Increasing HPV Vaccination Uptake among Adolescents: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- (P) The population addressed in this systematic review included the different possible targets of strategies aimed at increasing vaccination coverage in adolescents, including adolescents themselves, their parents, and healthcare professionals involved in vaccination services.
- (C) The usual strategy, namely, the absence of any specific strategy aimed at increasing coverage was included.
- (O) HPV vaccination coverage in terms of vaccination initiation and/or all doses completion was considered.
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria
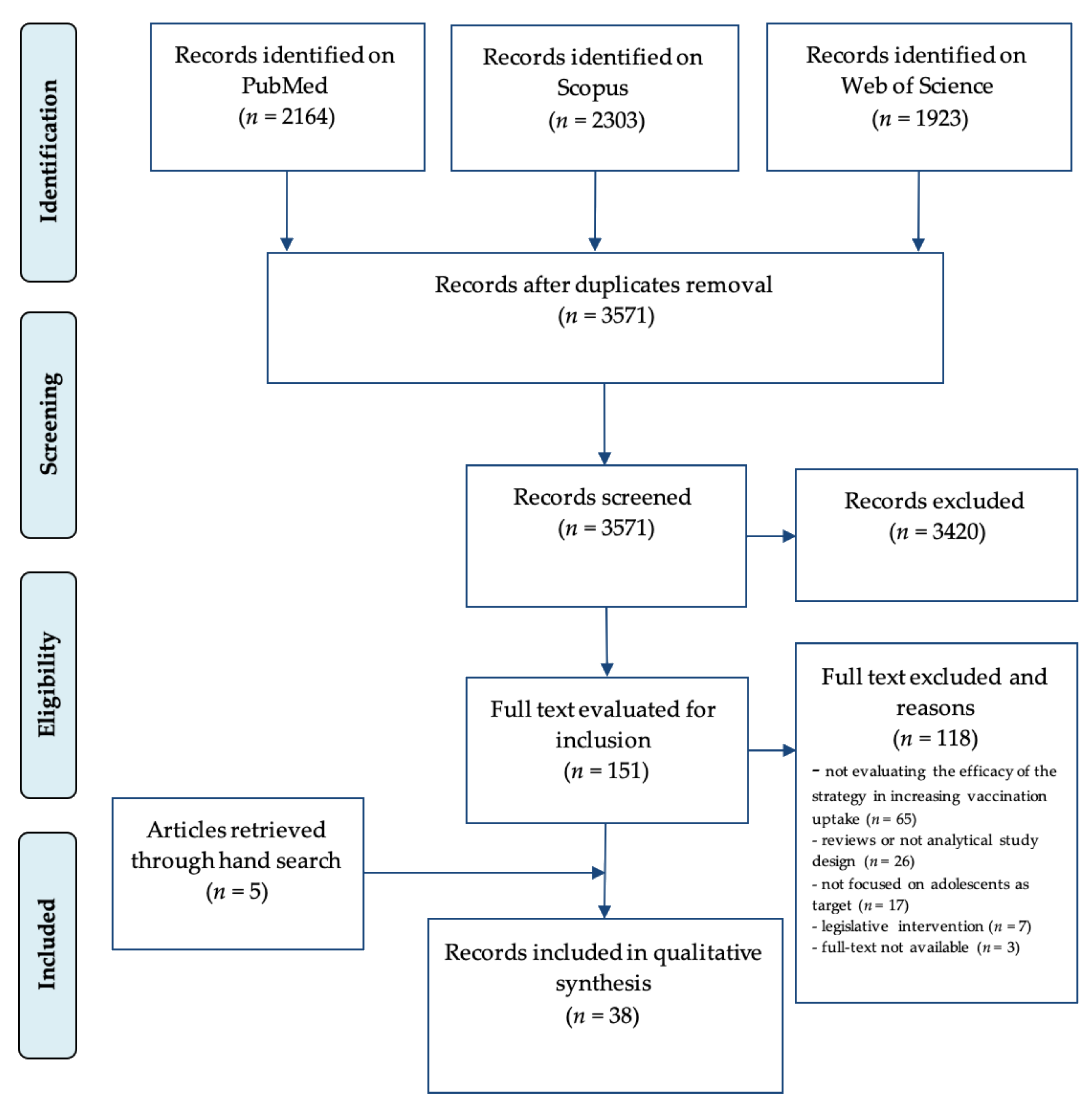
2.3. Selection Process and Data Extraction
3. Results
3.1. Characteristics of the Studies
3.2. Description of Identified Strategies and Synthesis of the Results
- (1)
- reminder-based interventions,
- (2)
- education, information, and communication strategies,
- (3)
- multicomponent interventions.
