Chronic Effect of Fatmax Training on Body Weight, Fat Mass, and Cardiorespiratory Fitness in Obese Subjects: A Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Manuscripts
2.2. Inclusion Criteria
- Humans randomized clinical trials
- Peer-reviewed articles
- People with BMI ≥ 25 kg∙m2
- Studies where fatmax was used as a training strategy
- Studies where MFO and fatmax were measures by indirect calorimetry
- Studies where BF and CRF were reported as a primary or secondary outcome
2.3. Exclusion Criteria
- Intervention period lower than eight weeks
- Unsupervised exercise sessions during trials
- Papers that did not specify the physical activities on the applied exercise protocol
- Studies where men and women were analyzed as a single group
- Studies performed on individuals with physical disabilities
- Documents written in languages other than English and Spanish
2.4. Data Extracted
2.5. BMI Classification
2.6. Risk of Bias
2.7. Statistical Analysis
3. Results
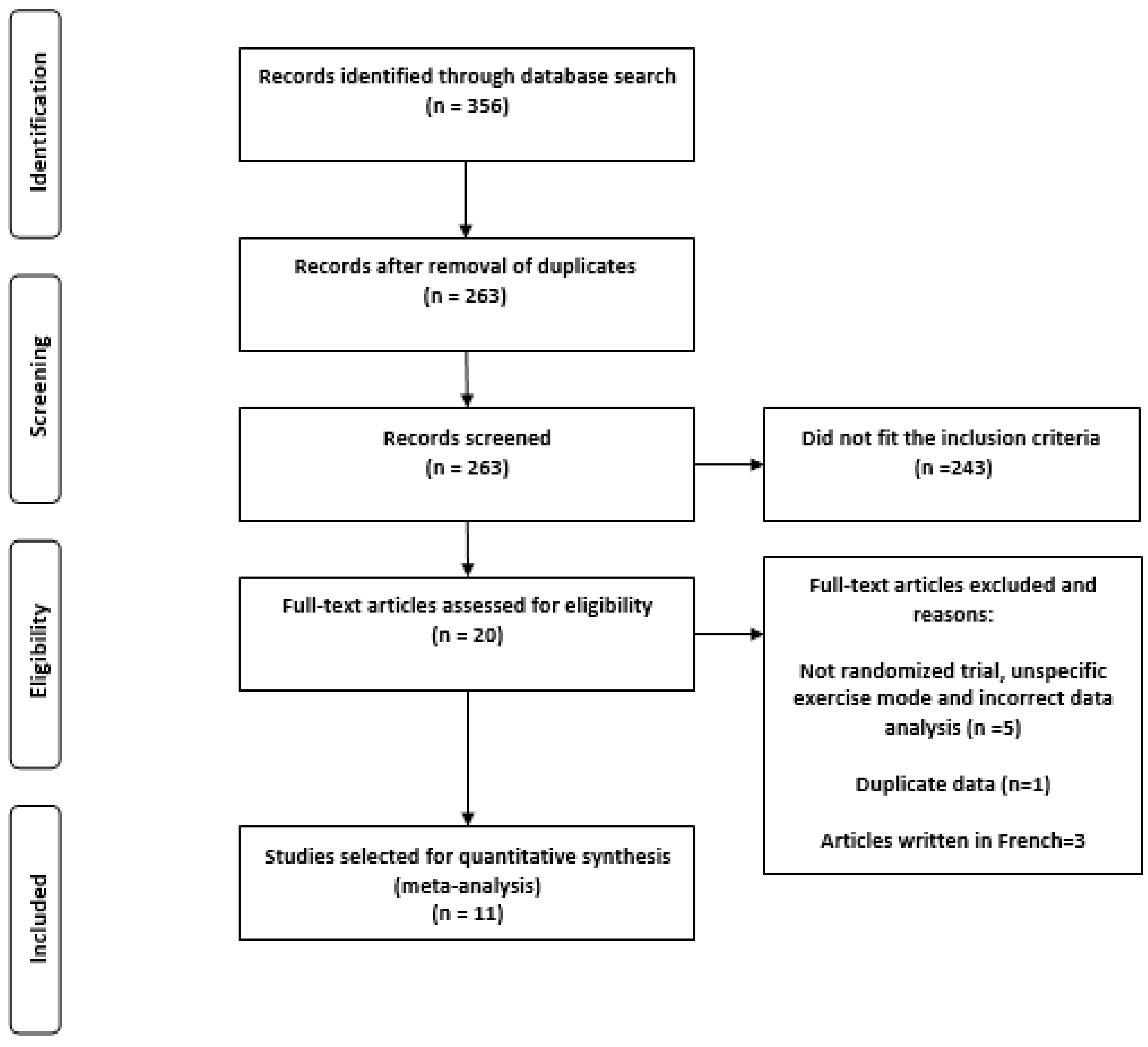
3.1. Characteristics of the Search
- Participants were not randomly assigned to the experimental and control groups
