UV-C Irradiation as a Tool to Reduce Biofilm Growth on Pompeii Wall Paintings
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical-Physical Analysis (XRF)
2.2. Cultivation of Algae
2.3. UV-C Treatments
2.4. Light Microscopy and Cell Count
2.5. Absorption of Main Photosynthetic Pigments
2.6. Colorimetric Analyses
3. Results
3.1. XRF Analyses of the Pigments
3.2. Effect of UV-C on Artificial Biofilm and Control Test Samples
3.3. Measurement of Chlorophyll Concentration
3.4. Colorimetric Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caneva, G.; Nugari, M.P.; Salvadori, O. La Biologia Vegetale per i Beni Culturali Vol. I, 2nd ed.; Nardini Editore: Firenze, Italy, 2007. [Google Scholar]
- Cennamo, P.; Montuori, N.; Trojsi, G.; Fatigati, G.; Moretti, A. Biofilms in churches built in grottoes. Sci. Total Environ. 2016, 543, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Borderie, F.; Těte, N.; Cailhol, D.; Alaoui-Sehmer, L.; Bousta, F.; Rieffel, D.; Aleya, L.; Alaoui-Sossé, B. Factors driving epilithic algal colonization in show caves and new insights into combating biofilm development with UV-C treatment. Sci. Total Environ. 2014, 484, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Mora, P. Causes of Deterioration of Mural Paintings; International Centre for the Study of the Preservation and the Restoration of Cultural Property: Roma, Italy, 1964. [Google Scholar]
- Borderie, F.; Alaoui-Sehmer, L.; Naoufal, R.; Bousta, F.; Orial, G.; Rieffel, D.; Alaoui-Sossé, B. UV-C irradiation as a tool to eradicate algae in caves. Int. Biodeterior. Biodegrad. 2011, 65, 579–584. [Google Scholar] [CrossRef]
- Pfendler, S.; Borderie, F.; Bousta, F.; Alaoui-Sosse, L.; Alaoui-Sosse, B.; Aleya, L. Comparison of biocides, allelopathic substances and UV-C a streatments for biofilm proliferation on heritage monuments. J. Cult. Herit. 2018, 33, 117–124. [Google Scholar] [CrossRef]
- Cappitelli, F.; Cattò, C.; Villa, F. The Controlof Cultural Heritage MicrobialDeterioration. Microorganisms 2020, 8, 1542. [Google Scholar] [CrossRef]
- Bischoff, H.W.; Bold, H.C. Some soil algae from Enchanted Rock and related algal species. In Phycological Studies IV; No. 6318; University of Texas: Austin, TX, USA, 1963; pp. 1–95. [Google Scholar]
- Castenholz, R.W. Culturing methods for Cyanobacteria. Methods Enzymol. 1988, 167, 68–93. [Google Scholar]
- Cennamo, P.; Caputo, P.; Giorgio, A.; Moretti, A.; Pasquino, N. Biofilms on Tuff Stones at Historical Sites: Identification and Removal by Non thermal effects of radiofrequencies. Microbial Ecol. 2013, 66, 659–668. [Google Scholar] [CrossRef]
- Carfagna, S.; Lanza, N.; Salbitani, G.; Basile, A.; Sorbo, S.; Vona, V. Physiological and morphological responses of Lead or Cadmium exposed Chlorella sorokiniana 211–8K (Chlorophyceae). SpringerpPlus 2013, 2, 147–154. [Google Scholar] [CrossRef]
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulae, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Nassau, K. Color for Science, Art and Technology; Elsevier Science: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Johnston-Feller, R. Color science in the examination of museum objects. In Nondestructive Procedures; The Getty Conservation Institute: Los Angeles, CA, USA, 2001. [Google Scholar]