3.2.1. Reminder-Based Strategies
3.2.2. Education, Information and Communication Strategies
3.2.3. Multicomponent Interventions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Formana, D.; de Martel, C.; Lacey, C.J.; Soerjomatarama, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30, F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Chesson, H.W.; Dunne, E.F.; Hariri, S.S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 2014, 41, 660. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.C.; Schiffman, M.; Herrero, R.; Wacholder, S.; Hildesheim, A.; Castle, P.E.; Solomon, D.; Burk, R. On behalf of the Proyecto Epidemiológico Guanacaste Group. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J. Natl. Cancer Inst. 2008, 100, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L.; Herrero, R.; Bray, F.; Bosch, F.X.; de Sanjosé, S.; Castellsagué, X. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef]
- IARC. IARC working group on the evaluation of carcinogenic risks to humans. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 61, 45–119. [Google Scholar]
- Muñoz, N.; Bosch, F.X.; De Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, A.; Bianchi, C.; Auzzi, N.; Fallani, M.G.; Bussani, C.; Fambrini, M.; Cariti, G.; Scarselli, G.; Petraglia, F.; Ghelardi, A. Indication of prophylactic vaccines as a tool for secondary prevention in HPV-linked disease. Arch. Gynecol. Obstet. 2018, 298, 1205–1210. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A.A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, J.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018; Available online: https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf (accessed on 20 May 2020).
- De Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 2007, 7, 453–459. [Google Scholar] [CrossRef]
- OMS. Guide to Introducing HPV Vaccine into National Immunization Programmes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; de Andrade, R.P.; Ault, K.A.; Bartholomew, D.; Cestero, R.M.; Fedrizzi, E.N.; Hirschberg, A.L.; et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: A randomised, double-blind trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef]
- National HPV Vaccination Program Register HPV Register. Coverage Data 2017. Available online: http://www.hpvregister.org.au/research/coverage-data (accessed on 20 May 2020).
- European Centre for Disease Prevention and Control. Guidance on HPV Vaccination in EU Countries: Focus on Boys, People Living with HIV and 9-Valent HPV Vaccine Introduction, 2020; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- Italian Ministry of Health. Piano Nazionale Prevenzione Vaccinale 20172–019. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf (accessed on 5 November 2018).
- Ciavattini, A.; Giannella, L.; De Vincenzo, R.; Di Giuseppe, J.; Papiccio, M.M.; Lukic, A.A.; Delli Carpini, G.; Perino, A.; Frega, A.; Sopracordevole, F.; et al. HPV vaccination: The position paper of the Italian society of colposcopy and cervico-vaginal pathology (SICPCV). Vaccines 2020, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministry of Health. Vaccinazione Contro il Papilloma Virus (HPV)—Coperture Vaccinali. Data di Ultimo Aggiornamento: Last Updated 6 Luglio 2020. Available online: http://www.salute.gov.it/imgs/C_17_tavole_27_1_0_file.pdf (accessed on 20 August 2020).