- The authors did not specify exercise mode
- The authors grouped men and women into the same groups for statistical analysis
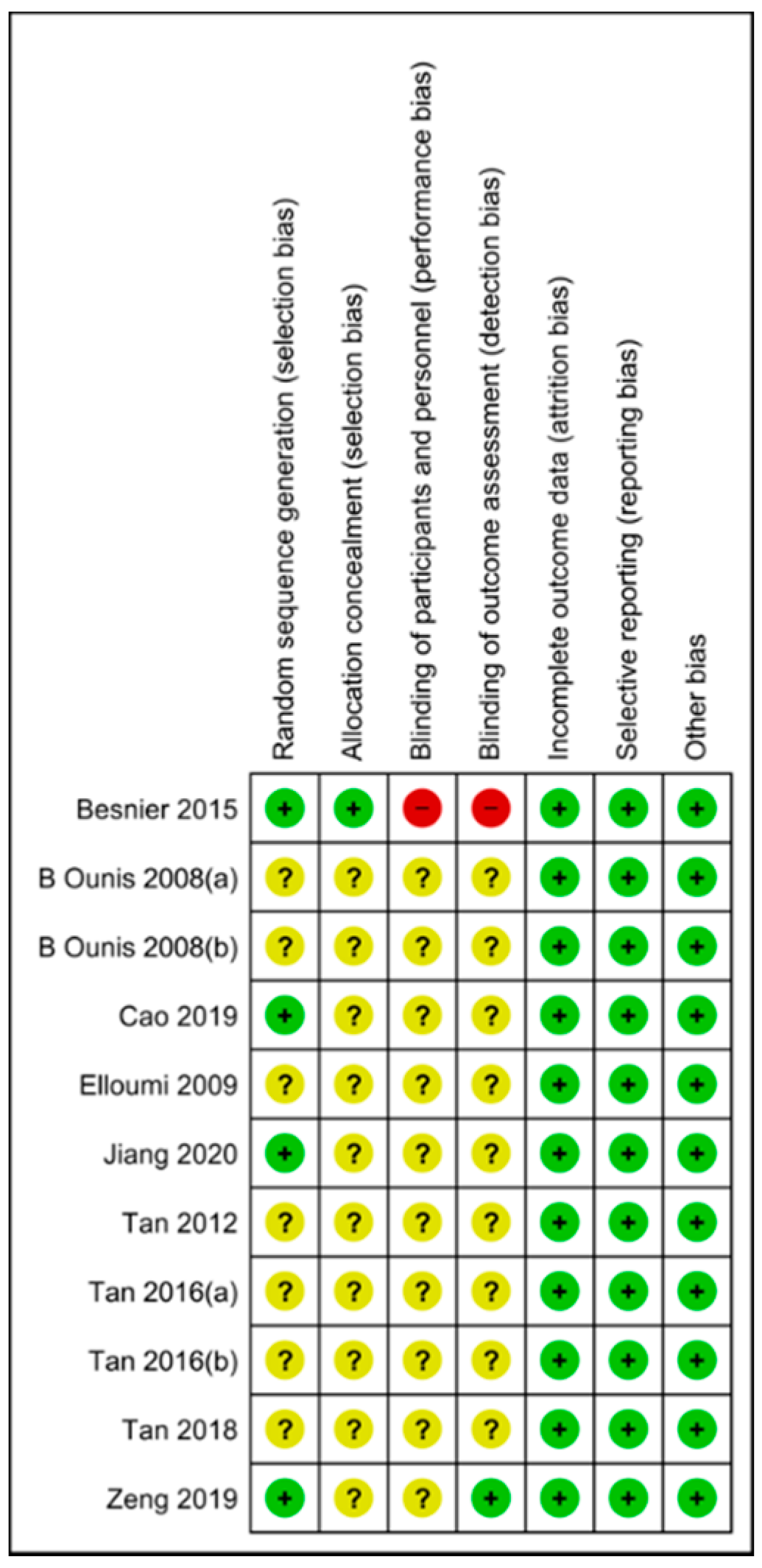
3.2. Risk of Bias
3.3. Subjects Characteristics
3.4. Study Design
3.5. Interventions Characteristics
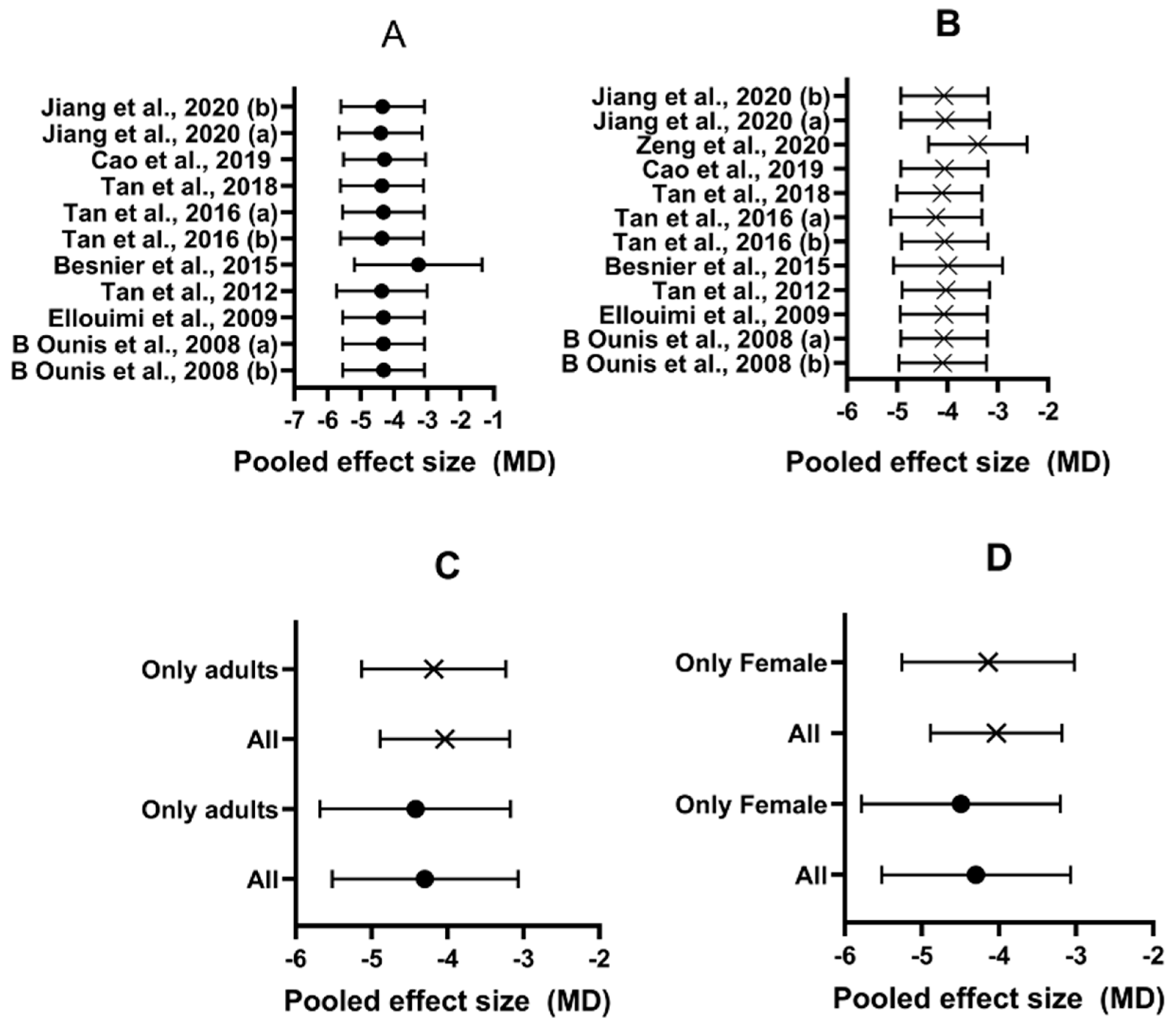
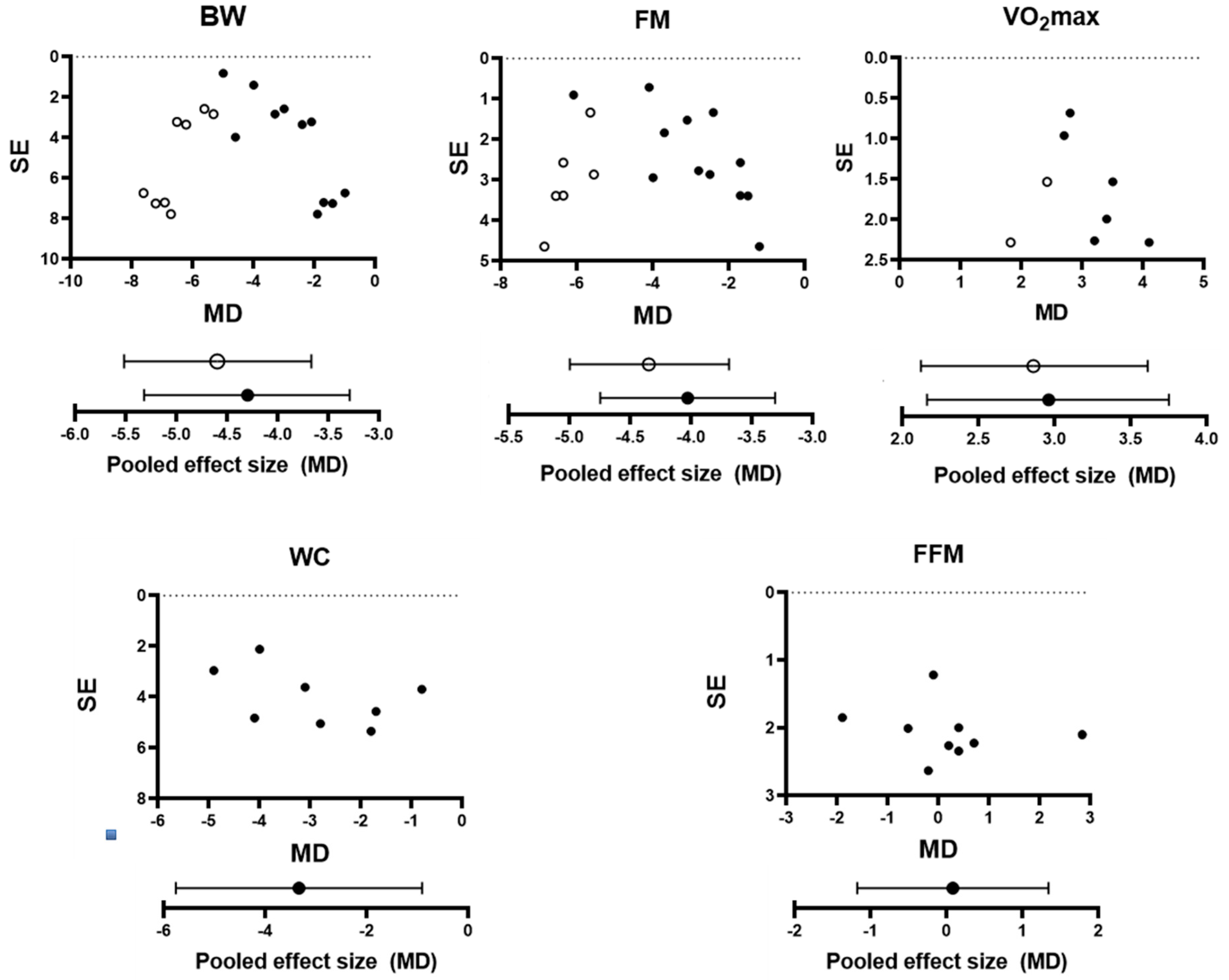
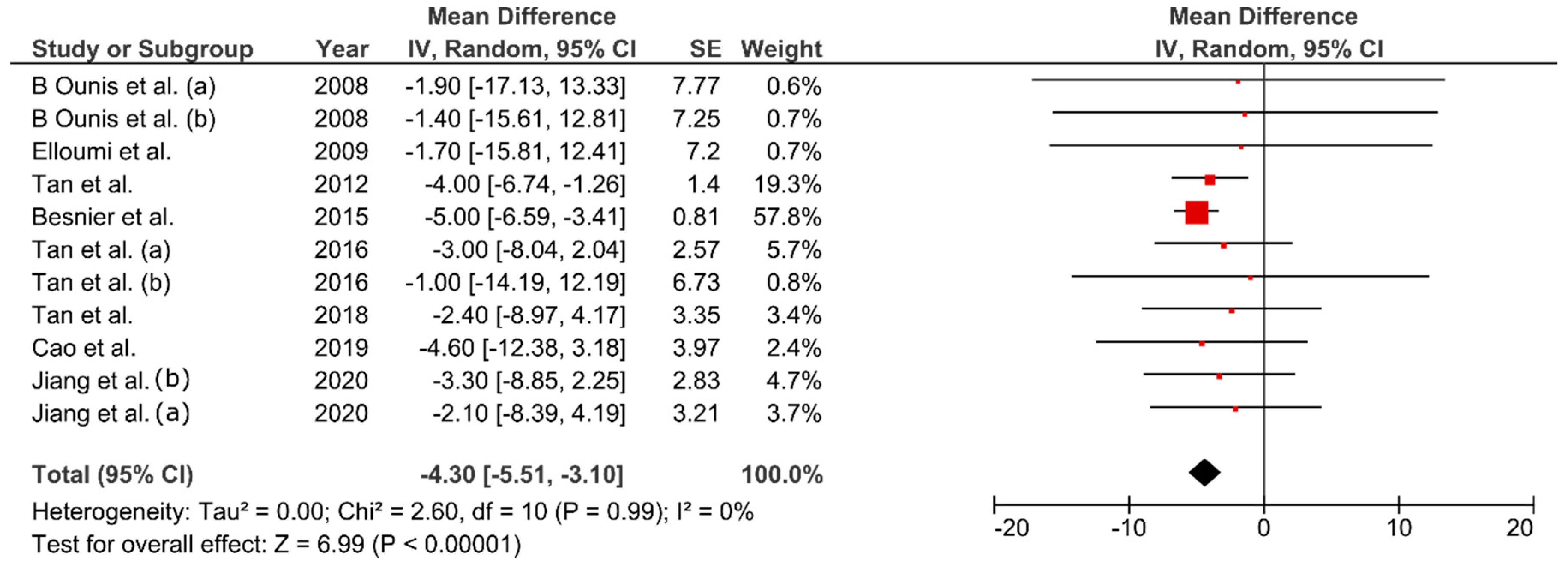
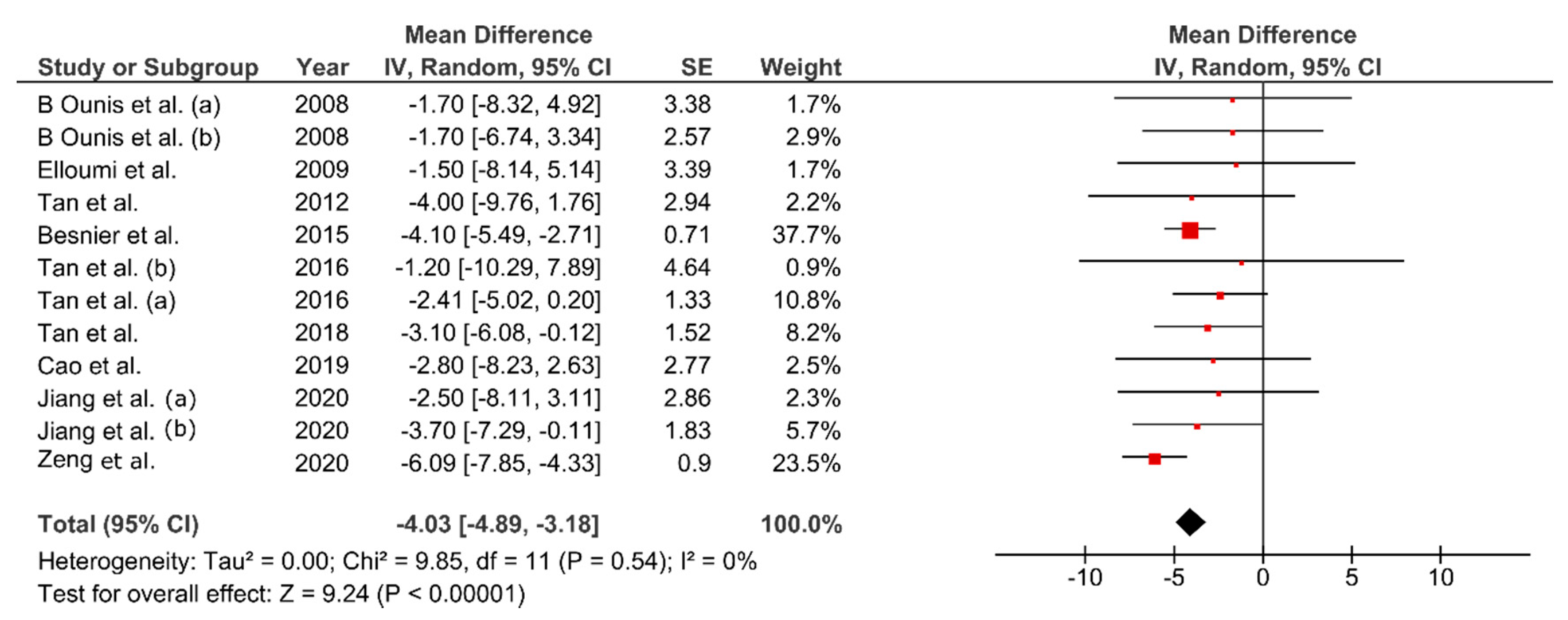
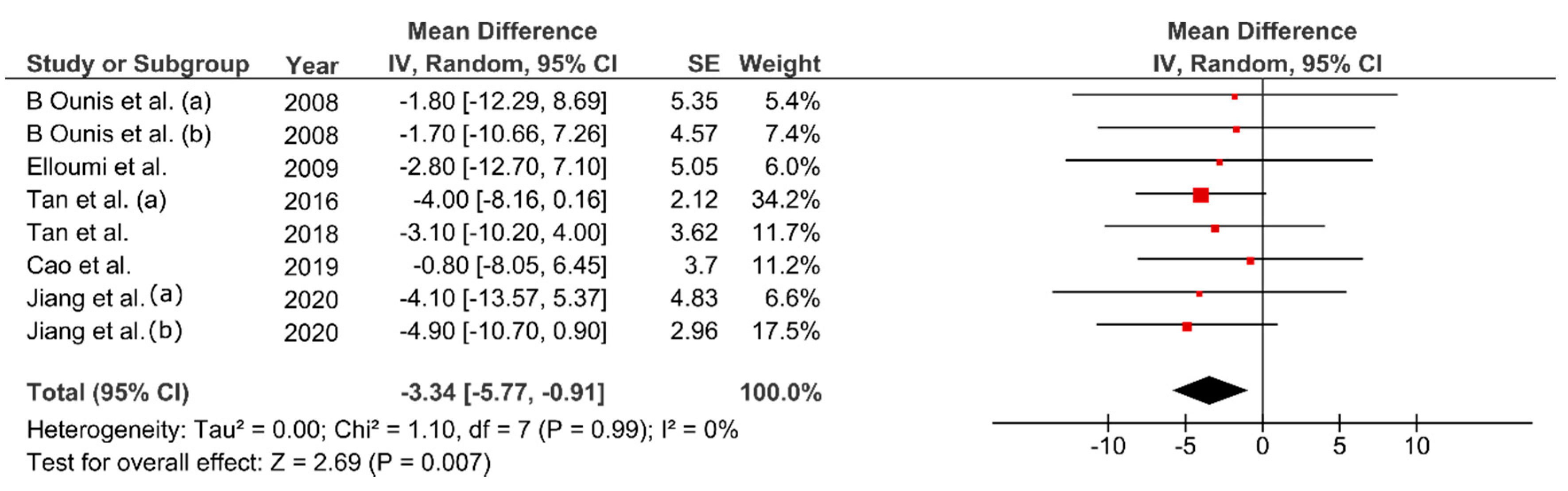
3.6. Effect of FMT on Body Composition
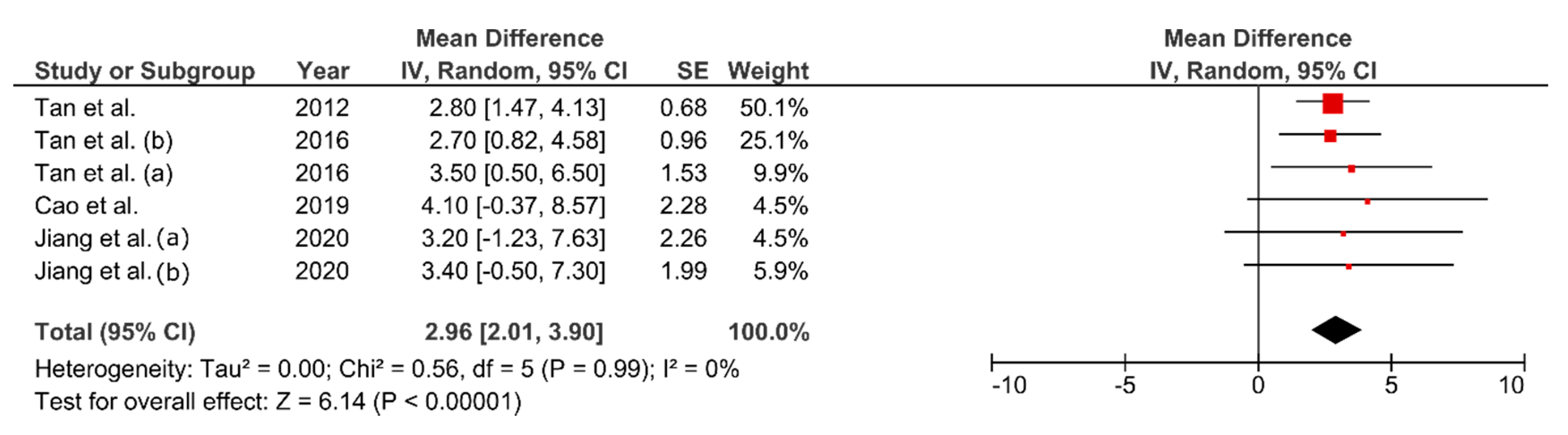
3.7. Effect of FMT on VO2max
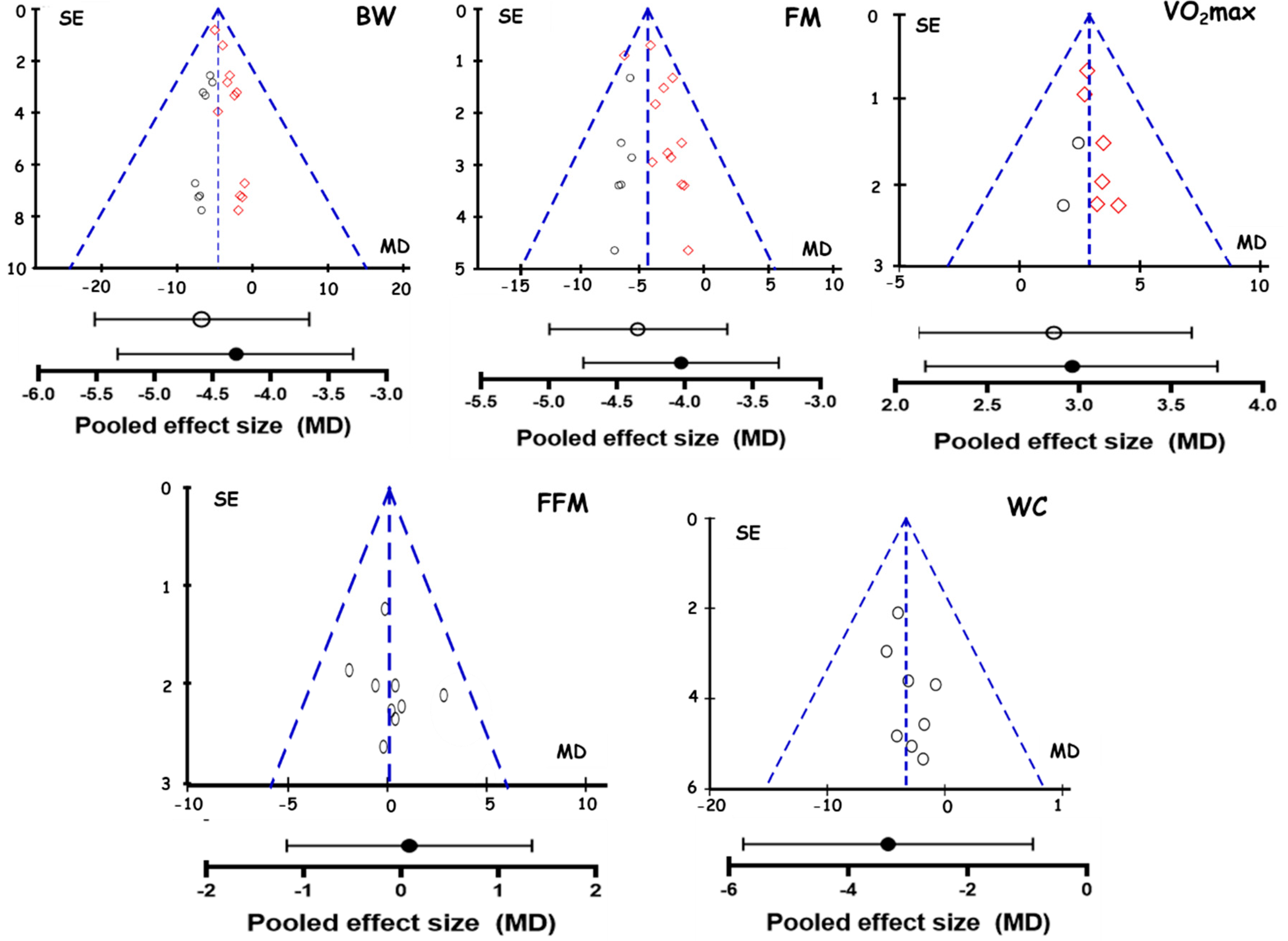
3.8. Publication Bias
4. Discussion
4.1. Effect of FMT on Body Composition
- A high heterogeneity among their studies (I2 = 85%)
- Mixing of randomized and non-randomized trials into the analysis
- Mixing of exercise + hypocaloric diet and only exercise treatments
- They did not evaluate the risk of bias of the studies
- Only one study had a duration > 3 months
4.2. Mechanisms for Body Fat Reduction
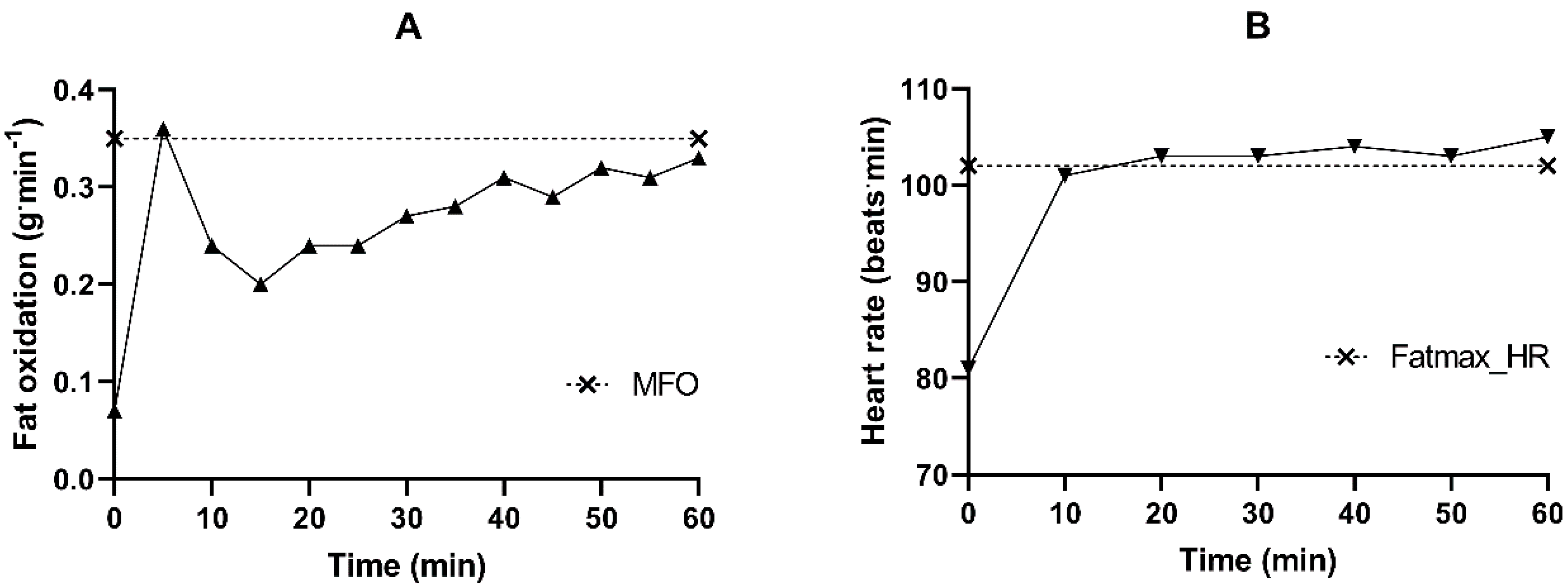
4.3. Effect of FMT on Cardiorespiratory Fitness
4.4. Weakness in the Analyzed Studies
