Prevalence of Sarcopenia in Community-Dwelling Older Adults in Valencia, Spain
Abstract
1. Introduction
2. Materials and Methods
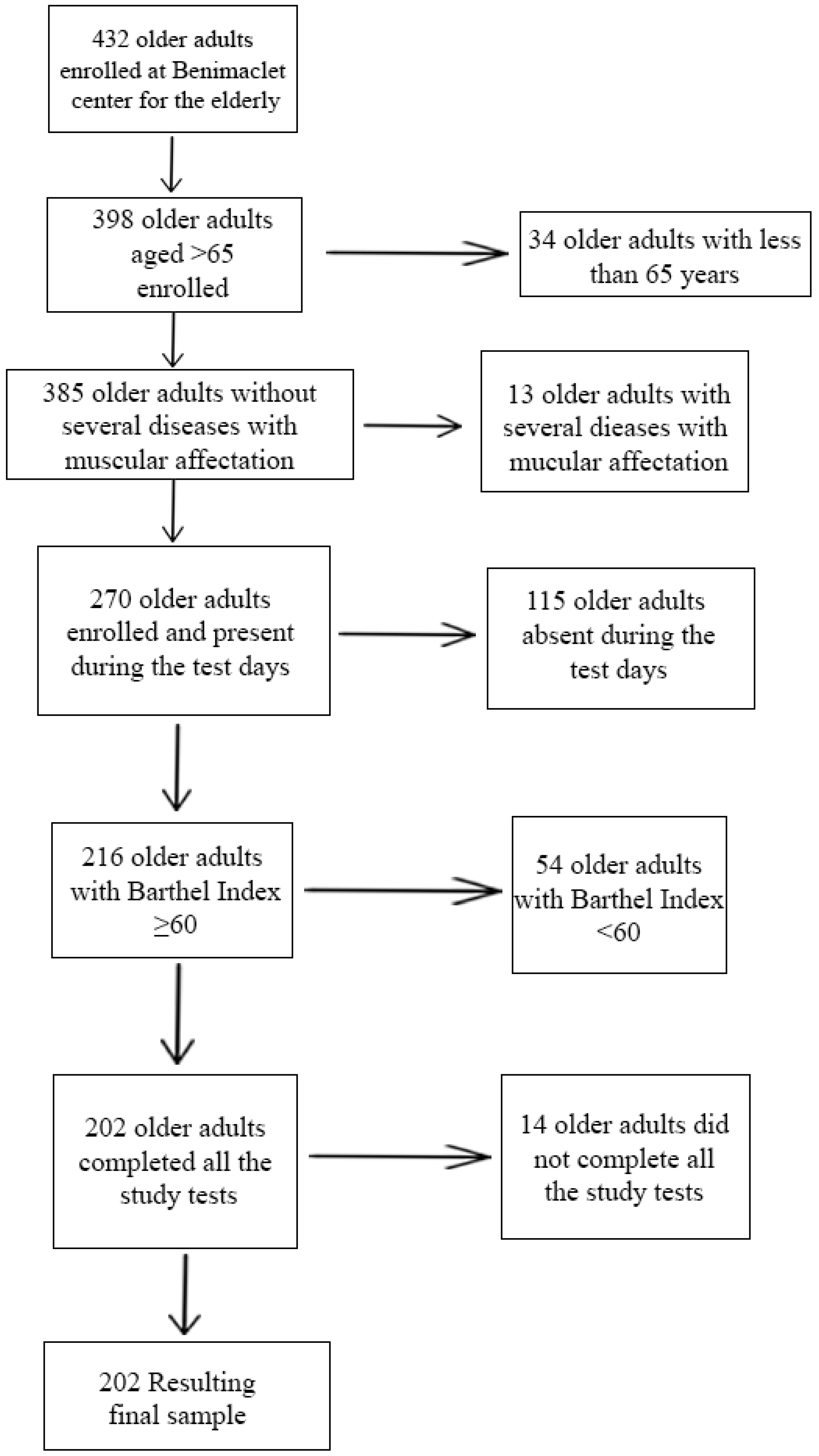
2.1. Study Population
2.2. Examination Protocol and Measurements
2.3. Degree of Dependence
2.4. Sarcopenia Screening
2.5. Diagnosis of Sarcopenic Pathology
2.6. Grip Force (Upper Train)
2.7. Lower Train Strength
2.8. Appendicular Skeletal Muscle Mass (ASMM)
2.9. Physical Performance
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cebrià i Iranzo, M.A.; Arnal-Gómez, A.; Tortosa-Chuliá, M.A.; Balasch-Bernat, M.; Forcano, S.; Sentandreu-Mañó, T.; Tomas, J.M.; Cezón-Serrano, N. Functional and clinical characteristics for predicting sarcopenia in institutionalised older adults: Identifying tools for clinical screening. Int. J. Environ. Res. Public Health 2020, 17, 4483. [Google Scholar]
- Rezuş, E.; Burlui, A.; Cardoneanu, A.; Rezuş, C.; Codreanu, C.; Pârvu, M.; Zota, G.R.; Tamba, B.I. Inactivity and skeletal muscle metabolism: A vicious cycle in old age. Int. J. Mol. Sci. 2020, 21, 592. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Bokshan, S.L.; Marcaccio, S.E.; DePasse, J.M.; Daniels, A.H. Diagnostic criteria and clinical outcomes in sarcopenia research: A literature review. J. Clin. Med. 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.K. Sarcopenia: A contemporary health problem among older adult populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M. What is dynapenia? Nutrition 2012, 28, 495–503. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M. Sarcopenia =/= dynapenia. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 829–834. [Google Scholar] [CrossRef]
- Scott, D. Sarcopenia in older adults. J. Clin. Med. 2019, 8, 1844. [Google Scholar] [CrossRef]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 324–333. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H.; Kawashima, Y.; Katsuyama, H.; Sako, A.; Goto, A.; Yanai, H. Association of handgrip strength with hospitalization, cardiovascular events, and mortality in Japanese patients with type 2 diabetes. Sci. Rep. 2017, 7, 7041. [Google Scholar] [CrossRef] [PubMed]
- da Silva Alexandre, T.; de Oliveira Duarte, Y.A.; Ferreira Santos, J.L.; Wong, R.; Lebrão, M.L. Sarcopenia according to the european working group on sarcopenia in older people (EWGSOP) versus Dynapenia as a risk factor for disability in the elderly. J. Nutr. Health Aging 2014, 18, 547–553. [Google Scholar] [CrossRef] [PubMed]
