Association of Allelic Variants of the Reelin Gene with Autistic Spectrum Disorder: A Systematic Review and Meta-Analysis of Candidate Gene Association Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Bibliographic Search and Data Extraction
2.3. Statistical Analysis
3. Results
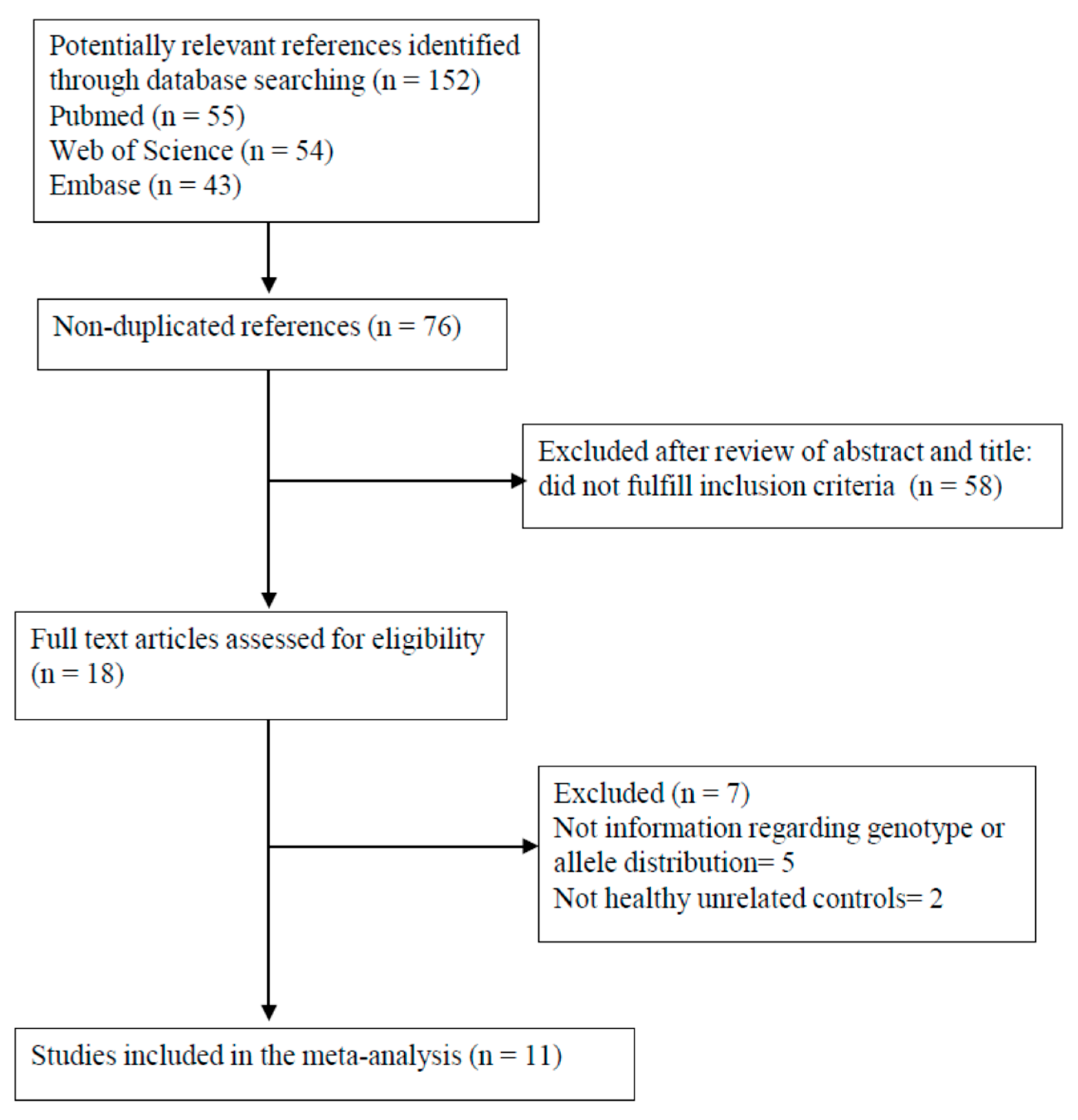
3.1. Study Identification and Selection
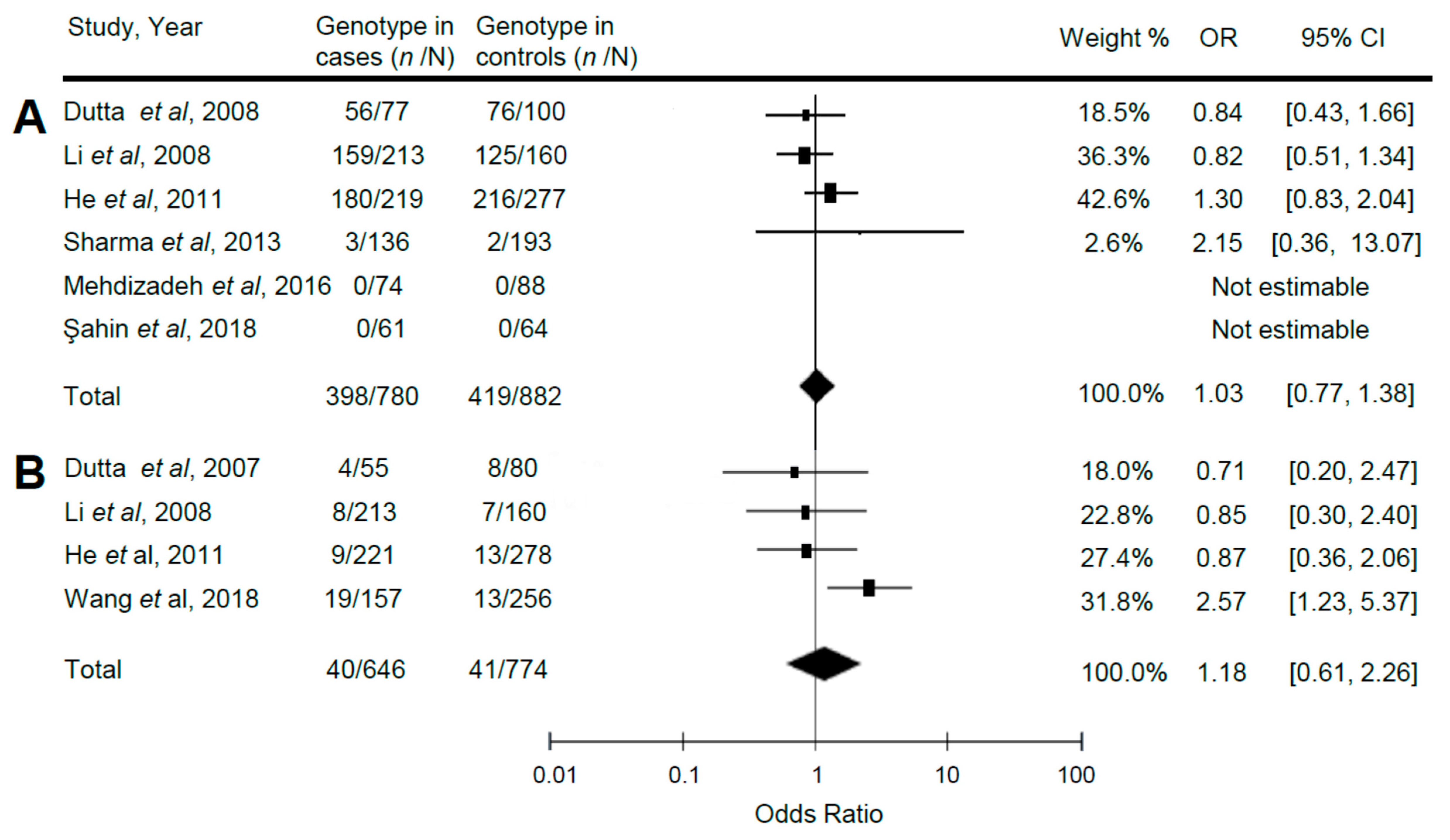
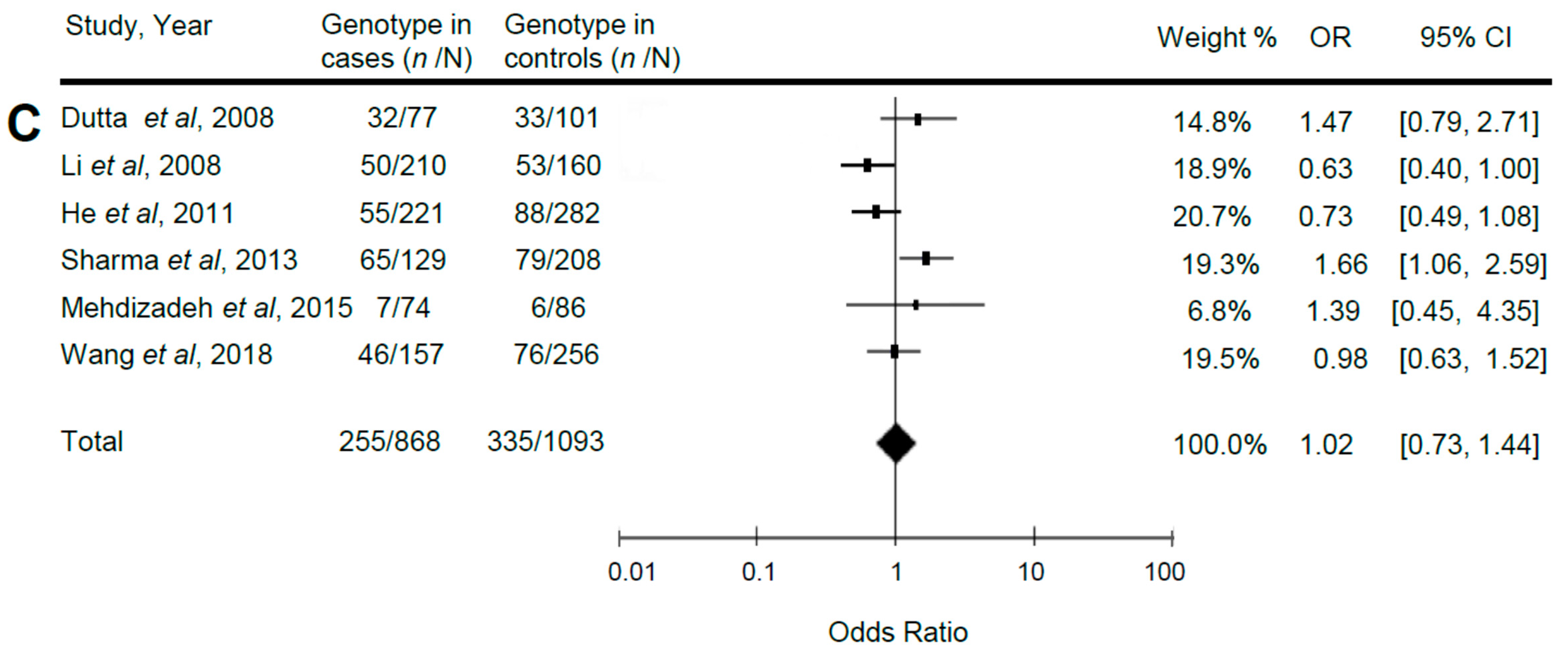
3.2. Relationship of Reelin Gene Polymorphisms with ASD
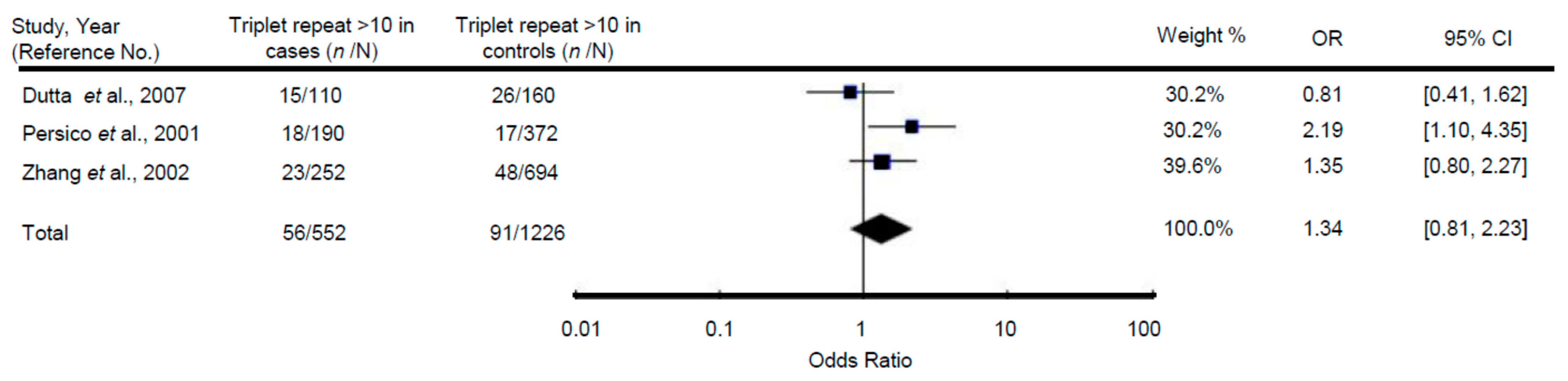
3.3. Relationship of Polymorphic Trinucleotide Repeat (CGG/GCC) within the Reelin Gene with ASD
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elsabbagh, M.; Divan, G.; Koh, Y.J.; Kim, Y.S.; Kauchali, S.; Marcín, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Manning-Courtney, P.; Murray, D.; Currans, K.; Johnson, H.; Bing, N.; Kroeger-Geoppinger, K.; Sorensen, R.; Bass, J.; Reinhold, J.; Johnson, A.; et al. Autism spectrum disorders. Curr. Probl. Pediatr. Adolesc. Health Care 2013, 43, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Croen, L.; Daniels, J.; Fallin, M.D.; Ladd-Acosta, C.; Lee, B.K.; Park, B.Y.; Snyder, N.W.; Schendel, D.; Volk, H.; et al. The Changing Epidemiology of Autism Spectrum Disorders. Annu Rev. Public Health 2017, 38, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Pettygrove, S.; Meaney, F.J.; Mancilla, K.; Gotschall, K.; Kessler, D.B.; Grebe, T.A.; Cunniff, C. Prevalence of autism spectrum disorders in Hispanic and non-Hispanic white children. Pediatrics 2012, 129, e629–e635. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Scott, F.J.; Allison, C.; Williams, J.; Bolton, P.; Matthews, F.E.; Brayne, C. Prevalence of autism-spectrum conditions: UK school-based population study. Br. J. Psychiatry 2009, 194, 500–509. [Google Scholar] [CrossRef]
- Narzisi, A.; Posada, M.; Barbieri, F.; Chericoni, N.; Ciuffolini, D.; Pinzino, M.; Romano, R.; Scattoni, M.L.; Tancredi, R.; Calderoni, S.; et al. Prevalence of Autism Spectrum Disorder in a large Italian catchment area: A school-based population study within the ASDEU project. Epidemiol. Psychiatr. Sci. 2020, 29, e5. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Allison, C. A review of the prevalence of Autism Spectrum Disorder in Asia. Res. Autism Spectr. Disord. 2010, 4, 156–167. [Google Scholar] [CrossRef]
- Qiu, S.; Lu, Y.; Li, Y.; Shi, J.; Cui, H.; Gu, Y.; Li, Y.; Zhong, W.; Zhu, X.; Liu, Y.; et al. Prevalence of autism spectrum disorder in Asia: A systematic review and meta-analysis. Psychiatry Res. 2020, 284, 112679. [Google Scholar] [CrossRef]
- Olusanya, B.O.; Davis, A.C.; Wertlieb, D.; Boo, N.Y.; Nair, M.K.C.; Halpern, R.; Kuper, H.; Breinbauer, C.; De Vries, P.J.; Gladstone, M.; et al. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Glob. Health 2018, 6, e1100–e1121. [Google Scholar] [CrossRef]
- Howlin, P.; Goode, S.; Hutton, J.; Rutter, M. Adult outcome for children with autism. J. Child. Psychol. Psychiatry 2004, 45, 212–229. [Google Scholar] [CrossRef]
- Gordon-Lipkin, E.; Marvin, A.R.; Law, J.K.; Lipkin, P.H. Anxiety and Mood Disorder in Children with Autism Spectrum Disorder and ADHD. Pediatrics 2018, 141, e20171377. [Google Scholar] [CrossRef]