- Lehmann, C.E.; Brady, R.C.; Battley, R.O.; Huggins, J.L. Adolescent vaccination strategies: Interventions to increase coverage. Pediatric Drugs 2016, 18, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Salam, R.A.; Arshad, A.; Lassi, Z.S.; Bhutta, Z.A. Systematic review and meta-analysis of interventions to improve access and coverage of adolescent immunizations. J. Adolesc. Health 2016, 59, S40–S48. [Google Scholar] [CrossRef]
- Walling, E.B.; Benzoni, N.; Dornfeld, J.; Bhandari, R.; Sisk, B.A.; Garbutt, J.; Colditz, G. Interventions to improve HPV vaccine uptake: A systematic review. Pediatrics 2016, 138, e20153863. [Google Scholar] [CrossRef]
- Smulian, E.A.; Mitchell, K.R.; Stokley, S.S. Interventions to increase HPV vaccination coverage: A systematic review. Hum. Vaccines Immunother. 2016, 12, 1566–1588. [Google Scholar] [CrossRef]
- Abdullahi, L.H.; Kagina, B.M.; Ndze, V.N.; Hussey, G.D.; Wiysonge, C.S. Improving vaccination uptake among adolescents. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, V.J.C.; Jacobson, R.M.; Coyne-Beasley, T.; Asafu-Adjei, J.K.; Szilagyi, P.G. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Niccolai, L.M.; Hansen, C.E. Practice-and community-based interventions to increase human papillomavirus vaccine coverage a systematic review. JAMA Pediatrics 2015, 169, 686–692. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.J.; Altman, D.G.; Grp, P.P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement (reprinted from annals of internal medicine). Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef]
- WHO. Human Papillomavirus Vaccines: WHO Position Paper; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Human papillomavirus vaccines: WHO position paper, May 2017—Recommendations. Vaccine 2017, 84, 118–131.
- The World Bank. Data: World Bank Country and Lending Groups; World Bank Country and Lending Groups: Washington, DC, USA, 2018. [Google Scholar]
- Lee, H.; Kim, M.; Cooley, M.E.; Kiang, C.P.N.; Kim, D.; Tang, S.; Shi, L.; Thiem, L.; Kan, P.; Peou, S.; et al. Using narrative intervention for HPV vaccine behavior change among Khmer mothers and daughters: A pilot RCT to examine feasibility, acceptability, and preliminary effectiveness. Appl. Nurs. Res. 2018, 40, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Rehn, M.; Uhnoo, I.; Kühlmann-Berenzon, S.; Wallensten, A.; Sparén, P.P.; Netterlid, E.E. Highest vaccine uptake after school-based delivery—A county-level evaluation of the implementation strategies for HPV catch-up vaccination in Sweden. PLoS ONE 2016, 11, e0149857. [Google Scholar] [CrossRef] [PubMed]
- Kempe, A.; O’Leary, S.T.; Shoup, J.A.; Stokley, S.; Lockhart, S.; Furniss, A.; Dickinson, L.M.; Barnard, J.; Daley, M.F. Parental choice of recall method for HPV vaccination: A pragmatic trial. Pediatrics 2016, 137, e20152857. [Google Scholar] [CrossRef] [PubMed]
- Rand, C.M.; Brill, H.; Albertin, C.; Humiston, S.G.; Schaffer, S.S.; Shone, L.P.; Blumkin, A.K.; Szilagyi, P.G. Effectiveness of centralized text message reminders on human papillomavirus immunization coverage for publicly insured adolescents. J. Adolesc. Health 2015, 56 (Suppl. 5), S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Rand, C.M.; Vincelli, P.; Goldstein, N.P.N.; Blumkin, A.A.; Szilagyi, P.G. Effects of phone and text message reminders on completion of the human papillomavirus vaccine series. J. Adolesc. Health 2017, 60, 113–119. [Google Scholar] [CrossRef]
