Current Pharmacological Treatment of Type 2 Diabetes Mellitus in Undocumented Migrants: Is It Appropriate for the Phenotype of the Disease?
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Pharmacological Treatment
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Patient Characteristics
3.2. Patient Clusters
3.3. Comparison between Italians and Undocumented Migrants
3.4. Pharmacological Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas 2019, 9th ed.; IDF: Brussels, Belgium, 2019. [Google Scholar]
- Ogurtsova, K.; Fernandes, J.D.R.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.; Cavan, D.; Shaw, J.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Gunaid, A.A.; Al-Kebsi, M.M.; Bamashmus, M.A.; Al-Akily, S.A.; Al-Radaei, A.N. Clinical phenotyping of newly diagnosed type 2 diabetes in Yemen. BMJ Open Diabetes Res. Care 2018, 6, e000587. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Karajamaki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Clustering of adult-onset diabetes into novel subgroups guides therapy and improves prediction of outcome. Lancet Diab. Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- NICE Guideline. Type 2 Diabetes in Adults: Management; NICE Guide 28. NG28: London/Manchester, UK, 2019; pp. 1–57. [Google Scholar]
- American Diabetes Association 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care 2019, 43, S98–S110. [Google Scholar] [CrossRef]
- Arno, C.R. Osservatorio ARNO Diabete; ARNO: Firenze, Italy, 2019. [Google Scholar]
- Chilunga, F.P.; Henneman, P.; Meeks, K.A.; Beune, E.; Requena-Méndez, A.; Smeeth, L.; Addo, J.; Bahendeka, S.; Danquah, I.; Schulze, M.B.; et al. Prevalence and determinants of type 2 diabetes among lean African migrants and non-migrants: The RODAM study. J. Glob. Heal. 2019, 9, 020426. [Google Scholar] [CrossRef]
- Lee, S.; Lacy, M.E.; Jankowich, M.; Correa, A.; Wu, W.-C. Association between obesity phenotypes of insulin resistance and risk of type 2 diabetes in African Americans: The Jackson Heart Study. J. Clin. Transl. Endocrinol. 2020, 19, 100210. [Google Scholar] [CrossRef]