4.5. Strengths
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- World Health Organization. Obesity and Overweight Fact Sheet. World Health Organization Web Site. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 May 2020).
- Borén, J.; Taskinen, M.R.; Olofsson, S.O.; Levin, M. Ectopic lipid storage and insulin resistance: A harmful relationship. J. Intern. Med. 2013, 274, 25–40. [Google Scholar] [CrossRef]
- Fava, M.C.; Agius, R.; Fava, S. Obesity and cardio-metabolic health. Br. J. Hosp. Med. 2019, 80, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A. Lipid oxidation is reduced in obese human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1039–E1044. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Tadaishi, M.; Kamei, Y.; Ezaki, O. Mechanisms of exercise- and training-induced fatty acid oxidation in skeletal muscle. J. Phys. Fit. Sports Med. 2014, 3, 43–53. [Google Scholar] [CrossRef]
- Bogdanis, G.C.; Vangelakoudi, A.; Marikadi, M. Peak fat oxidation rate during walking in sedentary overweight men and women. J. Sports Sci. Med. 2008, 7, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Engeli, S.; Budziarek, P.; Utz, W.; Schulz-Menger, J.; Hermsdorg, M.; Wiesner, S.; Otto, C.; Fuhrmann, J.C.; Luft, F.C.; et al. Determinants of exercise-induced fat oxidation in obese women and men. Horm. Metab. Res. 2010, 42, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Colberg-Ochs, S.R.; Ehrman, J.K.; Johann, J.; Kokkinos, P.; Liguori, G.; Pack, K.R. Exercise prescription for individuals with metabolic disease and cardiovascular disease risk factors. In ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Diebe, D., Ehrman, J.K., Liguori, G., Magal, M., Eds.; Wolters Kluwer: Beijing, China, 2018; pp. 287–291. [Google Scholar]
- Jeukendrup, A.; Achten, J. Fatmax: A new concept to optimize fat oxidation during exercise? Eur. J. Sport Sci. 2001, 1, 1–5. [Google Scholar] [CrossRef]
- Venables, M.C.; Jeukendrup, A.E. Endurance training and obesity: Effect on substrate metabolism and insulin sensitivity. Med. Sci. Sports Exerc. 2008, 40, 495–502. [Google Scholar] [CrossRef]
