How Many Urine Samples Are Needed to Accurately Assess Exposure to Non-Persistent Chemicals? The Biomarker Reliability Assessment Tool (BRAT) for Scientists, Research Sponsors, and Risk Managers
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. System Requirements
2.3. User Interface—Model Inputs
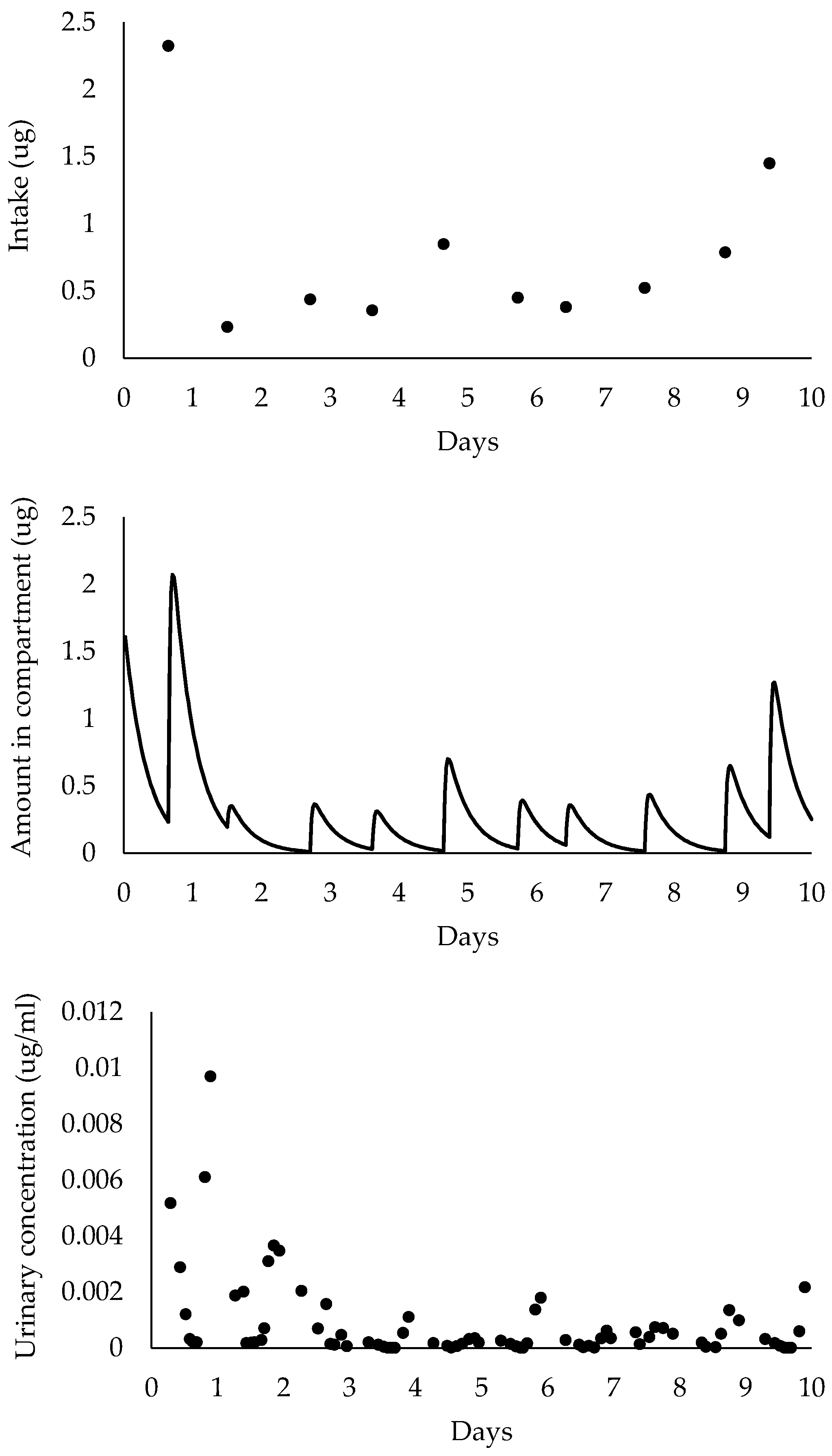
2.4. Pharmacokinetic Model Simulations
2.5. Model Outputs
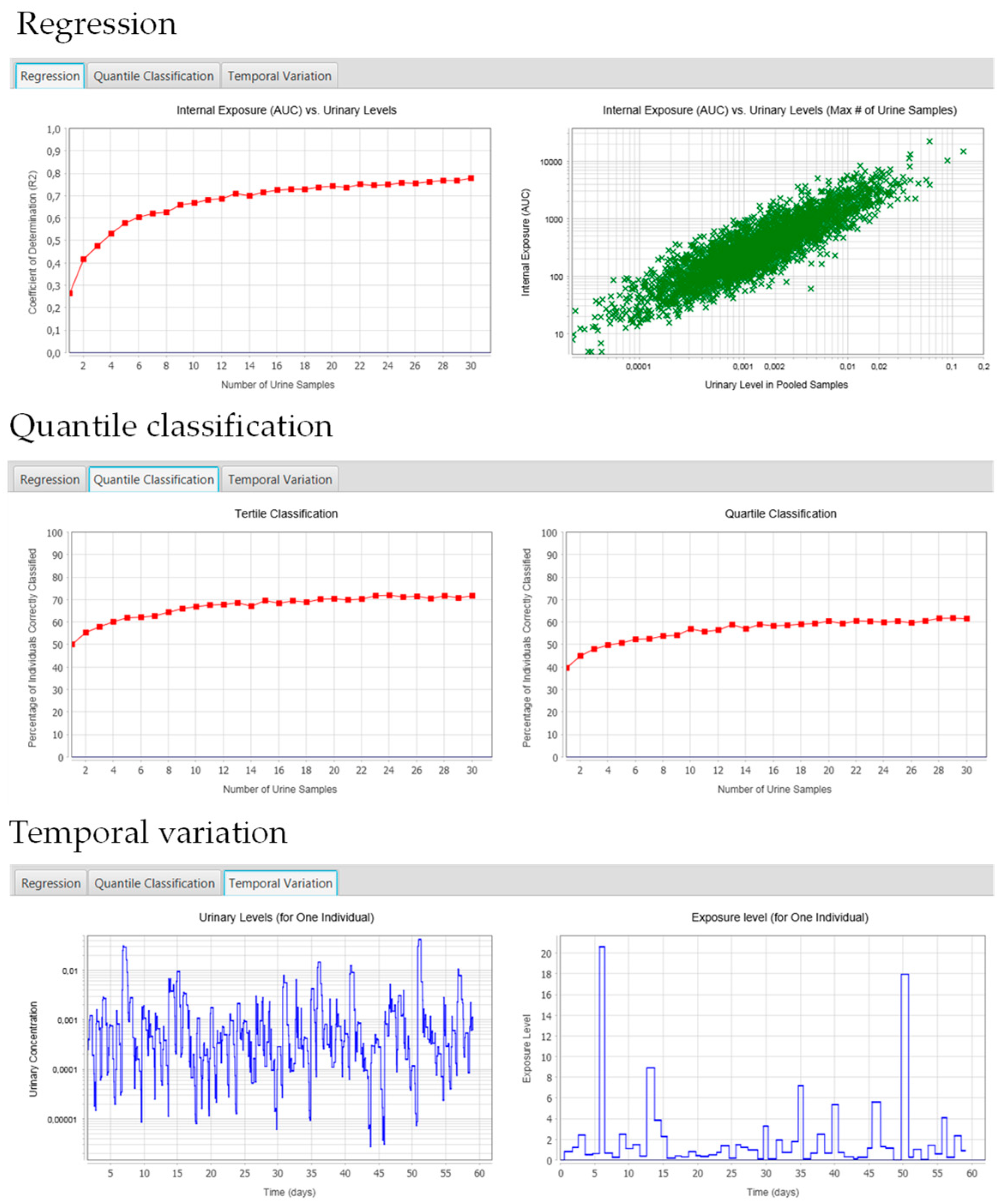
2.5.1. Regression Tab
2.5.2. Quantile Classification
2.5.3. Temporal Variation Tab
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aylward, L.L.; Hays, S.M.; Smolders, R.; Koch, H.M.; Cocker, J.; Jones, K.; Warren, N.; Levy, L.; Bevan, R. Sources of variability in biomarker concentrations. J. Toxicol. Environ. Health B Crit. Rev. 2014, 17, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wang, R.Y.; Needham, L.L. Biologic monitoring of exposure to environmental chemicals throughout the life stages: Requirements and issues for consideration for the National Children’s Study. Environ. Health Perspect. 2005, 113, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Longnecker, M.P.; Koch, H.M.; Swan, S.H.; Hauser, R.; Goldman, L.R.; Lanphear, B.P.; Rudel, R.A.; Engel, S.M.; Teitelbaum, S.L.; et al. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ. Health Perspect. 2005, 123, A166–A168. [Google Scholar] [CrossRef] [PubMed]
- LaKind, J.S.; Idri, F.; Naiman, D.Q.; Verner, M.A. Biomonitoring and Nonpersistent Chemicals-Understanding and Addressing Variability and Exposure Misclassification. Curr. Environ. Health Rep. 2019, 6, 16–21. [Google Scholar] [CrossRef] [PubMed]
- LaKind, J.S.; Goodman, M.; Mattison, D.R. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: A systematic review of epidemiologic research. Crit. Rev. Toxicol. 2014, 44, 121–150. [Google Scholar] [CrossRef]