- Williams, J.; Allen, L.; Wickramasinghe, K.; Mikkelsen, B.; Roberts, N.; Townsend, N. A systematic review of associations between non-communicable diseases and socioeconomic status within low- and lower-middle-income countries. J. Glob. Heal. 2018, 8, 020409. [Google Scholar] [CrossRef]
- Wang, K.; Hendrickson, Z.M.; Brandt, C.A.; Nunez-Smith, M. The relationship between non-permanent migration and non-communicable chronic disease outcomes for cancer, heart disease and diabetes a systematic review. BMC Public Heal. 2019, 19, 405. [Google Scholar] [CrossRef]
- Quiñones, A.R.; Botoseneanu, A.; Markwardt, S.; Nagel, C.L.; Newsom, J.T.; Dorr, D.A.; Allore, H.G. Racial/ethnic differences in multimorbidity development and chronic disease accumulation for middle-aged adults. PLoS ONE 2019, 14, e0218462. [Google Scholar] [CrossRef]
- Agyemang, C.; Meeks, K.; Beune, E.; Owusu-Dabo, E.; Mockenhaupt, F.P.; Addo, J.; Aikins, A.D.G.; Bahendeka, S.; Danquah, I.; Schulze, M.B.; et al. Obesity and type 2 diabetes in sub-Saharan Africans—Is the burden in today’s Africa similar to African migrants in Europe? The RODAM study. BMC Med. 2016, 14, 166. [Google Scholar] [CrossRef]
- Commodore-Mensah, Y.; Selvin, E.; Aboagye, J.; Turkson-Ocran, R.-A.; Li, X.; Himmelfarb, C.D.; Ahima, R.S.; Cooper, L.A. Hypertension, overweight/obesity, and diabetes among immigrants in the United States: An analysis of the 2010–2016 National Health Interview Survey. BMC Public Heal. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Raza, Q.; Nicolaou, M.; Dijkshoorn, H.; Seidell, J.C. Comparison of general health status, myocardial infarction, obesity, diabetes, and fruit and vegetable intake between immigrant Pakistani population in the Netherlands and the local Amsterdam population. Ethn. Heal. 2016, 22, 551–564. [Google Scholar] [CrossRef]
- Fedeli, U.; Casotto, V.; Ferroni, E.; Saugo, M.; Targher, G.; Zoppini, G. Prevalence of diabetes across different immigrant groups in North-eastern Italy. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bernardi, D.; Miccoli, R.; Rossi, E.; Vaccaro, O.; De Rosa, M.; Bonora, E.; Bruno, G. Under-treatment of migrants with diabetes in a universalistic health care system: The ARNO Observatory. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 393–399. [Google Scholar] [CrossRef]