- Szilagyi, P.G.; Humiston, S.G.; Gallivan, S.; Albertin, C.; Sandler, M.M.; Blumkin, A.A. Effectiveness of a citywide patient immunization navigator program on improving adolescent immunizations and preventive care visit rates. Arch. Pediatr. Adolesc. Med. 2011, 165, 547–553. [Google Scholar] [CrossRef]
- Szilagyi, P.G.; Albertin, C.; Humiston, S.G.; Rand, C.M.; Schaffer, S.S.; Brill, H.H.; Stankaitis, J.; Yoo, B.K.; Blumkin, A.; Stokley, S. A randomized trial of the effect of centralized reminder/recall on immunizations and preventive care visits for adolescents. Acad. Pediatr. 2013, 13, 204–213. [Google Scholar] [CrossRef]
- Ruffin, M.T.; Plegue, M.A.; Rockwell, P.G.; Young, A.P.; Patel, D.A.; Yeazel, M.W. Impact of an electronic health record (EHR) reminder on human papillomavirus (HPV) vaccine initiation and timely completion. J. Am. Board. Fam. Med. 2015, 28, 324–333. [Google Scholar] [CrossRef]
- Grandahl, M.; Rosenblad, A.; Stenhammar, C.; Tyden, T.; Westerling, R.; Larsson, M.; Oscarsson, M.; Andrae, B.; Dalianis, T.; Nevéus, T. School-based intervention for the prevention of HPV among adolescents: A cluster randomised controlled study. BMJ Open 2016, 6, e009875. [Google Scholar] [CrossRef]
- Parra-Medina, D.; Morales-Campos, D.Y.; Mojica, C.C.; Ramirez, A.G. Promotora outreach, education and navigation support for HPV vaccination to hispanic women with unvaccinated daughters. J. Cancer Educ. 2015, 30, 353–359. [Google Scholar] [CrossRef]
- Pot, M.; Paulussen, T.G.W.M.; Ruiter, R.A.C.; Eekhout, I.; De Melker, H.E.; Spoelstra, M.E.A.; van Keulen, H.M. Effectiveness of a web-based tailored intervention with virtual assistants promoting the acceptability of hpv vaccination among mothers of invited girls: Randomized controlled trial. J. Med. Internet Res. 2017, 19, e312. [Google Scholar] [CrossRef] [PubMed]
- Rickert, V.I.; Auslander, B.A.; Cox, D.S.; Rosenthal, S.L.; Rupp, R.E.; Zimet, G.D. School-based HPV immunization of young adolescents: Effects of two brief health interventions. Hum. Vaccin Immunother. 2015, 11, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Staras, S.A.S.; Vadaparampil, S.T.; Livingston, M.D.; Thompson, L.A.; Sanders, A.H.; Shenkman, E.A. Increasing human papillomavirus vaccine initiation among publicly insured Florida adolescents. J. Adolesc. Health. 2015, 56, S40–S46. [Google Scholar] [CrossRef] [PubMed]
- Winer, R.L.; Gonzales, A.A.; Noonan, C.J.; Buchwald, D.S. A cluster-randomized trial to evaluate a mother-daughter dyadic educational intervention for increasing HPV vaccination coverage in American Indian girls. J. Community Health 2016, 41, 274–281. [Google Scholar] [CrossRef]
- Cates, J.R.; Shafer, A.; Diehl, S.J.; Deal, A.M. Evaluating a county-sponsored social marketing campaign to increase mothers’ initiation of HPV vaccine for their pre-teen daughters in a primarily rural area. Soc. Mar. Q. 2011, 17, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Cates, J.R.; Diehl, S.J.; Crandell, J.L.; Coyne-Beasley, T.T. Intervention effects from a social marketing campaign to promote HPV vaccination in preteen boys. Vaccine 2014, 32, 4171–4178. [Google Scholar] [CrossRef]