- Lung, F.W.; Shu, B.C.; Chiang, T.L.; Lin, S.J. Prevalence of bullying and perceived happiness in adolescents with learning disability, intellectual disability, ADHD, and autism spectrum disorder: In the Taiwan Birth Cohort Pilot Study. Medicine (Baltimore) 2019, 98, e14483. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.E.; Law, J.K.; Yenokyan, G.; McGready, J.; Kaufmann, W.E.; Law, P.A. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch. pediatrics Adolesc. Med. 2009, 163, 907–914. [Google Scholar] [CrossRef]
- Lamb, J.A.; Parr, J.R.; Bailey, A.J.; Monaco, A.P. Autism: In search of susceptibility genes. Neuromolecular Med. 2002, 2, 11–28. [Google Scholar] [CrossRef]
- Matoba, N.; Liang, D.; Sun, H.; Aygün, N.; McAfee, J.C.; Davis, J.E.; Raffield, L.M.; Qian, H.; Piven, J.; Li, Y.; et al. Common genetic risk variants identified in the SPARK cohort support DDHD2 as a candidate risk gene for autism. Transl. Psychiatry 2020, 10, 265. [Google Scholar] [CrossRef]
- Yonan, A.L.; Alarcon, M.; Cheng, R.; Magnusson, P.K.; Spence, S.J.; Palmer, A.A.; Grunn, A.; Juo, S.H.H.; Terwilliger, J.D.; Liu, J.; et al. A genomewide screen of 345 families for autism-susceptibility loci. Am. J. Hum. Genet. 2003, 73, 886–897. [Google Scholar] [CrossRef]
- Barrett, S.; Beck, J.C.; Bernier, R.; Bisson, E.; Braun, T.A.; Casavant, T.L.; Childress, D.; Folstein, S.E.; Garcia, M.; Gardiner, M.B.; et al. An autosomal genomic screen for autism. Am. J. Med. Genet.—Neuropsychiatr. Genet. 2001, 105, 609–615. [Google Scholar] [PubMed]