- da Silva Alexandre, T.; de Oliveira Duarte, Y.A.; Ferreira Santos, J.L.; Wong, R.; Lebrão, M.L. Sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J. Nutr. Health Aging 2014, 18, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cabello, A.; Vicente Rodríguez, G.; Vila-Maldonado, S.; Casajús, J.A.; Ara, I. Envejecimiento y composición corporal: La obesidad sarcopénica en España. Nutrición Hospitalaria 2012, 27, 22–30. [Google Scholar] [PubMed]
- Anonymous. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Bernaola-Sagardui, I. Validation of the Barthel Index in the Spanish population. Enferm. Clin. 2018, 28, 210–211. [Google Scholar] [CrossRef]
- Mayhew, A.J.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.D.; de Souza, R.J.; Thabane, L.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing 2019, 48, 48–56. [Google Scholar] [CrossRef]
- Moreira, V.G.; Perez, M.; Lourenço, R.A. Prevalence of sarcopenia and its associated factors: The impact of muscle mass, gait speed, and handgrip strength reference values on reported frequencies. Clinics 2019, 74. [Google Scholar] [CrossRef]
- Kim, T.N.; Yang, S.J.; Yoo, H.J.; Lim, K.I.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: The Korean sarcopenic obesity study. Int. J. Obes. 2009, 33, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Villafañe, J.H.; Pirali, C.; Dughi, S.; Testa, A.; Manno, S.; Bishop, M.D.; Negrini, S. Association between malnutrition and Barthel Index in a cohort of hospitalized older adults article information. J. Phys. Ther. Sci. 2016, 28, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Mii, S.; Guntani, A.; Kawakubo, E.; Shimazoe, H. Barthel Index and outcome of open bypass for critical limb ischemia. Circ. J. 2017, 82, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, A.P.; Ibarz, E.; Gracia, L.; Mateo, J.; Herrera, A. The use of Barthel index for the assessment of the functional recovery after osteoporotic hip fracture: One year follow-up. PLoS ONE 2019, 14, e0212000. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Van Kan, G.A. Epidemiology and consequences of sarcopenia. J. Nutr. Health Aging 2009, 13, 708–712. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Lauretani, F.; Zamboni, V.; Bandinelli, S.; Bernabei, R.; Guralnik, J.M.; Ferrucci, L. Skeletal Muscle and Mortality Results from the InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 377–384. [Google Scholar] [CrossRef]