- Dumortier, M.; Brandou, F.; Perez-Martin, A.; Fedou, C.; Mercier, J.; Brun, J.F. Low intensity endurance exercise targeted for lipid oxidation improves body composition and insulin sensitivity in patients with the metabolic syndrome. Diabetes Metab. 2003, 29, 509–518. [Google Scholar] [CrossRef]
- Ben Ounis, O.; Elloumi, M.; Zouhal, H.; Makni, E.; Lac, G.; Tabka, Z.; Amri, M. Effect of an individualized physical training program on resting cortisol and growth hormone levels and fat oxidation during exercise in obese children. Ann. Endocrinol. 2011, 72, 34–41. [Google Scholar] [CrossRef]
- Wang, J.; Tan, S.; Cao, L. Exercise training at the maximal fat oxidation intensity improved health-related physical fitness in overweight middle-aged women. J. Exerc. Sci. Fit. 2015, 13, 111–116. [Google Scholar] [CrossRef]
- Bordenave, S.; Metz, L.; Flavier, S.; Lambert, K.; Ghanassia, E.; Dupuy, A.M.; Michel, F.; Puech-Cathala, A.M.; Raynaud, E.; Brun, J.F.; et al. Training-induced improvement in lipid oxidation in type 2 diabetes mellitus is related to alterations in muscle mitochondrial activity. Effect of endurance training in type 2 diabetes. Diabetes Metab. 