- Fiorini, G.; Cerri, C.; Bini, S.; Rigamonti, A.E.; Perlini, S.; Marazzi, N.; Sartorio, A.; Cella, S.G. The burden of chronic noncommunicable diseases in undocumented migrants: A 1-year survey of drugs dispensation by a non–governmental organization in Italy. Public Heal. 2016, 141, 26–31. [Google Scholar] [CrossRef]
- Fiorini, G.; Cerri, C.; Rigamonti, A.E.; Bini, S.; Marazzi, N.; Sartório, A.; Cella, S.G. Gender and age related differences in the use of medicines for chronic diseases among undocumented migrants. Int. J. Migr. Heal. Soc. Care 2018, 14, 221–229. [Google Scholar] [CrossRef]
- Atkinson, M.A.; MacLaren, N.K. Islet cell autoantigens in insulin-dependent diabetes. J. Clin. Investig. 1993, 92, 1608–1616. [Google Scholar] [CrossRef]
- Rossi, M.C.; Lucisano, G.; Comaschi, M.; Coscelli, C.; Cucinotta, D.; Di Blasi, P.; Bader, G.; Pellegrini, F.; Valentini, U.; Vespasiani, G.; et al. Quality of Diabetes Care Predicts the Development of Cardiovascular Events: Results of the AMD-QUASAR Study. Diabetes Care 2011, 34, 347–352. [Google Scholar] [CrossRef]
- Methodology WCC for DS. Guidelines for ATC Classification and DDD Assignment, 2020; WHO Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2019. [Google Scholar]
- Greenacre, M.J. Correspondence Analysis in Practice; Academic Press: London, UK, 1993; p. 195. [Google Scholar]
- Benzecri, J.P.L.; Benzecri, J.P. L’analyse des Données; Pensée: Paris, France, 2018. [Google Scholar]
- Kirk, I.K.; Simon, C.; Banasik, K.; Holm, P.C.; Haue, A.D.; Jensen, P.B.; Jensen, L.J.; Rodríguez, C.L.; Pedersen, M.K.; Eriksson, R.; et al. Linking glycemic dysregulation in diabetes to symptoms, comorbidities, and genetics through EHR data mining. eLife 2019, 8, e44941. [Google Scholar] [CrossRef]
- Nowakowska, M.; Zghebi, S.S.; Ashcroft, D.M.; Buchan, I.; Chew-Graham, C.; Holt, T.; Mallen, C.; Van Marwijk, H.; Peek, N.; Perera-Salazar, R.; et al. The comorbidity burden of type 2 diabetes mellitus: Patterns, clusters and predictions from a large English primary care cohort. BMC Med. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Cho, S.B.; Kim, S.C.; Chung, M.G. Identification of novel population clusters with different susceptibilities to type 2 diabetes and their impact on the prediction of diabetes. Sci. Rep. 2019, 9, 3329. [Google Scholar] [CrossRef]