- Trosclair, D.; Bellar, D.; Judge, L.W.; Smith, J.; Mazerat, N.; Brignac, A. Hand-Grip Strength as a Predictor of Muscular Strength and Endurance. J. Strength Cond. Res. 2011, 25, S99. [Google Scholar] [CrossRef]
- Gopinath, B.; Kifley, A.; Liew, G.; Mitchell, P. Handgrip strength and its association with functional independence, depressive symptoms and quality of life in older adults. Maturitas 2017, 106, 92–94. [Google Scholar] [CrossRef]
- Kwak, Y.; Kim, Y.; Chung, H. Sex-associated differences in the handgrip strength of elderly individuals. West J. Nurs. Res. 2019, 42, 262–268. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.M.; Roriz, A.K.C.; Barreto-Medeiros, J.M.; Ferreira, A.J.F.; Ramos, L. Sarcopenic obesity in community-dwelling older women, determined by different diagnostic methods. Nutr. Hosp. 2019, 36, 1267–1272. [Google Scholar]
- Cooper, C.; Fielding, R.; Visser, M.; van Loon, L.J.; Rolland, Y.; Orwoll, E.; Reid, K.; Boonen, S.; Dere, W.; Epstein, S.; et al. Tools in the assessment of sarcopenia. Calcif. Tissue Int. 2013, 93, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Hans, D.; Pichard, C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin. Nutr. 2003, 22, 537–543. [Google Scholar] [CrossRef]
- Alvero-Cruz, J.R.; Correas Gómez, L.; Ronconi, M.; Fernández Vázquez, R.; Porta i Manzañido, J. La bioimpedancia eléctrica como método de estimación de la composición corporal, normas prácticas de utilización. Rev. Andal. Med. Deporte 2011, 4, 167–174. [Google Scholar]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Lauretani, F.; Ticinesi, A.; Gionti, L.; Prati, B.; Nouvenne, A.; Tana, C.; Meschi, T.; Maggio, M. Short-Physical Performance Battery (SPPB) score is associated with falls in older outpatients. Aging Clin. Exp. Res. 2019, 31, 1435–1442. [Google Scholar] [CrossRef]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short physical performance battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef]
- Cuesta, F.; Formiga, F.; Lopez-Soto, A.; Masanes, F.; Ruiz, D.; Artaza, I.; Salvà, A.; Serra-Rexach, J.A.; Rojano, I.; Luque, X.; et al. Prevalence of sarcopenia in patients attending outpatient geriatric clinics: The ELLI study. Age Ageing 2015, 44, 807–809. [Google Scholar] [CrossRef][Green Version]