- Goodman, M.; Lakind, J.S.; Mattison, D.R. Do phthalates act as obesogens in humans? A systematic review of the epidemiological literature. Crit. Rev. Toxicol. 2014, 44, 151–175. [Google Scholar] [CrossRef]
- Goodman, M.; Naiman, D.Q.; LaKind, J.S. Systematic review of the literature on triclosan and health outcomes in humans. Crit. Rev. Toxicol. 2018, 48, 1–51. [Google Scholar] [CrossRef]
- Bertelsen, R.J.; Engel, S.M.; Jusko, T.A.; Calafat, A.M.; Hoppin, J.A.; London, S.J.; Eggesbø, M.; Aase, H.; Zeiner, P.; Reichborn-Kjennerud, T.; et al. Reliability of triclosan measures in repeated urine samples from Norwegian pregnant women. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 517–521. [Google Scholar] [CrossRef][Green Version]
- Vernet, C.; Philippat, C.; Calafat, A.M.; Ye, X.; Lyon-Caen, S.; Siroux, V.; Schisterman, E.F.; Slama, R. Within-Day, Between-Day, and Between-Week Variability of Urinary Concentrations of Phenol Biomarkers in Pregnant Women. Environ. Health Perspect. 2018, 126, 037005. [Google Scholar] [CrossRef]
- Morgan, M.K.; Sobus, J.R.; Barr, D.B.; Croghan, C.W.; Chen, F.L.; Walker, R.; Alston, L.; Andersen, E.; Clifton, M.S. Temporal variability of pyrethroid metabolite levels in bedtime, morning, and 24-h urine samples for 50 adults in North Carolina. Environ. Res. 2016, 144, 81–91. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Application of Systematic Review in TSCA Evaluations. EPA Document# 740-P1-8001; Office of Chemical Safety and Pollution Prevention: Washington, DC, USA, 2018.
- Casas, M.; Basagaña, X.; Sakhi, A.K.; Haug, L.S.; Philippat, C.; Granum, B.; Manzano-Salgado, C.B.; Brochot, C.; Zeman, F.; de Bont, J.; et al. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ. Int. 2018, 121 Pt 1, 561–573. [Google Scholar] [CrossRef]