- Fatemi, S.H.; Snow, A.V.; Stary, J.M.; Araghi-Niknam, M.; Reutiman, T.J.; Lee, S.; Brooks, A.I.; Pearce, D.A. Reelin signaling is impaired in autism. Biol. Psychiatry 2005, 57, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Persico, A.M.; D’Agruma, L.; Maiorano, N.; Totaro, A.; Militerni, R.; Bravaccio, C.; Wassink, T.H.; Schneider, C.; Melmed, R.; Trillo, S.; et al. Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol. Psychiatry 2001, 6, 150–159. [Google Scholar] [CrossRef]
- Serajee, F.J.; Zhong, H.; Mahbubul Huq, A.H. Association of Reelin gene polymorphisms with autism. Genomics 2006, 87, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.R.; Arieff, Z.; Gameeldien, H.; Davids, M.; Kaur, M.; van der Merwe, L. Association analysis of two single-nucleotide polymorphisms of the RELN gene with autism in the South African population. Genet. Test. Mol. Biomark. 2013, 17, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sinha, S.; Ghosh, S.; Chatterjee, A.; Ahmed, S.; Usha, R. Genetic analysis of reelin gene (RELN) SNPs: No association with autism spectrum disorder in the Indian population. Neurosci. Lett. 2008, 441, 56–60. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xun, G.; Xia, K.; Hu, Z.; Lv, L.; Deng, Z.; Zhao, J. No significant association between RELN polymorphism and autism in case-control and familybased association study in Chinese Han population. Psychiatry Res. 2011, 187, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Cochrane Training: Review Manager (RevMan). Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 16 August 2020).