- Adjei, D.N.; Stronks, K.; Adu, D.; Beune, E.; Meeks, K.; Smeeth, L.; Addo, J.; Owuso-Dabo, E.; Klipstein-Grobusch, K.; Mockenhaupt, F.P.; et al. Chronic kidney disease burden among African migrants in three European countries and in urban and rural Ghana: The RODAM cross-sectional study. Nephrol. Dial. Transplant. 2018, 33, 1812–1822. [Google Scholar] [CrossRef]
- Adjei, D.N.; Stronks, K.; Adu, D.; Beune, E.; Meeks, K.; Smeeth, L.; Addo, J.; Owusu-Dabo, E.; Klipstein-Grobusch, K.; Mockenhaupt, F.; et al. Cross-sectional study of association between psychosocial stressors with chronic kidney disease among migrant and non-migrant Ghanaians living in Europe and Ghana: The RODAM study. BMJ Open 2019, 9, e027931. [Google Scholar] [CrossRef]
- Magri, F.; Zerbini, F.; Gaiti, M.; Capelli, V.; Croce, L.; Bini, S.; Rigamonti, A.E.; Fiorini, G.; Cella, S.G.; Chiovato, L. Poverty and immigration as a barrier to iodine intake and maternal adherence to iodine supplementation. J. Endocrinol. Investig. 2018, 42, 435–442. [Google Scholar] [CrossRef]
- Rosano, A.; Dauvrin, M.; Buttigieg, S.C.; Ronda-Pérez, E.; Tafforeau, J.; Dias, S. Migrant’s access to preventive health services in five EU countries. BMC Heal. Serv. Res. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- LeDoux, C.; Pilot, E.; Diaz, E.; Krafft, T. Migrants’ access to healthcare services within the European Union: A content analysis of policy documents in Ireland, Portugal and Spain. Glob. Heal. 2018, 14, 57. [Google Scholar] [CrossRef]
- Fiorini, G.; Milani, S.; Pincelli, A.I.; Calella, D.; Galliani, S.; Badalamenti, S.; Rigamonti, A.E.; Marazzi, N.; Sartorio, A.; Cella, S.G. Will undocumented migrants contribute to change epidemiology, presentation and pharmacologic treatment of diabetes in Western countries? Prim. Care Diabetes 2020, 14, 21–28. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Grassi, G.; Ivanovic, B. Gender-specific therapeutic approach in arterialhypertension Challenges ahead. Pharmacol. Res. 2019, 141, 181–188. [Google Scholar] [CrossRef]
