −0.077, 0.083 | 0.936 |
| <50 (Premenopausal) | −0.006 | −0.098, 0.086 | 0.897 |
| ≥50 (Postmenopausal) | Reference | Reference | Reference |
| Variables | Coefficient Estimates | 95% CI | p-Value |
|---|---|---|---|
| Intercept | 0.741 | 0.436, 0.890 | <0.001 |
| Disease stage | |||
| Not-stated | −0.056 | −0.146, 0.032 | 0.209 |
| Late-stage | −0.264 | −0.342, −0.186 | <0.001 |
| Early-stage | Reference | Reference | Reference |
| Assessment method | |||
| EQ-5D | 0.033 | −0.153, 0.219 | 0.728 |
| SG | −0.012 | −0.224, 0.202 | 0.916 |
| RS | −0.114 | −0.309, 0.082 | 0.249 |
| TTO | Reference | Reference | Reference |
| Respondent type | |||
| Patient | 0.165 | 0.019, 0.3311 | 0.027 |
| Non-Patient | Reference | Reference | Reference |
| Lower and upper bounds of the scales | |||
| Others | −0.052 | −0.179, 0.075 | 0.413 |
| Death to perfect health | Reference | Reference | Reference |
| Age | |||
| Not-stated | −0.004 | −0.111, 0.105 | 0.923 |
| <50 (Premenopausal) | 0.005 | −0.111, 0.121 | 0.931 |
| ≥50 (Postmenopausal) | Reference | Reference | Reference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Han, J.; Lee, D.; Bae, S. A Meta-Regression Analysis of Utility Weights for Breast Cancer: The Power of Patients’ Experience. Int. J. Environ. Res. Public Health 2020, 17, 9412. https://doi.org/10.3390/ijerph17249412
Gong J, Han J, Lee D, Bae S. A Meta-Regression Analysis of Utility Weights for Breast Cancer: The Power of Patients’ Experience. International Journal of Environmental Research and Public Health. 2020; 17(24):9412. https://doi.org/10.3390/ijerph17249412
Chicago/Turabian StyleGong, Jiryoun, Juhee Han, Donghwan Lee, and Seungjin Bae. 2020. "A Meta-Regression Analysis of Utility Weights for Breast Cancer: The Power of Patients’ Experience" International Journal of Environmental Research and Public Health 17, no. 24: 9412. https://doi.org/10.3390/ijerph17249412
APA StyleGong, J., Han, J., Lee, D., & Bae, S. (2020). A Meta-Regression Analysis of Utility Weights for Breast Cancer: The Power of Patients’ Experience. International Journal of Environmental Research and Public Health, 17(24), 9412. https://doi.org/10.3390/ijerph17249412