- Cates, J.R.; Crandell, J.L.; Diehl, S.J.; Coyne-Beasley, T.T. Immunization effects of a communication intervention to promote preteen HPV vaccination in primary care practices. Vaccine 2018, 36, 122–127. [Google Scholar] [CrossRef]
- Sanderson, M.; Canedo, J.R.; Khabele, D.; Fadden, M.K.; Harris, C.; Beard, K.; Burress, M.; Pinkerton, H.; Jackson, C.; Mayo-Gamble, T.; et al. Pragmatic trial of an intervention to increase human papillomavirus vaccination in safety-net clinics. BMC Public Health 2017, 17, 158. [Google Scholar] [CrossRef]
- Cassidy, B.; Braxter, B.; Charron-Prochownik, D.D.; Schlenk, E.A. A quality improvement initiative to increase HPV vaccine rates using an educational and reminder strategy with parents of preteen girls. J. Pediatr. Health Care 2014, 28, 155–164. [Google Scholar] [CrossRef]
- Fujiwara, H.; Takei, Y.; Ishikawa, Y.; Saga, Y.; Machida, S.; Taneichi, A.; Suzuki, M. Community-based interventions to improve HPV vaccination coverage among 13- to 15-year-old females: Measures implemented by local governments in Japan. PLoS ONE 2013, 8, e84126. [Google Scholar] [CrossRef]
- Mantzari, E.E.; Vogt, F.M.T. Financial incentives for increasing uptake of HPV vaccinations: A randomized controlled trial. Health Psychol. 2015, 34, 160–171. [Google Scholar] [CrossRef]
- Tiro, J.A.; Sanders, J.M.; Pruitt, S.L.; Stevens, C.F.; Skinner, C.S.; Bishop, W.P.; Fuller, S.; Persaud, D. Promoting HPV vaccination in safety-net clinics: A randomized trial. Pediatrics 2015, 136, 850–859. [Google Scholar] [CrossRef]
- Vanderpool, R.C.; Breheny, P.J.; Tiller, P.A.; Huckelby, C.A.; Edwards, A.D.; Upchurch, K.D.; Phillips, C.A.; Weyman, C.F. Implementation and evaluation of a school-based human papillomavirus vaccination program in rural Kentucky. Am. J. Prev. Med. 2015, 49, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, F.; Baldacchini, F.; Campari, C.; Perilli, C.; Pascucci, M.G.; Finarelli, A.C.; Moscara, L.; Rossi, P.G. Association between mothers’ screening uptake and daughters’ HPV vaccination: A quasi-experimental study on the effect of an active invitation campaign. BMJ Open 2017, 7, e016189. [Google Scholar] [CrossRef] [PubMed]
- Whelan, N.W.; Steenbeek, A.; Martin-Misener, R.; Scott, J.; Smith, B.; D’Angelo-Scott, H.H. Engaging parents and schools improves uptake of the human papillomavirus (HPV) vaccine: Examining the role of the public health nurse. Vaccine 2014, 32, 4665–4671. [Google Scholar] [CrossRef]
- Zimmerman, R.K.; Moehling, K.K.; Lin, C.J.; Zhang, S.; Raviotta, J.M.; Reis, E.C.; Humiston, S.G.; Nowalk, M.P. Improving adolescent HPV vaccination in a randomized controlled cluster trial using the 4 PillarsTM practice transformation program. Vaccine 2017, 35, 109–117. [Google Scholar] [CrossRef][Green Version]
- Bundy, D.G.; Persing, N.M.; Solomon, B.S.; King, T.M.; Murakami, P.N.; Thompson, R.E.; Engineer, L.D.; Lehmann, C.U.; Miller, M.R. Improving immunization delivery using an electronic health record: The ImmProve project. Acad. Pediatr. 2013, 13, 458–465. [Google Scholar] [CrossRef]
- Gilkey, M.B.; Dayton, A.M.; Moss, J.L.; Sparks, A.C.; Grimshaw, A.H.; Bowling, J.M.; Brewer, N.T. Increasing provision of adolescent vaccines in primary care: A randomized controlled trial. Pediatrics 2014, 134, e346–e353. [Google Scholar] [CrossRef]