- Masanes, F.; Culla, A.; Navarro-Gonzalez, M.; Navarro-Lopez, M.; Sacanella, E.; Torres, B.; Lopez-Soto, A. Prevalence of sarcopenia in healthy community-dwelling elderly in an urban area of Barcelona (Spain). J. Nutr. Health Aging 2012, 16, 184–187. [Google Scholar] [CrossRef]
- Kim, M.; Won, C.W. Prevalence of sarcopenia in community-dwelling older adults using the definition of the European Working Group on Sarcopenia in Older People 2: Findings from the Korean Frailty and Aging Cohort Study. Age Ageing 2019, 48, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Bravo-José, P.; Moreno, E.; Espert, M.; Romeu, M.; Martínez, P.; Navarro, C. Prevalence of sarcopenia and associated factors in institutionalised older adult patients. Clin. Nutr. ESPEN 2018, 27, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; Rizzo, M.R.; et al. Prevalence and clinical correlates of sarcopenia, identified according to the EWGSOP definition and diagnostic algorithm, in hospitalized older people: The GLISTEN Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Garrido, J.; Navarro-Martínez, R.; Buigues-González, C.; Martínez-Martínez, M.; Ruiz-Ros, V.; Cauli, O. The value of neutrophil and lymphocyte count in frail older women. Exp. Gerontol. 2014, 54, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garrido, J.; Ruiz-Ros, V.; Navarro-Martínez, R.; Buigues, C.; Martínez-Martínez, M.; Verdejo, Y.; Sanantonio-Camps, L.; Mascarós, M.C.; Cauli, O. Frailty and leucocyte count are predictors of all-cause mortality and hospitalization length in non-demented institutionalized older women. Exp. Gerontol. 2018, 103, 80–86. [Google Scholar] [CrossRef]
- Dodds, R.M.; Murray, J.C.; Robinson, S.M.; Sayer, A.A. The identification of probable sarcopenia in early old age based on the SARC-F tool and clinical suspicion: Findings from the 1946 British birth cohort. Eur. Geriatr. Med. 2020, 11, 433–441. [Google Scholar] [CrossRef]
| 65–75 Years | 75–85 Years | Total | |
| Men (n) | 27 | 11 | 38 |
| Without sarcopenia 1 | 21 (77.8%) | 6 (54.5%) | 27 (71%) |
| Total sarcopenia 2 | 6 (28.6%) | 5 (45.4%) | 11 (28.9%) |
| Sarcopenia probable 3 | 5 (18.5%) | 3 (27.3%) | 8 (21.1%) |