- Luo, M.R.; Cui, G.; Rigg, B. The development of the CIE 2000 colour-difference formula CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Rajczy, M. The flora of Hungarian caves. Karszt és Barlang 1989, 69–72. [Google Scholar]
- Kubešová, S. Bryophyte flora at lamps in public caves in the Moravian Karst (Czech Republic). Acta Mus. Moraviae Sci. Boil. 2001, 86, 195–202. [Google Scholar]
- Cennamo, P.; Marzano, C.; Ciniglia, C.; Pinto, G.; Cappelletti, P.; Caputo, P.; Pollio, A. A survey of the algal flora of anthropogenic caves of Campi Flegrei (Naples, Italy) archeological district. J. Cave Karst Stud. 2012, 74, 243–250. [Google Scholar] [CrossRef]
- Beveridge, T.; Makin, S.A.; Kadurugamuwa, J.L.; Zusheng, L. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 1997, 20, 291–303. [Google Scholar] [CrossRef] [PubMed]
- La Farge, C.; Williams, K.H.; England, J.H. Regeneration of Little Ice Age bryophytes emerging from a polar glacier with implications of totipotency in extreme environments. Proc. Natl. Acad. Sci. USA 2013, 110, 9839–9844. [Google Scholar] [CrossRef] [PubMed]
- Warscheid, T.; Braams, J. Biodeterioration of Stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Stapleton, A.E. Ultraviolet radiation and plants: Burning question. Plant Cell 1992, 4, 1353–1358. [Google Scholar] [CrossRef]
- Takahashi, A.; Shibata, N.; Nishikawa, S.; Ohnishi, K.; Ishiokan, N.; Ohnishi, T. UV-B light induces an adaptative response to UV-C exposure via photoreactivation activity in Euglena gracilis. Photochem. Photobiol. Sci. 2006, 5, 467–471. [Google Scholar] [CrossRef]
- Hermann, H.; Häder, D.P.; Köfferlein, M.; Seidlitz, H.K.; Ghetti, F. Effects of UV radiation on photosynthesis of phytoplankton exposed to solar simulator light. J. Photochem. Photobiol. 1996, 34, 21–28. [Google Scholar] [CrossRef]
- Zvezdanović, J.; Cvetić, T.; Veljović-Jovanović, S.; Marković, D. Chlorophyll bleaching by UV-irradiation in vitro and in situ: Absorption and fluorescence studies. Radiat. Phys. Chem. 2009, 78, 25–32. [Google Scholar] [CrossRef]
- Schwartz, S.J.; Von Elbe, J.H. Kinetics of chlorophyll degradation to pyropheophytin in vegetables. J. Food Sci. 1983, 48, 1303–1306. [Google Scholar] [CrossRef]
- Chairat, B.; Nutthachai, P.; Varit, S. Effect of UV-C treatment on chlorophyll degradation, antioxidant enzyme activities and senescence in Chinese kale (Brassica oleracea var. alboglabra). Int. Food Res. J. 2013, 20, 623–628. [Google Scholar]
- Sarghein, S.H.; Carapetian, J.; Khara, J. Effects of UV-radiation on photosynthetic pigments and UV absorbing compounds in Capsicum longum (L.). Int. J. Bot. 2008, 4, 486–490. [Google Scholar] [CrossRef]
- Tinzl, C.; Oldenbourg, C.; Petersen, K.; Fricke-Tinzl, H.; Hilge, C.; Katkov, M. UV-C-irradiation for removal and control of lichen growths from wall paintings and stone sculptures: An alternative to using biocides? In Restauratorenblätter (Bd. 16); Koller, M., Prandtstetten, R., Eds.; Mayer & Comp.: Wien, Austria, 1995; pp. 127–138. [Google Scholar]
- Stewart, J.; More, A.; Simpson, P. The use of ultraviolet irradiation to control microbiological growth on mosaic pavements: A preliminary assessment at Newport Roman Villa. Unpublished.