- Bax, L.; Yu, L.M.; Ikeda, N.; Tsuruta, H.; Moons, K.G. Development and validation of MIX: Comprehensive free software for meta-analysis of causal research data. BMC Med. Res. Methodol. 2006, 6, 50. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Yu, J.; Fan, Y.; Liu, D.; Zhou, W.; Shi, T. Residential Radon and Histological Types of Lung Cancer: A Meta-Analysis of Case‒Control Studies. Int. J. Environ. Res. Public Health 2020, 17, 1457. [Google Scholar] [CrossRef]
- Bonora, E.; Beyer, K.S.; Lamb, J.A.; Parr, J.R.; Klauck, S.M.; Benner, A.; Paolucci, M.; Abbott, A.; Ragoussis, I.; Poustka, A.; et al. Analysis of reelin as a candidate gene for autism. Mol. Psychiatry 2003, 8, 885–892. [Google Scholar] [CrossRef][Green Version]
- Devlin, B.; Bennett, P.; Dawson, G.; Figlewicz, D.A.; Grigorenko, E.L.; McMahon, W.; Minshew, N.; Pauls, D.; Smith, M.; Spence, M.A.; et al. Alleles of a reelin CGG repeat do not convey liability to autism in a sample from the CPEA network. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2004, 126B, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Skaar, D.A.; Shao, Y.; Haines, J.L.; Stenger, J.E.; Jaworski, J.; Martin, E.R.; DeLong, G.R.; Moore, J.H.; McCauley, J.L.; Sutcliffe, J.S.; et al. Analysis of the RELN gene as a genetic risk factor for autism. Mol. Psychiatry 2005, 10, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Kelemenova, S.; Schmidtova, E.; Ficek, A.; Celec, P.; Kubranska, A.; Ostatnikova, D. Polymorphisms of candidate genes in Slovak autistic patients. Psychiatr. Genet. 2010, 20, 137–139. [Google Scholar] [CrossRef]
- Krebs, M.O.; Betancur, C.; Leroy, S.; Bourdel, M.C.; Gillberg, C.; Leboyer, M. Absence of association between a polymorphic GGC repeat in the 5’ untranslated region of the reelin gene and autism. Mol. Psychiatry 2002, 7, 801–804. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Nguyen, L.; Gleason, C.; Lotspeich, L.; Spiker, D.; Risch, N.; Myers, R.M. Lack of evidence for an association between WNT2 and RELN polymorphisms and autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2004, 126B, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Zhang, C.; Mundo, E.; Macciardi, F.; Grayson, D.R.; Guidotti, A.R.; Holden, J.J.A. Reelin gene alleles and susceptibility to autism spectrum disorders. Mol. Psychiatry 2002, 7, 1012–1017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dutta, S.; Guhathakurta, S.; Sinha, S.; Chatterjee, A.; Ahmed, S.; Ghosh, S.; Gangopadhyay, P.K.; Singh, M.; Usha, R. Reelin gene polymorphisms in the Indian population: A possible paternal 5’UTR-CGG-repeat-allele effect on autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 106–112. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Shao, J.; Li, R.; Qin, Y.; Xie, C.; Zhao, Z. The association analysis of RELN and GRM8 genes with autistic spectrum disorder in Chinese Han population. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147, 194–200. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Shahrokhi, H.; Adampourezare, M.; Hosseinpour, M.A.; Bonyadi, M.; Eslami, A. Association between Common Single-nucleotide Polymorphism of Reelin Gene, rs736707 (C/T) with Autism Spectrum Disorder in Iranian-Azeri Patients. Int. J. Pediatrics 2015, 3, 1065–1071. [Google Scholar]
- Mehdizadeh, L.; Hosseinpour, M.A.; Zare, M.A.; Shahrokhi, H. An Association Analysis of Reelin Gene (RELN) Exon 22 (G/C), Rs.362691, Polymorphism with Autism Spectrum Disorder among Iranian-Azeri Population. Int. J. Pediatrics 2016, 4, 2027–2033. [Google Scholar]