| Sarcopenia diagnosed 4 (SC 5 + SS 6) | 1 (3.7%) | 2 (18.2%) | 3 (7.9%) |
| Sarcopenia confirmed 7 | 0 (0%) | 0 (0%) | 0 (%) |
| Sarcopenia severe 8 | 1 (3.7%) | 2 (18.2%) | 3 (7.9%) |
| Women (n) | 101 | 63 | 164 |
| Without sarcopenia 1 | 80 (79.2%) | 42 (66.7%) | 122 (74.4%) |
| Total sarcopenia 2 | 21 (20.8%) | 21 (33.3%) | 42 (25.6%) |
| Sarcopenia probable 3 | 18 (17.8%) | 12 (19.0%) | 30 (18.3%) |
| Sarcopenia diagnosed 4 (SC 5 + SS 6) | 3 (3%) | 9 (14.3%) | 12 (7.3%) |
| Sarcopenia confirmed 7 | 2 (2%) | 4 (6.3%) | 6 (3.7%) |
| Sarcopenia severe 8 | 1 (1%) | 5 (7.9%) | 6 (3.7%) |
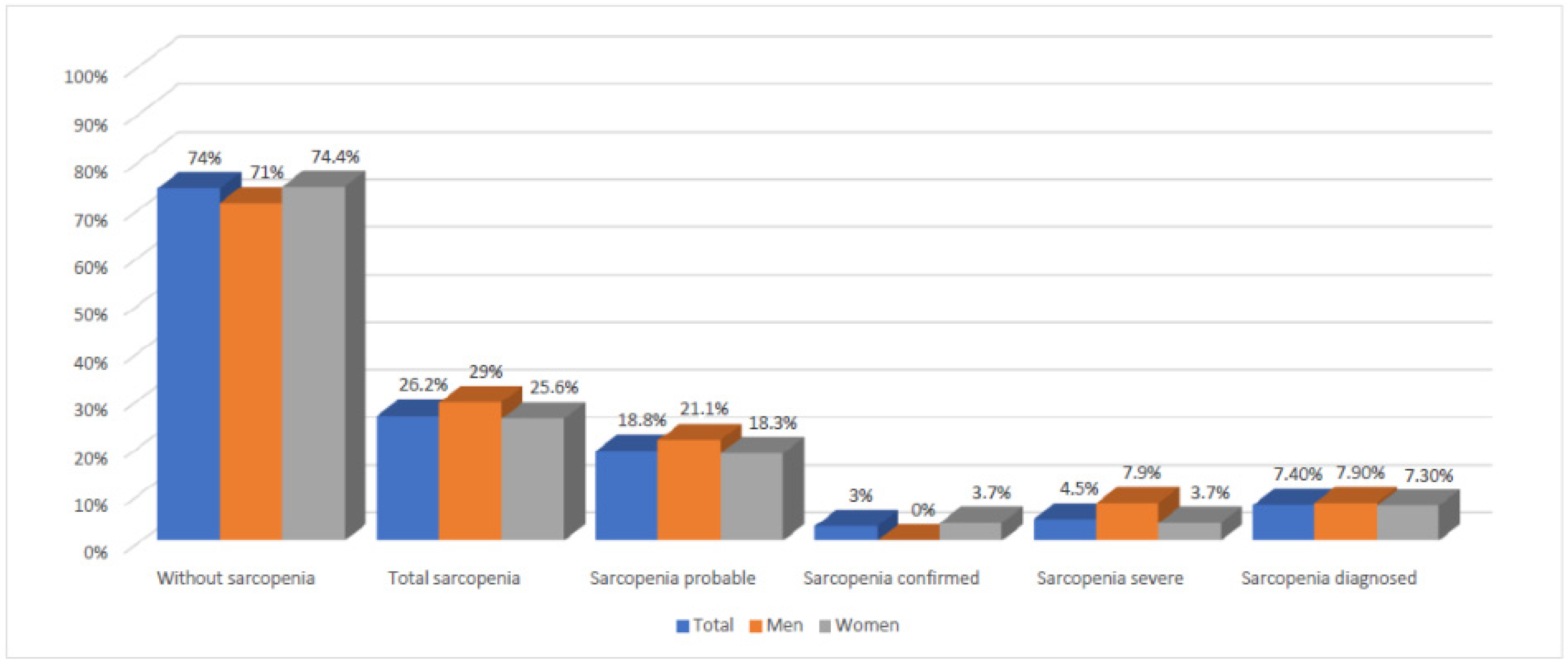
| Total (n) | 128 | 74 | 202 |
| Without sarcopenia 1 | 101 (78.9%) | 48 (64.9%) | 149 (73.8%) |
| Total sarcopenia 2 | 27 (21.1%) | 26 (35.1%) | 53 (26.2%) |
| Sarcopenia probable 3 | 23 (18%) | 15 (20.3%) | 18 (18.8%) |
| Sarcopenia diagnosed 4 (SC 5 + SS 6) | 4 (3.1%) | 11 (14.9%) | 15 (7.4%) |
| Sarcopenia confirmed 7 | 2 (1.6%) | 4 (5.4%) | 6 (3%) |
| Sarcopenia severe 8 | 2 (1.6%) | 7 (9.5%) | 9 (4.5%) |
| 65–75 years | 75–85 years | Total | |
| Men (n) | 27 | 11 | 38 |
| Without sarcopenia 1 | 21 (77.8%) | 6 (54.5%) | 27 (71%) |
| Total sarcopenia 2 | 6 (28.6%) | 5 (45.4%) | 11 (28.9%) |
| Sarcopenia probable 3 | 5 (18.5%) | 3 (27.3%) | 8 (21.1%) |
| Sarcopenia confirmed 4 | 0 (0%) | 0 (0%) | 0 (0%) |
| Sarcopenia severe 5 | 1 (3.7%) | 2 (18.18%) | 3 (7.9%) |
| Sarcopenia diagnosed 6 (SC 7 + SS 8) | 1 (3.7%) | 2 (18.2%) | 3 (7.9%) |
| Women (n) | 101 | 63 | 164 |
| Without sarcopenia 1 | 80 (79.2%) | 42 (66.7%) | 122 (74.4%) |
| Total sarcopenia 2 | 21 (20.8%) | 21 (33.3%) | 42 (25.6%) |
| Sarcopenia probable 3 | 18 (17.8%) | 12 (19.0%) | 30 (18.3%) |
| Sarcopenia confirmed 4 | 2 (2%) | 4 (6.3%) | 6 (3.7%) |
| Sarcopenia severe 5 | 1 (1%) | 5 (7.9%) | 6 (3.7%) |
| Sarcopenia diagnosed 6 (SC 7 + SS 8) | 3 (3%) | 9 (14.3%) | 12 (7.3%) |
| Total (n) | 128 | 74 | 202 |
| Without sarcopenia 1 | 101 (78.9%) | 48 (64.9%) | 149 (73.8%) |
| Total sarcopenia 2 | 27 (21.1%) | 26 (35.1%) | 53 (26.2%) |
| Sarcopenia probable 3 | 23 (18%) | 15 (20.3%) | 18 (18.8%) |
| Sarcopenia confirmed 4 | 2 (1.6%) | 4 (5.4%) | 6 (3%) |
| Sarcopenia severe 5 | 2 (1.6%) | 7 (9.5%) | 9 (4.5%) |
| Sarcopenia diagnosed 6 (SC 7 + SS 8) | 4 (3.1%) | 11 (14.9%) | 15 (7.4%) |
| GS 2 (kg) | STS 3 (s) | ASMM Total 4 (kg) | Gait Speed 5 | SPPB 6 (Score) | SARC-F (Score) | |