- Danquah, I.; Addo, J.; Boateng, D.; Klipstein-Grobusch, K.; Meeks, K.; Galbete, C.; Beune, E.; Bahendeka, S.; Spranger, J.; Mockenhaupt, F.P.; et al. Early-life factors are associated with waist circumference and type 2 diabetes among Ghanaian adults: The RODAM Study. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Piao, H.; Yun, J.M.; Shin, A.; Cho, B. Longitudinal Study of Diabetic Differences between International Migrants and Natives among the Asian Population. Biomol. Ther. 2019, 28, 110–118. [Google Scholar] [CrossRef]
- Fiorini, G.; Cerri, C.; Magri, F.; Chiovato, L.; Croce, L.; Rigamonti, A.E.; Sartorio, A.; Cella, S.G. Risk factors, awareness of disease and use of medications in a deprived population: Differences between indigent natives and undocumented migrants in Italy. J. Public Heal. 2019. [Google Scholar] [CrossRef]
- Hsueh, L.; Vrany, E.A.; Patel, J.S.; Hollingshead, N.A.; Hirsh, A.T.; De Groot, M.; Stewart, J.C. Associations between immigrant status and pharmacological treatments for diabetes in U.S. adults. Heal. Psychol. 2018, 37, 61–69. [Google Scholar] [CrossRef]
- Chung, J.O.; Cho, D.H.; Chung, D.J.; Chung, M.Y. Associations among Body Mass Index, Insulin Resistance, and Pancreatic ß-Cell Function in Korean Patients with New-Onset Type 2 Diabetes. Korean J. Intern. Med. 2012, 27, 66–71. [Google Scholar] [CrossRef]
- Abu Hassan, H.; Tohid, H.; Amin, R.M.; Bidin, M.B.L.; Leelavathi, M.; Omar, K. Factors influencing insulin acceptance among type 2 diabetes mellitus patients in a primary care clinic: A qualitative exploration. BMC Fam. Pr. 2013, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Pour, M.R.S.; Sadeghiyeh, T.; Hadavi, M.; Besharati, M.; Bidaki, R. The barriers against initiating insulin therapy among patients with diabetes living in Yazd, Iran. Diabetes, Metab. Syndr. Obes. Targets Ther. 2019, 12, 1349–1354. [Google Scholar] [CrossRef]
- Guerci, B.; Chanan, N.; Kaur, S.; Jasso-Mosqueda, J.G.; Lew, E. Lack of Treatment Persistence and Treatment Nonadherence as Barriers to Glycaemic Control in Patients with Type 2 Diabetes. Diabetes Ther. 2019, 10, 437–449. [Google Scholar] [CrossRef]