2008, 34, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Romain, A.J.; Carayol, M.; Desplan, M.; Fedou, C.; Ninot, G.; Mercier, J.; Avignon, A.; Brun, J.F. Physical Activity Targeted at Maximal Lipid Oxidation: A Meta-Analysis. J. Nutr. Metab. 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Macera, C.A. Quantifying cardiorespiratory fitness to predict mortality and cardiovascular events: A review. Clin. J. Sport Med. 2010, 20, 224. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.J.; Sanchez-Delgado, G.; Ara, I.; R Ruiz, J. Cardiorespiratory Fitness May Influence Metabolic Inflexibility during Exercise in Obese Persons. J. Clin. Endocrinol. Metab. 2019, 104, 5780–5790. [Google Scholar] [CrossRef]
- Jesus, Í.C.; Alle, L.F.; Munhoz, E.C.; Silva, L.; Lopes, W.A.; Tureck, L.V.; Purim, K.; Titski, A.; Leite, N. Trp64Arg polymorphism of the ADRB3 gene associated with maximal fat oxidation and LDL-C levels in non-obese adolescents. J. Pediatr. 2018, 94, 425–431. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Manual Cochrane de Revisiones Sistemáticas de Intervenciones. 2011. Available online: https://es.cochrane.org/sites/es.cochrane.org/files/public/uploads/Manual_Cochrane_510_reduit.pdf (accessed on 1 May 2020).