|---|---|---|---|---|---|---|
| Men | ||||||
| 65–75 years | ||||||
| Without Sarcopenia | 36.5 ± 7.5 | 10.2 ± 2.4 | 23.2 ± 3.1 | 1.1 ± 0.2 | 10.3 ± 1.5 | 1.2 ± 1.4 |
| Sarcopenia Probable | 32.8 ± 5 | 16.2 ± 4.6 | 25 ± 1.3 | 0.9 ± 0.2 | 9 ± 2 | 2.6 ± 1.3 |
| Sarcopenia Confirmed | - | - | - | - | - | - |
| Sarcopenia severe | 25 ± 0 | 21 ± 0 | 16.9 ± 0 | 0.8 ± 0 | 5 ± 0 | 4 ± 0 |
| Total Sarcopenia Diagnosed | 25 ± 0 | 21 ± 0 | 16.9 ± 0 | 0.8 ± 0 | 5 ± 0 | 4 ± 0 |
| Total Sarcopenia | 28.9 ± 5.5 | 18.6 ± 3.4 | 20.9 ± 5.7 | 0.8 ± 0.1 | 7 ± 2.8 | 3.3 ± 0.9 |
| 75–85 years | ||||||
| Without Sarcopenia | 32.7 ± 4.5 | 12.6 ± 2.7 | 23 ± 2.5 | 0.9 ± 0.1 | 9.3 ± 2.2 | 3.2 ± 2.1 |
| Sarcopenia Probable | 28.7 ± 6.1 | 18.9 ± 1.4 | 24.5 ± 2.4 | 0.9 ± 0.3 | 5.3 ± 1.2 | 5 ± 2 |
| Sarcopenia Confirmed | - | - | - | - | - | - |
| Sarcopenia severe | 28 ± 1.4 | 16.5 ± 0 | 19.6 ± 0.3 | 0.8 ± 0.1 | 6.5 ± 0.7 | 1 ± 1.4 |
| Total Sarcopenia Diagnosed | 28 ± 1.4 | 16.5 ± 0 | 19.6 ± 0.3 | 0.8 ± 0.1 | 6.5 ± 0.7 | 1 ± 1.4 |
| Total Sarcopenia | 28.3 ± 0.5 | 17.7 ± 1.7 | 22 ± 3.5 | 0.8 ± 0.1 | 5.9 ± 0.8 | 3 ± 2.8 |
| Women | ||||||
| 65–75 years | ||||||
| Without Sarcopenia | 22 ± 3.8 | 10.3 ± 2.1 | 16.8 ± 2.4 | 1.1 ± 0.2 | 10.4 ± 1.4 | 1.7 ± 1,2 |
| Sarcopenia Probable | 16.5 ± 5.8 | 14.1 ± 4.3 | 17.9 ± 2 | 1 ± 0.3 | 8.7 ± 1.7 | 3.2 ± 1.5 |
| Sarcopenia Confirmed | 12.7 ± 3.2 | 11.6 ± 1.1 | 14.2 ± 2.2 | 1 ± 0.1 | 9.7 ± 2.1 | 1.3 ± 1.5 |
| Sarcopenia severe | 15 ± 0 | 19.3 ± 0 | 14.4 ± 0 | 0.7 ± 0 | 5 ± 0 | 4 ± 0 |
| Total Sarcopenia Diagnosed | 13.9 ± 1.6 | 15.5 ± 0.6 | 14.3 ± 1.1 | 0.9 ± 0.1 | 7.4 ± 1.1 | 2.7 ± 0.8 |
| Total Sarcopenia | 14.7 ± 1.9 | 15 ± 3.9 | 15.5 ± 2.1 | 0.9 ± 0.2 | 7.5 ± 2.1 | 3.5 ± 0.5 |
| 75–85 years | ||||||
| Without Sarcopenia | 20.2 ± 3.6 | 11 ± 2 | 15.9 ± 2.2 | 1 ± 0.2 | 10 ± 1.4 | 2.6 ± 1.3 |
| Sarcopenia Probable | 14.9 ± 3 | 17.2 ± 4.8 | 17.7 ± 2.4 | 0.7 ± 0.2 | 7.4 ± 1.7 | 4.1 ± 1 |
| Sarcopenia Confirmed | 14.8 ± 1.5 | 12.7 ± 1.7 | 13 ± 2.4 | 0.9 ± 0.1 | 10.3 ± 1.5 | 3.8 ± 0.5 |
| Sarcopenia severe | 12.6 ± 1.3 | 15.3 ± 4.6 | 13.9 ± 1.7 | 0.8 ± 0.2 | 7.8 ± 1.1 | 2.8 ± 2.3 |
| Total Sarcopenia Diagnosed | 13.7 ± 1.4 | 14 ± 3.2 | 13.5 ± 2.1 | 0.9 ± 0.2 | 9.1 ± 1.3 | 3.3 ± 1.4 |
| Total Sarcopenia | 14.1 ± 1.3 | 15.1 ± 2.3 | 14.9 ± 2.5 | 0.8 ± 0.1 | 8.5 ± 1.6 | 3.6 ± 0.7 |