- Roumie, C.L.; Chipman, J.; Min, J.Y.; Hackstadt, A.J.; Hung, A.M.; Greevy, R.A.; Grijalva, C.G.; Elasy, T.; Griffin, M.R. Association of Treatment With Metformin vs Sulfonylurea With Major Adverse Cardiovascular Events Among Patients With Diabetes and Reduced Kidney Function. JAMA 2019, 322, 1167–1177. [Google Scholar] [CrossRef]
- Salourou, M.; Archontakis, S.; Sideris, S.; Parisis, I.; Siasos, G.; Oikonomou, E.; Mourouzis, K.; Sideris, K.; Stefanadis, C.; Tousoulis, D. The effect of diet, lifestyle and psychological factors in the prognosis of ischemic heart failure. Metab. Open 2019, 1, 11–18. [Google Scholar] [CrossRef]
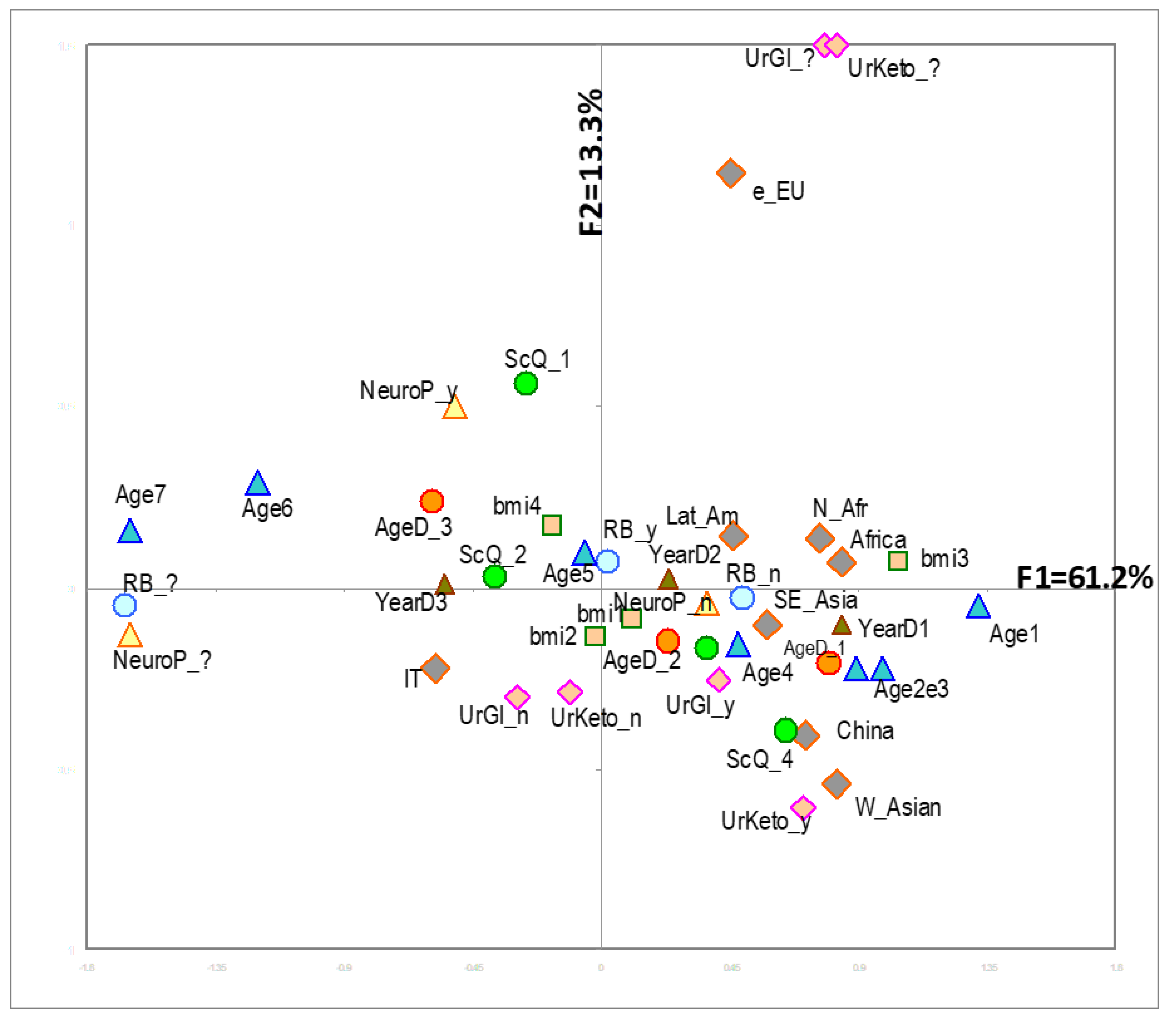


| Percentage of Contribution to the Explanation of the 3 First Factorial Axes (MCA) | Weighted Overall Contribution to the Explanation of the First 3 Axes | ||||||
|---|---|---|---|---|---|---|---|
| VARIABLE | Label | Abbreviation in Figures | % (n = 822) | F1 | F2 | F3 | All Three Axes |
| TOTAL variability explained | 61.2 | 13.3 | 8.8 | 83.3 | |||
| Sex | female | female | 39.4 | 0.9 | 0.7 | 0.0 | 0.6 |
| male | male | 60.6 | |||||
| Family history of diabetes | missing | FamD_? | 6.6 | 3.6 | 3.7 | 4.2 | 3.1 |
| NO | FamD_n | 32.0 | |||||
| YES | FamD_y | 61.4 | |||||
| Cardiovascular | missing | RFCV_? | 0.4 | 5.5 | 0.1 | 1.0 | 3.5 |
| risk factors | NO | RFCV_n | 12.2 | ||||
| YES | RFCV_y | 87.4 | |||||
| Unhealthy lifestyles | missing | RB_? | 16.4 | 17.7 | 0.1 | 0.0 | 10.9 |
| NO | RB_n | 53.2 | |||||
| YES | RB_y | 30.4 | |||||
| Glycosuria | missing | UrGl_? | 9.6 | 4.8 | 37.5 | 10.7 | 8.9 |
| NO | UrGl_n | 63.4 | |||||
| YES | UrGl_y | 27.0 | |||||
| Ketonuria | missing | UrKeto_? | 10.1 | 2.7 | 37.1 | 1.8 | 6.8 |
| NO | UrKeto_n | 87.7 | |||||
| YES | UrKeto_y | 2.2 | |||||
| Cardiovascular | NO | CVD_n | 76.8 | 2.7 | 5.1 | 2.1 | 2.5 |
| Disease | YES | CVD_y | 23.2 | ||||
| Nephropathy | missing | NephP_? | 2.2 | 1.0 | 2.5 | 9.1 | 1.7 |
| NO | NephP_n | 81.9 | |||||
| YES | NephP_y | 15.9 | |||||
| Retinopathy | missing | RetP_? | 5.0 | 1.8 | 1.5 | 10.0 | 2.2 |
| NO | RetP_n | 75.2 | |||||
| YES | RetP_y | 19.8 | |||||
| Neuropathy | missing | NeurP_? | 14.1 | 15.6 | 1.4 | 1.3 | 9.8 |
| NO | NeurP_n | 75.9 | |||||
| YES | NeurP_y | 10.0 | |||||
| Ulcers | missing | Ulc_? | 1.0 | 0.5 | 0.7 | 6.7 | 1.0 |
| NO | Ulc_n | 92.9 | |||||