- Borderie, F.; Alaoui-Sossé, B.; Aleya, L. Heritage material and biofouling mitigation through UV-C irradiation in show caves: State-of-the-art practices and future challenges. Environ. Sci. Pollut. R 2015, 22, 4144–4172. [Google Scholar] [CrossRef] [PubMed]


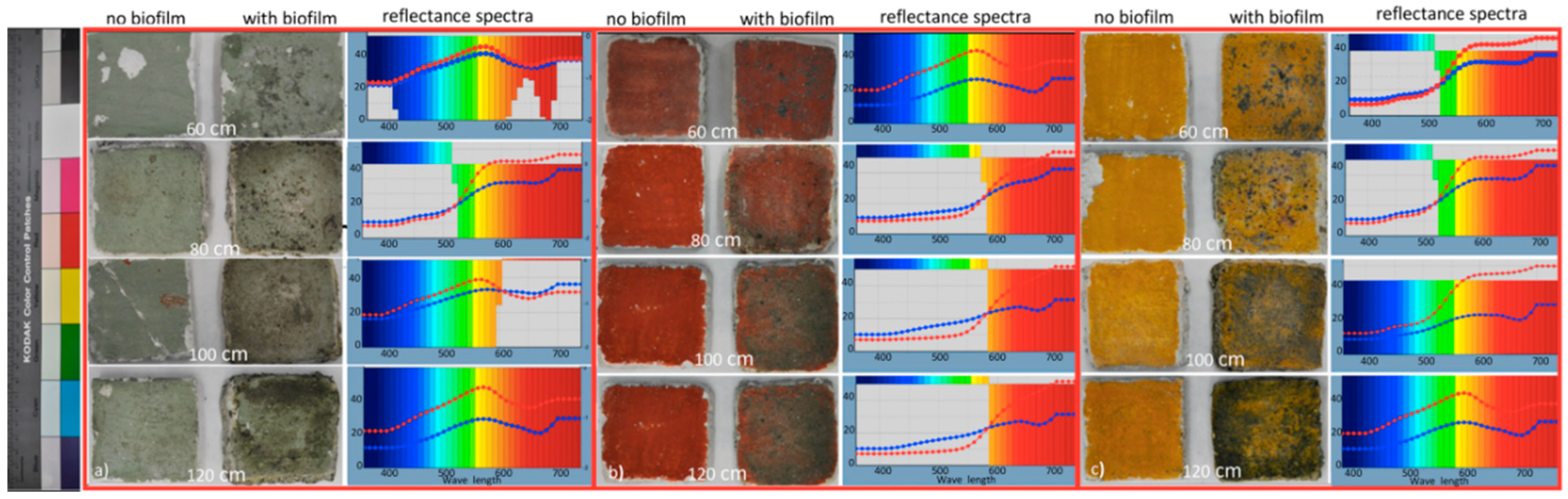
| Sample | ∆L* | ∆a* | ∆b* | ∆E |
|---|---|---|---|---|
| Green | ||||
| green 60 cm | −2.40 | 1.10 | 0.01 | 2.64 |
| green 80 cm | −1.40 | 2.70 | 0.35 | 3.14 |
| green 100 cm | −3.51 | 5.23 | 2.08 | 6.63 |
| green 120 cm | −12.69 | 1.22 | 3.68 | 13.27 |
| Red | ||||
| red 60 cm | 5.20 | −1.47 | −1.38 | 5.57 |
| red 80 cm | 1.31 | −11.96 | −7.28 | 14.06 |
| red 100 cm | 3.38 | −21.30 | −9.5 | 23.56 |
| red 120 cm | 1.33 | −17.15 | −4.89 | 17.88 |
| Yellow | ||||
| yellow 60 cm | −5.20 | −5.69 | −15.01 | 16.95 |
| yellow 80 cm | −6.15 | −6.09 | −16.23 | 18.39 |
| yellow 100 cm | −15.38 | −9.09 | −12.87 | 21.82 |
| yellow 120 cm | −12.69 | −1.22 | −3.68 | 13.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cennamo, P.; Ebbreo, M.; Quarta, G.; Trojsi, G.; De Rosa, A.; Carfagna, S.; Caputo, P.; Martelli Castaldi, M. UV-C Irradiation as a Tool to Reduce Biofilm Growth on Pompeii Wall Paintings. Int. J. Environ. Res. Public Health 2020, 17, 8392. https://doi.org/10.3390/ijerph17228392
Cennamo P, Ebbreo M, Quarta G, Trojsi G, De Rosa A, Carfagna S, Caputo P, Martelli Castaldi M. UV-C Irradiation as a Tool to Reduce Biofilm Growth on Pompeii Wall Paintings. International Journal of Environmental Research and Public Health. 2020; 17(22):8392. https://doi.org/10.3390/ijerph17228392
Chicago/Turabian StyleCennamo, Paola, Marta Ebbreo, Giovanni Quarta, Giorgio Trojsi, Alessandro De Rosa, Simona Carfagna, Paolo Caputo, and Monica Martelli Castaldi. 2020. "UV-C Irradiation as a Tool to Reduce Biofilm Growth on Pompeii Wall Paintings" International Journal of Environmental Research and Public Health 17, no. 22: 8392. https://doi.org/10.3390/ijerph17228392
APA StyleCennamo, P., Ebbreo, M., Quarta, G., Trojsi, G., De Rosa, A., Carfagna, S., Caputo, P., & Martelli Castaldi, M. (2020). UV-C Irradiation as a Tool to Reduce Biofilm Growth on Pompeii Wall Paintings. International Journal of Environmental Research and Public Health, 17(22), 8392. https://doi.org/10.3390/ijerph17228392