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Ben Ounis, O.; Elloumi, M.; Ben Chiekh, I.; Zbidi, A.; Amri, M.; Lac, G.; Tabka, Z. Effects of two-month physical-endurance and diet-restriction programmes on lipid profiles and insulin resistance in obese adolescent boys. Diabetes Metab. 2008, 34, 595–600. [Google Scholar] [CrossRef]
- Ben Ounis, O.; Elloumi, M.; Amri, M.; Zbidi, A.; Tabka, Z.; Lac, G. Impact of diet, exercise and diet combined with exercise programs on plasma lipoprotein and adiponectin levels in obese girls. J. Sport Sci. Med. 2008, 7, 437–445. [Google Scholar]
- Elloumi, M.; Ben Ounis, O.; Makni, E.; Van Praagh, E.; Tabka, Z.; Lac, G. Effect of individualized weight-loss programmes on adiponectin, leptin and resistin levels inobese adolescent boys. Acta Paediatr. 2009, 98, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, X.; Wang, J. Effects of supervised exercise training at the intensity of maximal fat oxidation in overweight young women. J. Exerc. Sci. Fit. 2012, 10, 64–69. [Google Scholar] [CrossRef]
- Besnier, F.; Lenclume, V.; Gérardin, P.; Fianu, A.; Martinez, J.; Naty, N.; Porcherat, S.; Boussaid, K.; Schneebeli, S.; Jarlet, E.; et al. Individualized exercise training at maximal fat oxidation combined with fruit and vegetable-rich diet in overweight or obese women: The LIPOXmax-réunion randomized controlled trial. PLoS ONE 2015, 10, e0139246. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, J.; Cao, L.; Guo, Z.; Wang, Y. Positive effect of exercise training at maximal fat oxidation intensity on body composition and lipid metabolism in overweight middle-aged women. Clin. Physiol. Funct. Imaging 2016, 36, 225–230. [Google Scholar] [CrossRef]
- Tan, S.; Wang, J.; Cao, L. Exercise training at the intensity of maximal fat oxidation in obese boys. Appl. Physiol. Nutr. Metab. 2016, 41, 49–54. [Google Scholar] [CrossRef]
- Tan, S.; Du, P.; Zhao, W.; Pang, J.; Wang, J. Exercise training at maximal fat oxidation intensity for older women with type 2 diabetes. Int. J. Sports Med. 2018, 39, 374–381. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, Y.; Li, Q.; Wang, J.; Tan, S. Exercise training at maximal fat oxidation intensity for overweight or obese older women: A randomized study. J. Sport Sci. Med. 2019, 18, 413–418. [Google Scholar]
- Zeng, J.; Peng, L.; Zhao, Q.; Chen, Q.G. Effects over 12 weeks of different types and durations of exercise intervention on body composition of young women with obesity. Sci. Sports 2020. [Google Scholar] [CrossRef]
- Jiang, Y.; Tan, S.; Wang, Z.; Guo, Z.; Li, Q.; Wang, J. Aerobic exercise training at maximal fat oxidation intensity improves body composition, glycemic control, and physical capacity in older people with type 2 diabetes. J. Exerc. Sci. Fit. 2020, 18, 7–13. [Google Scholar] [CrossRef]
- He, W.; Li, Q.; Yang, M.; Jiao, J.; Ma, X.; Zhou, Y.; Song, A.; Heymsfield, S.B.; Zhang, S.; Zhu, S. Lower BMI cutoffs to define overweight and obesity in China. Obesity 2015, 23, 684–691. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, J.C.; Martinez-Vizcaino, V.; García-Hermoso, A.; Sánchez-López, M.; Arias-Palencia, N.; Fonseca, J.; Mora-Rodriguez, R. Energy Expenditure in Playground Games in Primary School Children Measured by Accelerometer and Heart Rate Monitors. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 467–474. [Google Scholar] [CrossRef]
- Hammoudi, L.; Brun, J.F.; Noirez, P.; Bui, G.; Chevalier, C.; Gimet, F.; Mercier, J.; de Mauverger, R.E. Effects of 2 years endurance training targeted at the level of maximal lipid oxidation on body composition. Sci. Sport 2020. [Google Scholar] [CrossRef]
- Thorogood, A.; Mottillo, S.; Shimony, A.; Filion, K.B.; Joseph, L.; Genest, J.; Pilote, L.; Poirier, P.; Schiffrin, E.L.; Eisenberg, M.J. Isolated aerobic exercise and weight loss: A systematic review and meta-analysis of randomized controlled trials. Am. J. Med. 2011, 124, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Harber, M.P. Skeletal Muscle Hypertrophy After Aerobic Exercise Training. Exerc. Sport Sci. Rev. 2014, 42, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K. Carbohydrate and fat utilization during rest and physical activity. e-Spen. Eur. e-J. Clin. Nutr. Metab. 2011, 6, e45–e52. [Google Scholar] [CrossRef]
- Lazzer, S.; Lafortuna, C.; Busti, C.; Galli, R.; Tinozzi, T.; Agosti, F.; Sartorio, A. Fat oxidation rate during and after a low- or high-intensity exercise in severely obese Caucasian adolescents. Eur. J. Appl. Physiol. 2010, 108, 383–391. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- O’Donovan, G.; Owen, A.; Bird, S.R.; Kearney, E.M.; Nevill, A.M.; Jones, D.W.; Woolf-May, K. Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J. Appl. Physiol. 2005, 98, 1619–1625. [Google Scholar] [CrossRef]