| YES | Ulc_y | 6.1 | |||||
| Age (years) | ≤29 | Age1 | 1.5 | 19.6 | 1.6 | 6.9 | 12.8 |
| 30–39 | Age2 | 3.6 | |||||
| 40–49 | Age3 | 13.6 | |||||
| 50–59 | Age4 | 29.3 | |||||
| 60–69 | Age5 | 29.3 | |||||
| 70–79 | Age6 | 16.1 | |||||
| ≥80 | Age7 | 6.6 | |||||
| Age at Diagnosis (years) | ≤35 | AgeD1 | 18.2 | 8.6 | 1.9 | 14.9 | 6.8 |
| 36–50 | AgeD2 | 40.6 | |||||
| >50 | AgeD3 | 41.2 | |||||
| BMI | <18.5 | bmi1 | 4.7 | 2.0 | 9.6 | 1.5 | 1.5 |
| 18.5–24.9 | bmi2 | 20.1 | |||||
| 25–29.9 | bmi3 | 37.0 | |||||
| ≥30 | bmi4 | 38.2 | |||||
| Disease duration | <1 | YearD1 | 13.4 | 7.3 | 0.1 | 9.5 | 5.3 |
| (years from diagnosis) | 2–9 | YearD2 | 45.7 | ||||
| >10 | YearD3 | 40.9 | |||||
| Glycated | ≤6.5 | HbA_1 | 28.1 | 0.9 | 1.3 | 11.3 | 1.7 |
| haemoglobin | 6.6–7.0 | HbA_2 | 18.2 | ||||
| (%) | >7.0 | HbA_3 | 53.7 | ||||
| Q Score | ≤10 | ScQ_1 | 15.7 | 4.8 | 3.7 | 8.9 | 4.2 |
| 11–20 | ScQ_2 | 41.1 | |||||
| 21–30 | ScQ_3 | 31.4 | |||||
| 31–40 | ScQ_4 | 11.8 | |||||
| Ethnicity | Italy | IT | 51.3 | ||||
| East Europe | e_EU | 11.6 | |||||
| North Africa | N_Afr | 7.3 | |||||
| Sub-Saharan Africa | Africa | 5.3 | |||||
| Latin America | Lat Am | 11.8 | |||||
| West Asia | W Asian | 5.7 | |||||
| China | China | 3.3 | |||||
| South-East Asia | SE Asia | 3.7 | |||||
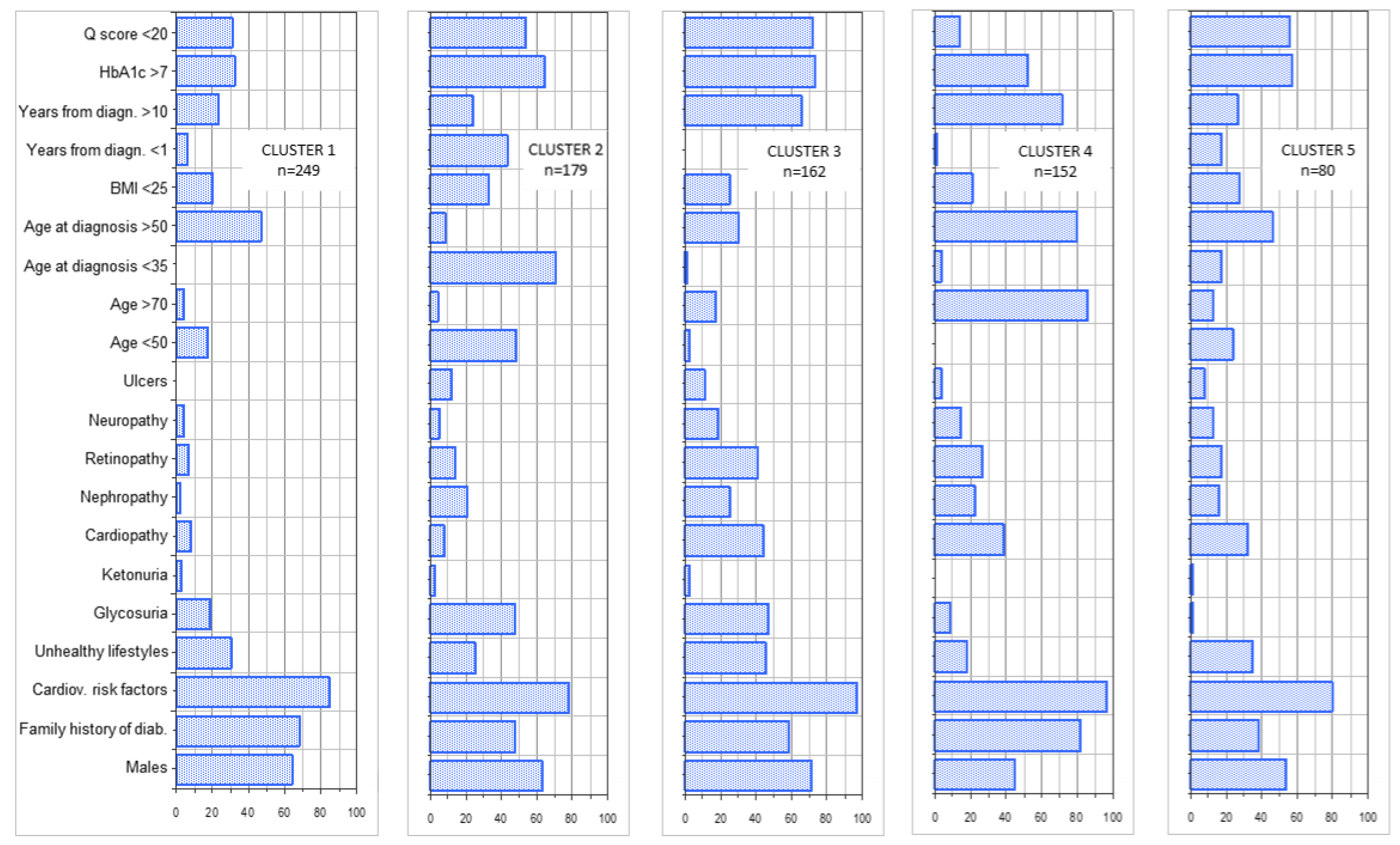
| Cluster 1 n 249 | Cluster 2 n 179 | Cluster 3 n 162 | Cluster 4 n 152 | Cluster 5 n 80 | |
|---|---|---|---|---|---|
| % | % | % | % | % | |
| Fast acting insulin | 16.5 | 26.3 | 29.6 | 35.5 | 27.5 |
| Long acting | 30.1 | 36.9 | 53.7 | 52.6 | 37.5 |
| Metformin | 77.11 | 66.48 | 67.28 | 63.82 | 71.25 |
| SGLPT 2 inhibitors | 25.3 | 17.3 | 28.4 | 18.4 | 8.7 |
| Other antidiabetic agents | 24.1 | 25.7 | 24.7 | 28.9 | 30.0 |
| Antihypertensives | 55.8 | 40.9 | 67.9 | 82.2 | 62.5 |
| Lipid lowering drugs | 48.6 | 25.7 | 62.3 | 79.6 | 35.0 |
| Anti-platelet agents | 21.3 | 11.7 | 48.1 | 67.7 | 26.2 |
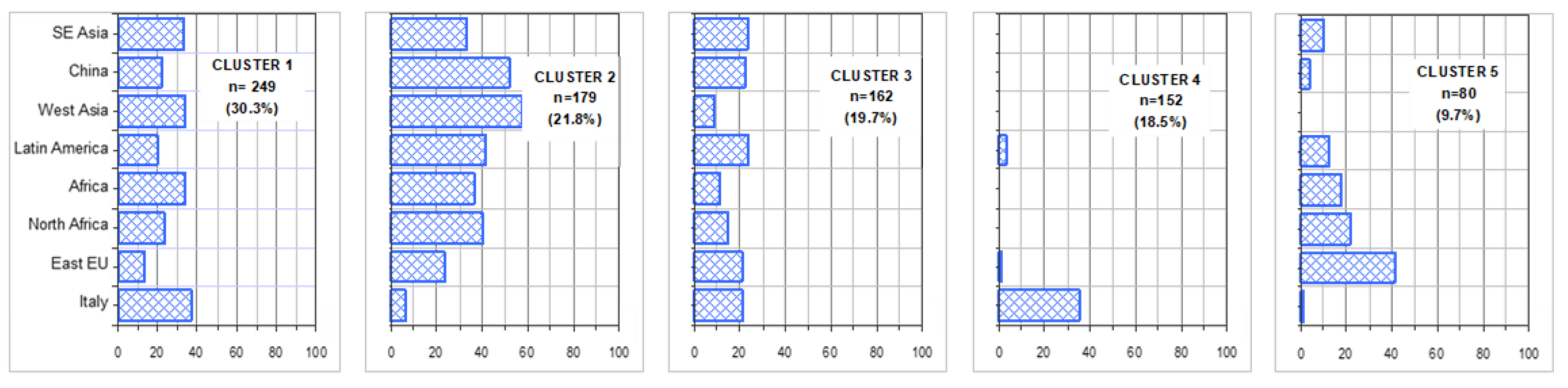
| Italy | East Europe | North Africa | Africa | Latin America | West Asia | China | South-East Asia | |
|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | |
| Fast acting insulin | 24.6 | 26.3 | 31.7 | 25.0 | 26.8 | 27.7 | 33.3 | 16.7 |