- Irving, S.A.; Groom, H.C.; Stokley, S.; McNeil, M.; Gee, J.; Smith, N.; Naleway, A.L. Human papillomavirus vaccine coverage and prevalence of missed opportunities for vaccination in an integrated healthcare system. Acad Pediatr. 2018, 18, S21–S22. [Google Scholar] [CrossRef]
- Perkins, R.B.; Zisblatt, L.; Legler, A.; Trucks, E.; Hanchate, A.; Gorin, S.S. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine 2015, 33, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Rand, C.M.; Tyrrell, H.; Wallace-Brodeur, R.; Goldstein, N.P.N.; Darden, P.M.; Humiston, S.G.; Albertin, C.S.; Stratbucker, W.; Schaffer, S.J.; Davis, W.; et al. A learning collaborative model to improve human papillomavirus vaccination rates in primary care. Acad. Pediatr. 2018, 18, S46–S52. [Google Scholar] [CrossRef] [PubMed]
- Krantz, L.; Ollberding, N.J.; Beck, A.F.; Burkhardt, C.M.M. Increasing HPV vaccination coverage through provider-based interventions. Clin. Pediatr. (Phila) 2018, 57, 319–326. [Google Scholar] [CrossRef]
- Choi, N.; Curtis, C.R.; Loharikar, A.; Fricchione, M.; Jones, E.; Balzer, E.; Liu, Y.; Levin, M.; Chavez-Torres, M.; Morita, J.; et al. Successful use of interventions in combination to improve human papillomavirus vaccination coverage rates among adolescents-Chicago, 2013 to 2015. Acad. Pediatr. 2018, 18, S93–S100. [Google Scholar] [CrossRef] [PubMed]
- Jacobs-Wingo, J.L.; Jim, C.C.; Groom, A.V. Human papillomavirus vaccine uptake: Increase for American Indian adolescents, 2013–2015. Am. J. Prev. Med. 2017, 53, 162–168. [Google Scholar] [CrossRef]
- McLean, H.Q.; VanWormer, J.J.; Chow, B.D.W.; Birchmeier, B.; Vickers, E.; DeVries, E.; Meyer, J.; Moore, J.; McNeil, M.M.; Stokley, S.; et al. Improving human papillomavirus vaccine use in an integrated health system: Impact of a provider and staff intervention. J. Adolesc. Heal. 2017, 61, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Farmar, A.-L.M.; Love-Osborne, K.; Chichester, K.; Breslin, K.; Bronkan, K.K.; Hambidge, S.J. Achieving high adolescent HPV vaccination coverage. Pediatrics 2016, 138, e20152653. [Google Scholar] [CrossRef]
- Paskett, E.D.; Krok-Schoen, J.L.; Pennell, M.L.; Tatum, C.M.; Reiter, P.L.; Peng, J.; Bernardo, B.M.; Weier, R.C.; Richardson, M.S.; Katz, M.L. Results of a multilevel intervention trial to increase human papillomavirus (HPV) vaccine uptake among adolescent girls. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 593–602. [Google Scholar] [CrossRef]
- Varman, M.; Sharlin, C.; Fernandez, C.; Vasudevan, J.; Wichman, C.C. Human papilloma virus vaccination among adolescents in a community clinic before and after intervention. J. Community Health 2018, 43, 455–458. [Google Scholar] [CrossRef]
- Hopfer, S.S. Effects of a narrative HPV vaccination intervention aimed at reaching college women: A randomized controlled trial. Prev. Sci. 2012, 13, 173–182. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Immunization Services Division. Assessment, Feedback, Incentives Exchange (AFIX) Program. Policies and Procedures Guide; U.S. Department of Health and Human Services: Washington, DC, USA, 2013. [Google Scholar]
- Centers for Disease Control and Prevention Immunization Services Division. Immunization Quality Improvement for Providers (IQIP). Available online: https://www.cdc.gov/vaccines/programs/iqip/index.html (accessed on 20 August 2020).