| Men | ||||||
| WS 7 (all sample sizes) | 34.6 ± 6 | 11.4 ± 2.6 | 23.1 ± 2.8 | 1 ± 0.2 | 9.8 ± 1.9 | 2.2 ± 2.8 |
| TSD 8 (all sample sizes) | 26.5 ± 0.7 | 18.8 ± 0 | 18.3 ± 0.15 | 0.8 ± 0.1 | 5.8 ± 0.4 | 2.5 ± 0.7 |
| TS 9 (all sample sizes) | 28.63 ±0.4 | 18.15 ± 0.6 | 21.5 ± 0.8 | 0.85 ± 0.0 | 6.45 ± 0.8 | 3.15 ± 0.2 |
| Women | ||||||
| WS 7 (all sample sizes) | 21.1 ± 3.4 | 10.7 ± 2.1 | 16.4 ± 2.3 | 1.1 ± 0.2 | 10.2 ± 1.4 | 2.2 ± 1.3 |
| TSD 8 (all sample sizes) | 13.8 ± 1.5 | 14.8 ± 1.9 | 13.9 ± 1.6 | 0.9 ± 0.2 | 8.3 ± 1.2 | 3 ± 1.1 |
| TS 9 (all sample sizes) | 14.4 ± 0.4 | 15 ± 0 | 15.2 ± 0.4 | 0.9 ± 0.1 | 8 ± 0.7 | 3.5 ± 0.1 |
| Total (n) | SARC-F Score | Positive SARC-F (≥4) | Negative SARC-F (<4) | |
|---|---|---|---|---|
| Without sarcopenia 2 | 149 | 1.95 ± 1.42 | 23 (15.4%) | 126 (84.6%) |
| Total sarcopenia 3 | 53 | 2.93 ± 1.46 | 32 (60.4%) | 21 (39.6%) |
| Sarcopenia probable 4 | 38 | 2.5 ± 1.52 | 25 (65.8%) | 13 (34.2%) |
| Sarcopenia diagnosed 5 (SC 6 + SS 7) | 15 | 2.8 ± 1.78 | 7 (46.7%) | 8 (53.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillamón-Escudero, C.; Diago-Galmés, A.; Tenías-Burillo, J.M.; Soriano, J.M.; Fernández-Garrido, J.J. Prevalence of Sarcopenia in Community-Dwelling Older Adults in Valencia, Spain. Int. J. Environ. Res. Public Health 2020, 17, 9130. https://doi.org/10.3390/ijerph17239130
Guillamón-Escudero C, Diago-Galmés A, Tenías-Burillo JM, Soriano JM, Fernández-Garrido JJ. Prevalence of Sarcopenia in Community-Dwelling Older Adults in Valencia, Spain. International Journal of Environmental Research and Public Health. 2020; 17(23):9130. https://doi.org/10.3390/ijerph17239130
Chicago/Turabian StyleGuillamón-Escudero, Carlos, Angela Diago-Galmés, Jose M. Tenías-Burillo, Jose M. Soriano, and Julio J. Fernández-Garrido. 2020. "Prevalence of Sarcopenia in Community-Dwelling Older Adults in Valencia, Spain" International Journal of Environmental Research and Public Health 17, no. 23: 9130. https://doi.org/10.3390/ijerph17239130
APA StyleGuillamón-Escudero, C., Diago-Galmés, A., Tenías-Burillo, J. M., Soriano, J. M., & Fernández-Garrido, J. J. (2020). Prevalence of Sarcopenia in Community-Dwelling Older Adults in Valencia, Spain. International Journal of Environmental Research and Public Health, 17(23), 9130. https://doi.org/10.3390/ijerph17239130