- Li, A.J.; Martinez-Moral, M.P.; Kannan, K. Variability in urinary neonicotinoid concentrations in single-spot and first-morning void and its association with oxidative stress markers. Environ. Int. 2020, 135, 105415. [Google Scholar] [CrossRef]
- Perrier, F.; Giorgis-Allemand, L.; Slama, R.; Philippat, C. Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology 2016, 27, 378–388. [Google Scholar] [CrossRef]
- Spaan, S.; Fransman, W.; Warren, N.; Cotton, R.; Cocker, J.; Tielemans, E. Variability of biomarkers in volunteer studies: The biological component. Toxicol. Lett. 2010, 198, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P.; Jones, R.D.; Jones, H.M.; Gibson, C.R.; Rowland, M.; Chien, J.Y.; Ring, B.J.; Adkison, K.K.; Ku, M.S.; He, H.; et al. PHRMA CPCDC initiative on predictive models of human pharmacokinetics, part 5: Prediction of plasma concentration-time profiles in human by using the physiologically-based pharmacokinetic modeling approach. J. Pharm. Sci. 2011, 100, 4127–4157. [Google Scholar] [CrossRef] [PubMed]
- Smolders, R.; Koch, H.M.; Moos, R.K.; Cocker, J.; Jones, K.; Warren, N.; Levy, L.; Bevan, R.; Hays, S.M.; Aylward, L.L. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 1: Metals. Toxicol. Lett. 2014, 231, 249–260. [Google Scholar] [CrossRef]
- Persad, A.S.; Cooper, G.S. Use of epidemiologic data in Integrated Risk Information System (IRIS) assessments. Toxicol. Appl. Pharmacol. 2008, 233, 137–145. [Google Scholar] [CrossRef] [PubMed]
- EFSA PPR Panel [EFSA Panel on Plant Protection Products and their Residues]; Ockleford, C.; Adriaanse, P.; Berny, P.; Brock, T.; Duquesne, S.; Grilli, S.; Hougaard, S.; Klein, M.; Kuhl, T.; et al. Scientific Opinion of the PPR Panel on the follow-up of the findings of the External Scientific Report ‘Literature review of epidemiological studies linking exposure to pesticides and health effects’. EFSA J. 2017, 15, 5007. [Google Scholar]
