Associations between High Serum Adipocyte Fatty Acid Binding Protein and First Hospitalization in Kidney Transplantation Patients: A 5-Year Follow-up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Participants
2.3. Anthropometric Analysis
2.4. Biochemical Analyses
2.5. First Hospitalization Event Monitoring
2.6. Statistical Analysis
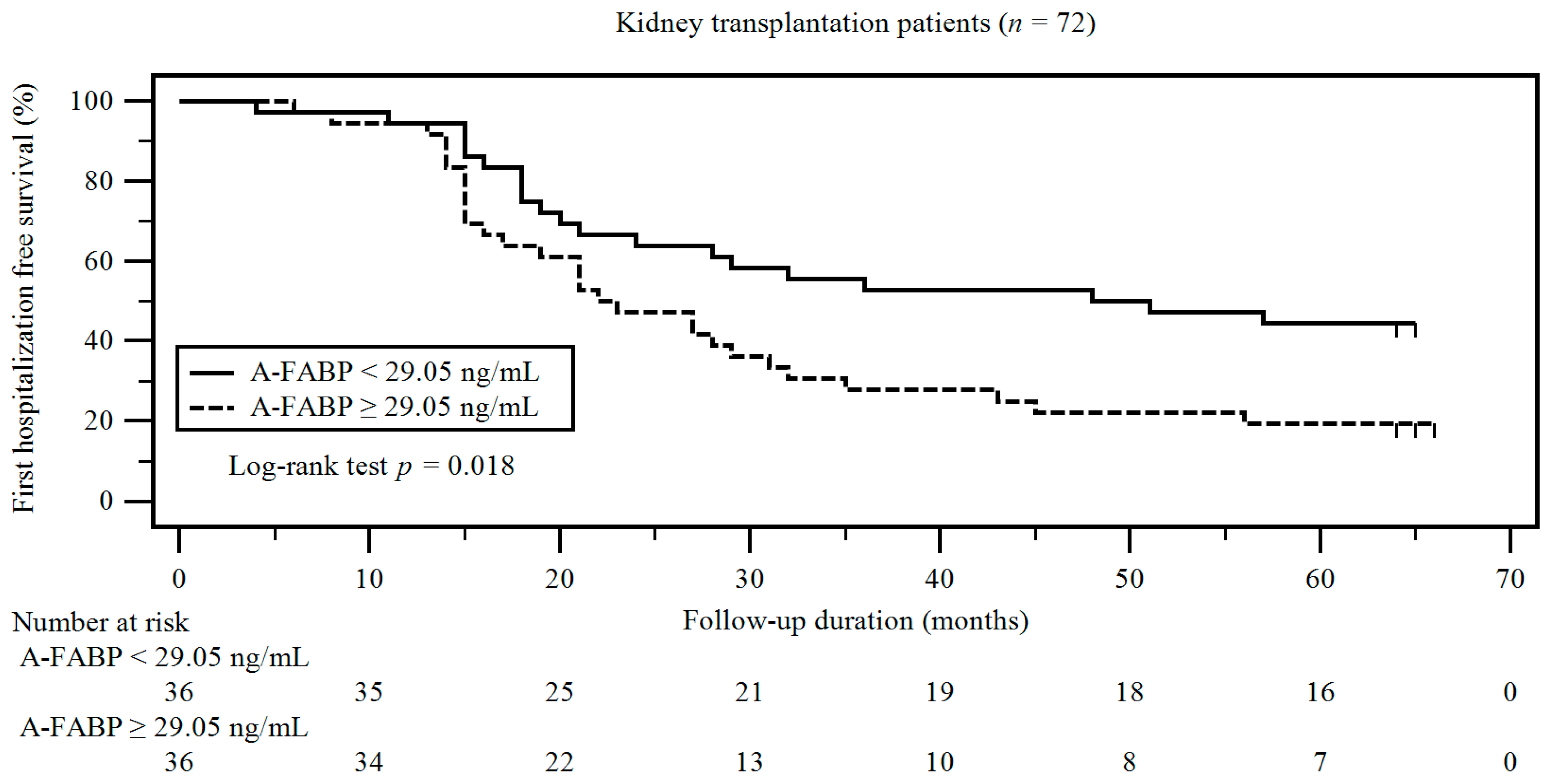
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kralisch, S.; Fasshauer, M. Adipocyte fatty acid binding protein: A novel adipokine involved in the pathogenesis of metabolic and vascular disease? Diabetologia 2013, 56, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Vanhoutte, P.M. Adiponectin and adipocyte fatty acid binding protein in the pathogenesis of cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1231–H1240. [Google Scholar] [CrossRef]
- Ebert, T.; Hopf, L.M.; Wurst, U.; Bachmann, A.; Kralisch, S.; Lössner, U.; Platz, M.; Kratzsch, J.; Stolzenburg, J.U.; Dietel, A.; et al. Circulating adipocyte fatty acid binding protein is increased in chronic and acute renal dysfunction. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1027–1034. [Google Scholar] [CrossRef]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier-Kriesche, H.U.; Schold, J.D.; Srinivas, T.R.; Reed, A.; Kaplan, B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am. J. Transplant. 2004, 4, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Helantera, I.; Raiha, J.; Finne, P.; Lempinen, M. Early failure of kidney transplants in the current era-a national cohort study. Transpl. Int. 2018, 31, 880–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aalten, J.; Hoogeveen, E.K.; Roodnat, J.I.; Weimar, W.; Borm, G.F.; de Fijter, J.W.; Hoitsma, A.J. Associations between pre-kidney-transplant risk factors and post-transplant cardiovascular events and death. Transpl. Int. 2008, 21, 985–991. [Google Scholar] [CrossRef]
- Israni, A.K.; Snyder, J.J.; Skeans, M.A.; Kasiske, B.L. Clinical diagnosis of metabolic syndrome: Predicting new-onset diabetes, coronary heart disease, and allograft failure late after kidney transplant. Transpl. Int. 2012, 25, 748–757. [Google Scholar] [CrossRef]
- Liefeldt, L.; Budde, K. Risk factors for cardiovascular disease in renal transplant recipients and strategies to minimize risk. Transpl. Int. 2010, 23, 1191–1204. [Google Scholar] [CrossRef]
- Khanna, A.; Plummer, M.; Bromberek, C.; Bresnahan, B.; Hariharan, S. Expression of TGF-beta and fibrogenic genes in transplant recipients with tacrolimus and cyclosporine nephrotoxicity. Kidney Int. 2002, 62, 2257–2263. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Sunaga, H.; Hanaoka, H.; Yamaguchi, A.; Kuwahara, S.; Umbarawan, Y.; Nakajima, K.; Machida, T.; Murakami, M.; Saito, A.; et al. Circulating FABP4 is eliminated by the kidney via glomerular filtration followed by megalin-mediated reabsorption. Sci. Rep. 2018, 6, 16451. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. I. Diagnosis and classification of diabetes mellitus: Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Chen, M.C.; Hsu, B.G.; Lee, C.J.; Yang, C.F.; Wang, J.H. High serum adipocyte fatty acid binding protein level as a potential biomarker of aortic arterial stiffness in hypertensive patients with metabolic syndrome. Clin. Chim. Acta 2017, 473, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Hsu, B.G.; Lee, C.J.; Ho, C.C.; Ho, G.J.; Lee, M.C. Serum adipocyte fatty acid-binding protein level is associated with arterial stiffness quantified with cardio-ankle vascular index in kidney transplant patients. Clin. Exp. Nephrol. 2018, 22, 188–195. [Google Scholar] [CrossRef]