- Holman, D.M.; Benard, V.; Roland, K.B.; Watson, M.; Liddon, N.; Stokley, S.S. Barriers to human papillomavirus vaccination among us adolescents a systematic review of the literature. JAMA Pediatrics 2014, 168, 76–82. [Google Scholar] [CrossRef]
- Cassidy, B.B.; Schlenk, E.A. Uptake of the human papillomavirus vaccine: A review of the literature and report of a quality assurance project. J. Pediatr. Health Care 2012, 26, 92–101. [Google Scholar] [CrossRef]
- Vänskä, S.; Luostarinen, T.; Baussano, I.; Apter, D.; Eriksson, T.T.; Natunen, K.; Nieminen, P.; Paavonen, J.; Pimenoff, V.N.; Pukkala, E.; et al. Vaccination with moderate coverage eradicates oncogenic human papillomaviruses if a gender-neutral strategy is applied. J. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
| Target | Number of Studies | Study Design | Size (Range) | Proven Efficacy of the Strategy * |
|---|---|---|---|---|
| Reminder-based strategies (n = 6) | ||||
| A/P | 5 | 5 RCT | 374–7546 | 4/5 |
| (31–35) | (80%) | |||
| HCP | 1 | 1 CS | 15,021 | 1/1 |
| (36) | (100%) | |||
| Education/Information and Communication strategies (n = 11) | ||||
| A/P | 7 | 5 RCT; 2 Q-E | 19–8062 | 2/7 |
| (29,37–42) | (28.6%) | |||
| A/P; HCP | 4 | 2 Q-E; 1 C-S; | 225–25,869 | 2/4 |
| (43–46) | 1 NRT | (50%) | ||
| Multicomponent interventions (n = 21) | ||||
| A/P | 9 | 3 RCT; 2 Q-E; | 53–325,229 | 7/9 |
| 2 CS; 1 C-S | ||||
| (30,47–54) | (77.8%) | |||
| 1 Ecologic | ||||
| HCP | 6 | 3 RCT; 3 Q-E | 50–107,443 | 4/6 |
| (55–60) | (1 nr) | (66.7%) | ||
| A/P; HCP | 6 | 4 Q-E; 1 RCT; | 105–16,041 | 3/6 |
| (61–66) | 1 CS | (1 nr) | (50%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acampora, A.; Grossi, A.; Barbara, A.; Colamesta, V.; Causio, F.A.; Calabrò, G.E.; Boccia, S.; de Waure, C. Increasing HPV Vaccination Uptake among Adolescents: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 7997. https://doi.org/10.3390/ijerph17217997
Acampora A, Grossi A, Barbara A, Colamesta V, Causio FA, Calabrò GE, Boccia S, de Waure C. Increasing HPV Vaccination Uptake among Adolescents: A Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(21):7997. https://doi.org/10.3390/ijerph17217997
Chicago/Turabian StyleAcampora, Anna, Adriano Grossi, Andrea Barbara, Vittoria Colamesta, Francesco Andrea Causio, Giovanna Elisa Calabrò, Stefania Boccia, and Chiara de Waure. 2020. "Increasing HPV Vaccination Uptake among Adolescents: A Systematic Review" International Journal of Environmental Research and Public Health 17, no. 21: 7997. https://doi.org/10.3390/ijerph17217997
APA StyleAcampora, A., Grossi, A., Barbara, A., Colamesta, V., Causio, F. A., Calabrò, G. E., Boccia, S., & de Waure, C. (2020). Increasing HPV Vaccination Uptake among Adolescents: A Systematic Review. International Journal of Environmental Research and Public Health, 17(21), 7997. https://doi.org/10.3390/ijerph17217997