- Faÿs, F.; Palazzi, P.; Hardy, E.M.; Schaeffer, C.; Phillipat, C.; Zeimet, E.; Vaillant, M.; Beausoleil, C.; Rousselle, C.; Slama, R.; et al. Is there an optimal sampling time and number of samples for assessing exposure to fast elimination endocrine disruptors with urinary biomarkers? Sci. Total Environ. 2020, 747, 141185. [Google Scholar] [CrossRef]
- Van Haarst, E.P.; Heldeweg, E.A.; Newling, D.W.; Schlatmann, T.J. The 24-h frequency-volume chart in adults reporting no voiding complaints: Defining reference values and analysing variables. BJU Int. 2004, 93, 1257–1261. [Google Scholar] [CrossRef]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]
- LaKind, J.S.; Burns, C.J.; Erickson, H.; Graham, S.E.; Jenkins, S.; Johnson, G.T. Bridging the Epidemiology Risk Assessment Gap: An NO2 Case Study of the Matrix. Glob. Epidemiol. 2020, 2, 100017. [Google Scholar] [CrossRef]
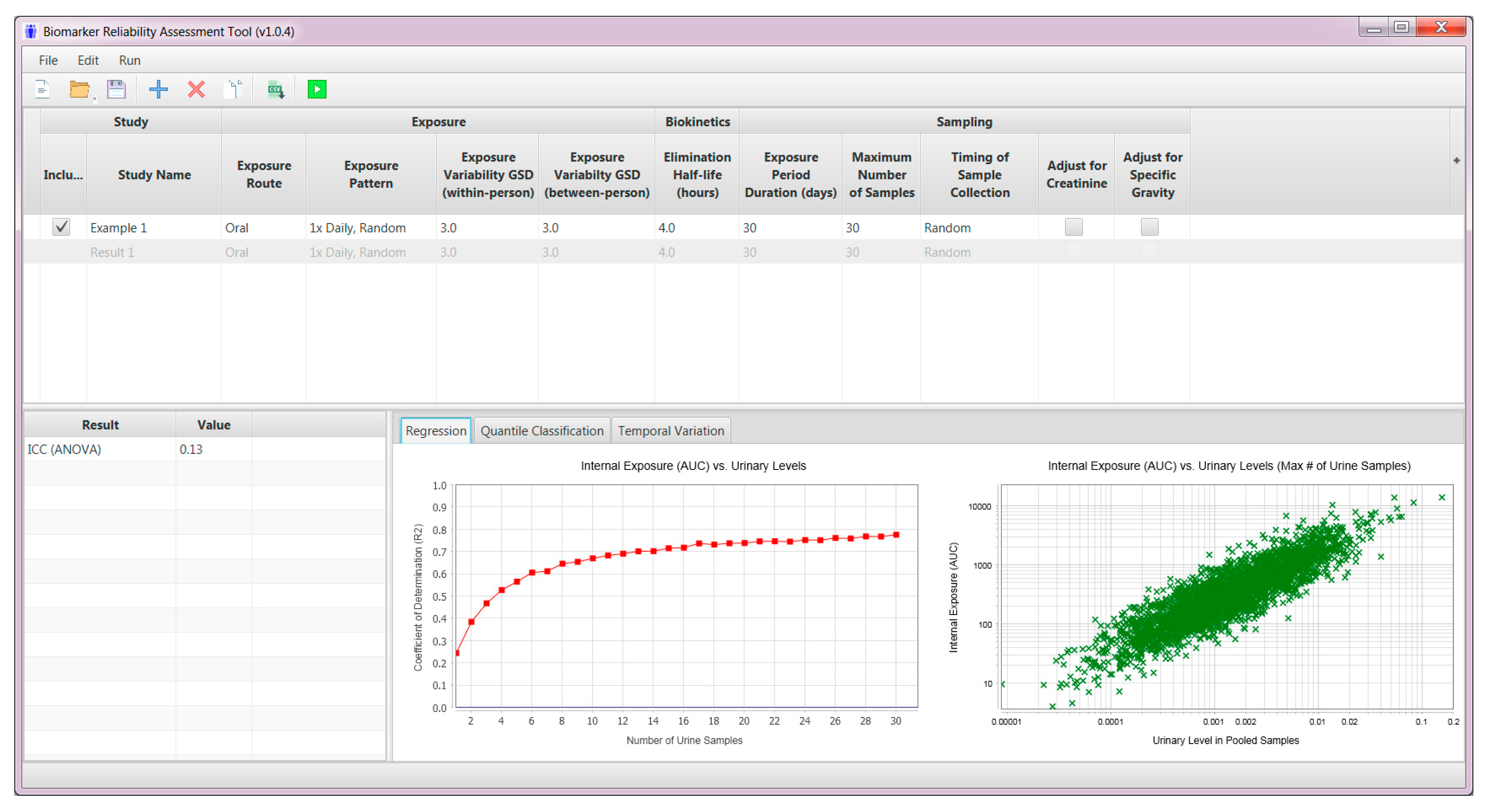
| Parameter | Input |
|---|---|
| Exposure route | At present, the tool accommodates the oral route of exposure |
| Exposure pattern |
|
| Within-person variability in exposure levels | Geometric standard deviation for a distribution of within-person exposure levels (including all exposure events) |
| Between-person variability in exposure levels | Geometric standard deviation for a distribution of geometric mean exposure levels in individuals |
| Biological half-life | Biological half-life in hours |
| Exposure period of interest | Duration of the toxicologically relevant period of exposure in days |
| Maximum number of samples that can be collected | The maximum number of urine samples that can realistically be collected from participants |
| Timing of sample collection |
|