- Groennebaek, T.; Vissing, K. Impact of resistance training on skeletal muscle mitochondrial biogenesis, content, and function. Front. Physiol. 2017, 8, 713. [Google Scholar] [CrossRef]
- Murphy, M.H.; Nevill, A.M.; Murtagh, E.M.; Holder, R.L. The effect of walking on fitness, fatness and resting blood pressure: A meta-analysis of randomised, controlled trials. Prev. Med. 2007, 44, 377–385. [Google Scholar] [CrossRef]
- Özdemir, Ç.; Özgünen, K.; Günaştı, Ö.; Eryılmaz, S.; Kılcı, A.; Kurdak, S. Changes in substrate utilization rates during 40 min of walking within the Fatmax range. Physiol. Int. 2019, 106, 294–304. [Google Scholar] [CrossRef]
- Horowitz, J.F.; Mora-Rodriguez, R.; Byerley, L.O.; Coyle, E.F. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E768–E775. [Google Scholar] [CrossRef]
| Study | Participants, Age (year) | BMI (kg∙m−2) | Exercise Protocol | ∆ BW (kg) | ∆ FM (kg) | ∆ Vo2max (mL∙kg−1∙min−1) |
|---|---|---|---|---|---|---|
| Ounis et al., (2008)a [22] | 8 boys (13.1 ± 0.1) | 30.2 ± 4.2 | FMT: playground activities; 4 day/week; 90 min/session; 8 weeks | −1.90 ± 15.54 | −1.70 ± 6.76 | NR |
| Ounis et al., (2008)b [23] | 6 girls (13.1 ± 0.1) | 30.6 ± 2.3 | FMT: playground activities; 4 day/week; 90 min/session; 8 weeks | −1.40 ± 12.55 | −1.70 ± 4.45 | NR |
| Elloumi et al., (2009) [24] | 7 boys (13.1 ± 0.7) | 30.3 ± 3.2 | FMT: playground activities; 4 day/week; 90 min/session; 8 weeks | −1.70 ± 13.46 | −1.50 ± 6.34 | NR |
| Tan et al. (2012) [25] | 29 women (20–23) | 27.5 ± 1.9 | FMT: running; 3 day/week; 60 min/session; 8 weeks | −4.00 ± 5.33 * | −4.00 ± 11.19 * | 2.80 ± 2.58 * |
| Besnier et al., (2015) [26] | 33 women (30.5 ± 5.9) | 33.3 ± 3.8 | FMT: cycling; 4 day/week; 55 min/session; 20 weeks | −5.00 ± 3.29 * | −4.10 ± 2.88 * | NR |
| Tan et al., (2016)a [27] | 15 women (50.7 ± 5.5) | 28.5 ± 2.1 | FMT: running; 5 day/week; 60 min/session; 12 weeks | −3.00 ± 7.03 * | −2.41 ± 3.64 * | 3.50 ± 4.19 * |
| Tan et al., (2016)b [28] | 11 boys (9.0 ± 1.0) | 27.1 ± 4.3 | FMT: playground activities; 5 day/week; 60 min/session; 10 weeks | −1.00 ± 15.78 * | −1.20 ± 10.88 * | 2.7 ± 2.25 * |
| Tan et al. (2018) [29] | 16 elderly women with T2D (63.0 ± 2.3) | 26.6 ± 3.1 | FMT: running; 3 day/week; 60 min/session; 12 weeks | −2.4 ± 9.47 * | −3.10 ± 4.29 * | NR |
| Cao et al. (2019) [30] | 13 women (63.8 ± 5.9) | 28.0 ± 2.9 | FMT: running; 3 day/week; 60 min/session; 12 weeks | −4.60 ± 10.12 * | −2.80 ± 5.78 * | 4.1 ± 5.81 * |
| Zeng et al. (2019) [31] | 18 women (21.1 ± 1.6) | 26.6 ± 3.1 | FMT: running; 3 day/week; 45 min/session; 12 weeks | NR | −6.09 ± 2.7 * | NR |
| Jiang et al. (2020) [32] | 13 elderly women with T2D a (63.9 ± 6.1) | 26.6 ± 2.2 | FMT: running; 3 day/week; 60 min/session; 16 weeks | −2.10 ± 8.18 * | −2.50 ± 7.29 * | 3.4 ± 5.07 * |
| 14 elderly men with T2D b (63.9 ± 6.1) | 26.9 ± 2.1 | FMT: running; 3 day/week; 60 min/session; 16 weeks | −3.30 ± 7.84 * | −3.7 ± 4.84 * | 3.2 ± 5.97 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Guevara, I.A.; Urquidez-Romero, R.; Pérez-León, J.A.; González-Rodríguez, E.; Moreno-Brito, V.; Ramos-Jiménez, A. Chronic Effect of Fatmax Training on Body Weight, Fat Mass, and Cardiorespiratory Fitness in Obese Subjects: A Meta-Analysis of Randomized Clinical Trials. Int. J. Environ. Res. Public Health 2020, 17, 7888. https://doi.org/10.3390/ijerph17217888
Chávez-Guevara IA, Urquidez-Romero R, Pérez-León JA, González-Rodríguez E, Moreno-Brito V, Ramos-Jiménez A. Chronic Effect of Fatmax Training on Body Weight, Fat Mass, and Cardiorespiratory Fitness in Obese Subjects: A Meta-Analysis of Randomized Clinical Trials. International Journal of Environmental Research and Public Health. 2020; 17(21):7888. https://doi.org/10.3390/ijerph17217888
Chicago/Turabian StyleChávez-Guevara, Isaac A., René Urquidez-Romero, Jorge A. Pérez-León, Everardo González-Rodríguez, Verónica Moreno-Brito, and Arnulfo Ramos-Jiménez. 2020. "Chronic Effect of Fatmax Training on Body Weight, Fat Mass, and Cardiorespiratory Fitness in Obese Subjects: A Meta-Analysis of Randomized Clinical Trials" International Journal of Environmental Research and Public Health 17, no. 21: 7888. https://doi.org/10.3390/ijerph17217888
APA StyleChávez-Guevara, I. A., Urquidez-Romero, R., Pérez-León, J. A., González-Rodríguez, E., Moreno-Brito, V., & Ramos-Jiménez, A. (2020). Chronic Effect of Fatmax Training on Body Weight, Fat Mass, and Cardiorespiratory Fitness in Obese Subjects: A Meta-Analysis of Randomized Clinical Trials. International Journal of Environmental Research and Public Health, 17(21), 7888. https://doi.org/10.3390/ijerph17217888