- Şahin, N.; Kara, M.; Kara, B.; Topal, H. Evaluation of RELN gene polymorphism in children with autism spectrum disorder. Anadolu Psikiyatri Dergisi 2018, 19, 599–606. [Google Scholar] [CrossRef]
- Wang, G.F.; Ye, S.; Gao, L.; Han, Y.; Guo, X.; Dong, X.P.; Su, Y.Y.; Zhang, X. Two Single-Nucleotide Polymorphisms of the RELN Gene and Symptom-Based and Developmental Deficits Among Children and Adolescents with Autistic Spectrum Disorders in the Tianjin, China. Behav. Brain Res. 2018, 350, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Stary, J.M.; Egan, E.A. Reduced blood levels of reelin as a vulnerability factor in pathophysiology of autistic disorder. Cell. Mol. Neurobiol. 2002, 22, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.; Luthert, P.; Dean, A.; Harding, B.; Janota, I.; Montgomery, M.; Rutter, M.; Lantos, P. A clinicopathological study of autism. Brain 1998, 121, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Risch, N.; Spiker, D.; Lotspeich, L.; Nouri, N.; Hinds, D.; Hallmayer, J.; Kalaydjieva, L.; McCague, P.; Dimiceli, S.; Pitts, T.; et al. A genomic screen of autism: Evidence for a multilocus etiology. Am. J. Hum. Genet. 1999, 65, 493–507. [Google Scholar] [CrossRef]
- Yu, C.E.; Dawson, G.; Munson, J.; D’Souza, I.; Osterling, J.; Estes, A.; Leutenegger, A.L.; Flodman, P.; Smith, M.; Raskind, W.H.; et al. Presence of large deletions in kindreds with autism. Am. J. Hum. Genet. 2002, 71, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K.; Day, C.P. Candidate gene case-control association studies: Advantages and potential pitfalls. Br. J. Clin. Pharmacol. 2001, 52, 489–499. [Google Scholar] [CrossRef]
| First Author, Year | N | Number of CGG Repeats | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3/10 | 4/8 | 4/10 | 6/10 | 7/8 | 7/10 | 8/8 | 8/9 | 8/10 | 8/11 | 8/11-15 | 8/12 | 8/13 | 8/14 | 9/10 | 9/13 | 10/10 | ||||
| (Persico et al., 2001) [20] | 1. Patients with ASD a | 95 | 1 | 1 | 16 | 44 | 0 | 5 | 2 | 0 | 16 | |||||||||
| 2. Healthy controls | 186 | 0 | 0 | 36 | 85 | 3 | 1 | 3 | 1 | 48 | ||||||||||
| (Zhang et al., 2002) [37] | 1. Patients with ASD 2. Healthy controls | 126 347 | 0 1 | 1 0 | 0 1 | 0 1 | 1 0 | 16 60 | 0 2 | 44 138 | 8 16 | 1 1 | 1 0 | 40 97 | ||||||
| (Dutta el al., 2007) [38] | 1. Patients with ASD 2. Healthy controls | 55 80 | 0 1 | 10 12 | 31 42 | |||||||||||||||
| First Author, Year | N | Number of CGG Repeats | ||||||||||||||||||
| 10/11 | 10/11-16 | 10/12 | 10/13 | 10/23 | 12/10 | 12/12 | 12/13 | 13/8 | 13/10 | 13/13 | 14/10 | 15/10 | 16/10 | |||||||
| (Persico et al., 2001) [20] | 1. Patients with ASD a | 95 | 0 | 5 | 3 | 1 | 1 | |||||||||||||
| 2. Healthy controls | 186 | 1 | 2 | 6 | 0 | 0 | ||||||||||||||
| (Zhang et al., 2002) [37] | 1. Patients with ASD 2. Healthy controls | 126 347 | 14 28 | 0 2 | ||||||||||||||||
| (Dutta el al., 2007) [38] | 1. Patients with ASD 2. Healthy controls | 55 80 | 0 3 | 2 4 | 11 14 | 1 1 | 0 1 | 0 1 | 0 1 | |||||||||||
| First Author, Year | N | Number of CGG Repeats | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 23 | |||
| (Persico et al., 2001) [20] | 1. Patients with ASD a | 95 | 2 | 84 | 86 | 0 | 11 | 6 | 0 | 1 | |||||||
| 2. Healthy controls | 186 | 0 | 165 | 190 | 4 | 3 | 9 | 1 | 0 | ||||||||
| (Zhang et al., 2002) [37] | 1. Patients with ASD | 126 | 0 | 1 | 0 | 1 | 85 | 2 | 140 | 4 | 5 | 13 | 0 | 0 | 1 | ||