| Standardization for urine dilution |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verner, M.-A.; Salame, H.; Housand, C.; Birnbaum, L.S.; Bouchard, M.F.; Chevrier, J.; Aylward, L.L.; Naiman, D.Q.; LaKind, J.S. How Many Urine Samples Are Needed to Accurately Assess Exposure to Non-Persistent Chemicals? The Biomarker Reliability Assessment Tool (BRAT) for Scientists, Research Sponsors, and Risk Managers. Int. J. Environ. Res. Public Health 2020, 17, 9102. https://doi.org/10.3390/ijerph17239102
Verner M-A, Salame H, Housand C, Birnbaum LS, Bouchard MF, Chevrier J, Aylward LL, Naiman DQ, LaKind JS. How Many Urine Samples Are Needed to Accurately Assess Exposure to Non-Persistent Chemicals? The Biomarker Reliability Assessment Tool (BRAT) for Scientists, Research Sponsors, and Risk Managers. International Journal of Environmental Research and Public Health. 2020; 17(23):9102. https://doi.org/10.3390/ijerph17239102
Chicago/Turabian StyleVerner, Marc-André, Hassan Salame, Conrad Housand, Linda S. Birnbaum, Maryse F. Bouchard, Jonathan Chevrier, Lesa L. Aylward, Daniel Q. Naiman, and Judy S. LaKind. 2020. "How Many Urine Samples Are Needed to Accurately Assess Exposure to Non-Persistent Chemicals? The Biomarker Reliability Assessment Tool (BRAT) for Scientists, Research Sponsors, and Risk Managers" International Journal of Environmental Research and Public Health 17, no. 23: 9102. https://doi.org/10.3390/ijerph17239102
APA StyleVerner, M.-A., Salame, H., Housand, C., Birnbaum, L. S., Bouchard, M. F., Chevrier, J., Aylward, L. L., Naiman, D. Q., & LaKind, J. S. (2020). How Many Urine Samples Are Needed to Accurately Assess Exposure to Non-Persistent Chemicals? The Biomarker Reliability Assessment Tool (BRAT) for Scientists, Research Sponsors, and Risk Managers. International Journal of Environmental Research and Public Health, 17(23), 9102. https://doi.org/10.3390/ijerph17239102