| Long acting insulin | 48.1 | 32.6 | 36.7 | 43.2 | 29.9 | 31.9 | 48.1 | 20.0 |
| Metformin | 75.6 | 69.5 | 51.7 | 59.1 | 69.1 | 70.2 | 29.6 | 80.0 |
| SGLPT 2 inhibitors | 29.6 | 8.4 | 5.0 | 13.6 | 17.5 | 21.3 | 7.4 | 13.3 |
| Other antidiabetic agents | 22.7 | 29.5 | 40.0 | 20.4 | 32.0 | 36.2 | 14.8 | 16.7 |
| Antihypertensives | 72.7 | 60.0 | 51.7 | 43.2 | 41.2 | 38.3 | 33.3 | 53.3 |
| Lipid lowering drugs | 76.1 | 36.8 | 15.0 | 25.0 | 24.7 | 14.9 | 18.5 | 16.7 |
| Anti-platelet agents | 49.3 | 35.8 | 11.7 | 11.4 | 9.3 | 6.4 | 25.9 | 10.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorini, G.; Cortinovis, I.; Corrao, G.; Franchi, M.; Pincelli, A.I.; Perotti, M.; Rigamonti, A.E.; Sartorio, A.; Cella, S.G. Current Pharmacological Treatment of Type 2 Diabetes Mellitus in Undocumented Migrants: Is It Appropriate for the Phenotype of the Disease? Int. J. Environ. Res. Public Health 2020, 17, 8169. https://doi.org/10.3390/ijerph17218169
Fiorini G, Cortinovis I, Corrao G, Franchi M, Pincelli AI, Perotti M, Rigamonti AE, Sartorio A, Cella SG. Current Pharmacological Treatment of Type 2 Diabetes Mellitus in Undocumented Migrants: Is It Appropriate for the Phenotype of the Disease? International Journal of Environmental Research and Public Health. 2020; 17(21):8169. https://doi.org/10.3390/ijerph17218169
Chicago/Turabian StyleFiorini, Gianfrancesco, Ivan Cortinovis, Giovanni Corrao, Matteo Franchi, Angela Ida Pincelli, Mario Perotti, Antonello Emilio Rigamonti, Alessandro Sartorio, and Silvano Gabriele Cella. 2020. "Current Pharmacological Treatment of Type 2 Diabetes Mellitus in Undocumented Migrants: Is It Appropriate for the Phenotype of the Disease?" International Journal of Environmental Research and Public Health 17, no. 21: 8169. https://doi.org/10.3390/ijerph17218169
APA StyleFiorini, G., Cortinovis, I., Corrao, G., Franchi, M., Pincelli, A. I., Perotti, M., Rigamonti, A. E., Sartorio, A., & Cella, S. G. (2020). Current Pharmacological Treatment of Type 2 Diabetes Mellitus in Undocumented Migrants: Is It Appropriate for the Phenotype of the Disease? International Journal of Environmental Research and Public Health, 17(21), 8169. https://doi.org/10.3390/ijerph17218169