| 2. Healthy controls | 347 | 1 | 0 | 1 | 1 | 277 | 3 | 363 | 7 | 12 | 26 | 1 | 2 | 0 | |||
| (Dutta et al., 2007) [38] | 1. Patients with ASD | 55 | 12 | 83 | 0 | 15 | 0 | 0 | 0 | ||||||||
| 2. Healthy controls | 80 | 18 | 116 | 3 | 20 | 1 | 1 | 1 | |||||||||
| First Author, Year | N | rs736707 (intron 59) | rs362691(exon22) L997V | rs2229864 (exon 50) | |||||||||||||
| Genotype distribution | Allele distribution | Genotype distribution | Allele distribution | Genotype distribution | Allele distribution | ||||||||||||
| CC | CT | TT | C | T | CC | CG | GG | C | G | TT | CT | CC | T | C | |||
| (Dutta et al., 2007) [38] | 1. Patients with ASD a | 55 | 4 | 24 | 27 | 32 | 78 | ||||||||||
| 2. Healthy controls | 80 | 8 | 31 | 41 | 47 | 113 | |||||||||||
| (Dutta et al., 2008) [23] | 1. Patients with ASD | 77 | 11 | 34 | 32 | 56 | 98 | 56 | 20 | 1 | 132 | 22 | |||||
| 2. Healthy controls (exon 22 N= 100) | 101 | 19 | 49 | 33 | 87 | 115 | 76 | 23 | 1 | 175 | 25 | ||||||
| (Li et al., 2008) [39] | 1. Patients with ASD (intron 59 N= 210) | 213 | 52 | 108 | 50 | 212 | 208 | 159 | 47 | 7 | 365 | 61 | 8 | 76 | 129 | 92 | 334 |
| 2. Healthy controls | 160 | 29 | 78 | 53 | 136 | 184 | 125 | 30 | 5 | 280 | 40 | 7 | 53 | 100 | 67 | 253 | |
| (He et al., 2011) [24] | 1. Patients with ASD (exon 22 N= 219) | 221 | 50 | 116 | 55 | 216 | 226 | 180 | 36 | 3 | 396 | 42 | 9 | 73 | 139 | 92 | 350 |
| 2. Healthy controls (exon 22 N= 277) (exon 50 N= 278) | 282 | 48 | 146 | 88 | 242 | 322 | 216 | 53 | 8 | 485 | 69 | 13 | 87 | 178 | 113 | 443 | |
| (Sharma et al., 2013) [22] | 1. Patients with ASD (intron 59 N= 129) | 136 | 14 | 50 | 65 | 78 | 180 | 3 | 16 | 117 | 22 | 250 | |||||
| 2. Healthy controls (intron 59 N= 208) | 193 | 35 | 94 | 79 | 164 | 252 | 2 | 34 | 157 | 38 | 348 | ||||||
| (Mehdizadeh et al., 2015) [40] | 1. Patients with ASD | 74 | 41 | 26 | 7 | 108 | 40 | ||||||||||
| 2. Healthy controls | 86 | 52 | 28 | 6 | 132 | 40 | |||||||||||
| (Mehdizadeh et al., 2016) [41] | 1. Patients with ASD | 74 | 0 | 16 | 58 | 16 | 132 | ||||||||||
| 2. Healthy controls | 88 | 0 | 28 | 60 | 28 | 148 | |||||||||||
| (Wang et al., 2018) [43] | 1. Patients with ASD | 157 | 33 | 78 | 46 | 144 | 170 | 19 | 70 | 68 | 108 | 206 | |||||
| 2. Healthy controls | 256 | 54 | 126 | 76 | 234 | 278 | 13 | 76 | 167 | 102 | 410 | ||||||
| (Şahin et al., 2018) [42] | 1. Patients with ASD | 61 | 0 | 10 | 51 | 10 | 112 | ||||||||||
| 2. Healthy controls | 64 | 0 | 8 | 56 | 8 | 120 | |||||||||||
| First Author, Year | Country | Criteria for ASD a Definition | Ethnicity | Female/Male Ratio | Age (Mean [SD]) | ||
|---|---|---|---|---|---|---|---|
| Patients with ASD | Healthy Controls | Patients with ASD | Healthy Controls | ||||
| Persico et al., 2001 [20] | Italy | DSM–IV b criteria for Autistic disorder | Caucasian | 6/89 | 89/97 | 6.25 (2.8) | 51.7 (19.6) |
| Zhang et al., 2002 [37] | Canada | ADI–R c algorithm / ADOS d | N/A e | N/A | 170/177 | N/A | N/A |
| Dutta et al., 2007 [38] | India | DSM–IV criteria for Autistic disorder | Indian | N/A | N/A | N/A | N/A |
| Dutta et al., 2008 [23] | India | DSM–IV criteria for Autistic disorder | Indian | 13/64 | N/A | 5.8 (2.9) | N/A |
| Li et al., 2008 [39] | China | DSM–IV criteria for Autistic disorder or ICD-10 f | Chinese Han | 32/181 | 25/135 | 5.3 (N/A) | 6.7 (N/A) |
| He et al., 2011 [24] | China | DSM–IV criteria for Autistic disorder | Chinese Han | 35/197 | 43/240 | N/A | 32.8 (10.5) |
| Sharma et al., 2013 [22] | South Africa | DSM–IV criteria for Autistic disorder | Black, white, and mixed ancestry | N/A | N/A | N/A | N/A |
| Mehdizadeh et al., 2015 [40] | Iran | DSM–IV criteria for Autistic disorder | Caucasian | 18/53 | 65/21 | 8.57 (N/A) | N/A |
| Mehdizadeh et al., 2016 [41] | Iran | DSM–IV criteria for Autistic disorder | Caucasian | 18/53 | 66/22 | 8.57 (0.07) | 7.79 (0.14) |
| Wang et al., 2018 [43] | China | DSM–IV criteria for Autistic disorder | Chinese Han | 21/108 | 72/184 | 8.4 (3.9) | 8.3 (3.9) |
| Şahin et al., 2018 [42] | Turkey | g DSM–5 criteria for Autistic disorder | N/A | 5/56 | 12/52 | 5.54 (3.1) | 6.43 (4.0) |
| Polymorphisms | OR | 95% CI | Poverall effect | Q | Pheterogeneity |
|---|---|---|---|---|---|
| Exon 22 | |||||
| C vs. G | 0.95 | 0.76, 1.20 | 0.68 | 5.32 | 0.38 |
| CC vs. CG + GG | 1.03 | 0.77, 1.38 | 0.83 | 2.85 | 0.42 |
| GG vs. CG + CC | 1.20 | 0.83, 1.75 | 0.34 | 4.08 | 0.54 |
| Exon 50 | |||||
| C vs. T | 0.81 | 0.55, 1.19 | 0.28 | 13.56 | 0.004 |
| CC vs. CT + TT | 0.75 | 0.48, 1.16 | 0.19 | 11.50 | 0.009 |
| TT vs. CT + CC | 1.18 | 0.61, 2.26 | 0.63 | 5.70 | 0.13 |
| Intron 59 | |||||
| C vs. T | 0.98 | 0.77, 1.24 | 0.84 | 16.05 | 0.007 |
| CC vs. CT + TT | 1.02 | 0.76, 1.37 | 0.88 | 7.84 | 0.17 |
| TT vs. CT + CC | 1.02 | 0.73, 1.44 | 0.90 | 13.08 | 0.02 |
| Triplet repeat number | |||||
| 4 | 9.09 | 1.00, 82.50 | 0.05 | 0.01 | 0.94 |
| 8 | 0.86 | 0.69, 1.08 | 0.19 | 1.30 | 0.52 |
| 10 | 1.00 | 0.78, 1.29 | 0.98 | 2.77 | 0.25 |
| 11 | 0.89 | 0.14, 5.59 | 0.90 | 1.63 | 0.20 |
| 12 | 1.68 | 0.30, 9.50 | 0.56 | 7.41 | 0.02 |
| 13 | 1.26 | 0.81, 1.97 | 0.31 | 0.22 | 0.89 |
| 14 | 0.66 | 0.10, 4.20 | 0.66 | 0.08 | 0.96 |
| 15 | 0.52 | 0.06, 4.69 | 0.56 | 0.00 | 0.95 |
| 16 | 2.00 | 0.12, 32.62 | 0.63 | 1.52 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-García, I.; Chamorro, A.-J.; Ternavasio-de la Vega, H.G.; Carbonell, C.; Marcos, M.; Mirón-Canelo, J.-A. Association of Allelic Variants of the Reelin Gene with Autistic Spectrum Disorder: A Systematic Review and Meta-Analysis of Candidate Gene Association Studies. Int. J. Environ. Res. Public Health 2020, 17, 8010. https://doi.org/10.3390/ijerph17218010
Hernández-García I, Chamorro A-J, Ternavasio-de la Vega HG, Carbonell C, Marcos M, Mirón-Canelo J-A. Association of Allelic Variants of the Reelin Gene with Autistic Spectrum Disorder: A Systematic Review and Meta-Analysis of Candidate Gene Association Studies. International Journal of Environmental Research and Public Health. 2020; 17(21):8010. https://doi.org/10.3390/ijerph17218010
Chicago/Turabian StyleHernández-García, Ignacio, Antonio-Javier Chamorro, Hugo Guillermo Ternavasio-de la Vega, Cristina Carbonell, Miguel Marcos, and José-Antonio Mirón-Canelo. 2020. "Association of Allelic Variants of the Reelin Gene with Autistic Spectrum Disorder: A Systematic Review and Meta-Analysis of Candidate Gene Association Studies" International Journal of Environmental Research and Public Health 17, no. 21: 8010. https://doi.org/10.3390/ijerph17218010
APA StyleHernández-García, I., Chamorro, A.-J., Ternavasio-de la Vega, H. G., Carbonell, C., Marcos, M., & Mirón-Canelo, J.-A. (2020). Association of Allelic Variants of the Reelin Gene with Autistic Spectrum Disorder: A Systematic Review and Meta-Analysis of Candidate Gene Association Studies. International Journal of Environmental Research and Public Health, 17(21), 8010. https://doi.org/10.3390/ijerph17218010