- Chen, M.C.; Wang, J.H.; Lee, C.J.; Hsu, B.G. Association between hyperleptinemia and cardiovascular outcomes in patients with coronary artery disease. Ther. Clin. Risk Manag. 2018, 14, 1855–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Luo, N.; Lopes-Virella, M.F.; Garvey, W.T. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis 2002, 165, 259–269. [Google Scholar] [CrossRef]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009, 23, 3865–3873. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Tso, A.W.; Cheung, B.M.; Wang, Y.; Wat, N.M.; Fong, C.H.; Yeung, D.C.; Janus, E.D.; Sham, P.C.; Lam, K.S. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: A 5-year prospective study. Circulation 2007, 115, 1537–1543. [Google Scholar] [CrossRef]
- Park, S.E.; Rhee, E.J.; Lee, W.Y.; Kim, W.J.; Yoo, S.H.; Bae, J.C.; Choi, E.S.; Park, C.Y.; Oh, K.W.; Park, S.W.; et al. The role of serum adipocyte fatty acid-binding protein on the development of metabolic syndrome is independent of pro-inflammatory cytokines. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Cabré, A.; Babio, N.; Lázaro, I.; Bulló, M.; Garcia-Arellano, A.; Masana, L.; Salas-Salvadó, J. FABP4 predicts atherogenic dyslipidemia development. The PREDIMED study. Atherosclerosis 2012, 222, 229–234. [Google Scholar] [CrossRef]
- Iwamoto, M.; Miyoshi, T.; Doi, M.; Takeda, K.; Kajiya, M.; Nosaka, K.; Nakayama, R.; Hirohata, S.; Usui, S.; Kusachi, S.; et al. Elevated serum adipocyte fatty acid-binding protein concentrations are independently associated with renal dysfunction in patients with stable angina pectoris. Cardiovasc. Diabetol. 2012, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Yeung, D.C.; Xu, A.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Fong, C.H.; Tam, S.; Sham, P.C.; Lam, K.S. Circulating levels of adipocyte and epidermal fatty acid-binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. Diabetes Care 2009, 32, 132–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabre, A.; Lazaro, I.; Girona, J.; Manzanares, J.M.; Marimon, F.; Plana, N.; Heras, M.; Masana, L. Plasma fatty acid-binding protein 4 increases with renal dysfunction in type 2 diabetic patients without microalbuminuria. Clin. Chem. 2008, 54, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, G.; Ziegelmeier, M.; Bachmann, A.; Kralisch, S.; Lossner, U.; Kratzsch, J.; Blüher, M.; Stumvoll, M.; Fasshauer, M. Serum levels of adipocyte fatty acid-binding protein (AFABP) are increased in chronic haemodialysis (CD). Clin. Endocrinol. 2008, 69, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Dimeny, E.; Fellstrom, B. Metabolic abnormalities in renal transplant recipients. Risk factors and predictors of chronic graft dysfunction? Nephrol. Dial. Transplant. 1997, 12, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Wissing, K.M.; Abramowicz, D.; Broeders, N.; Vereerstraeten, P. Hypercholesterolemia is associated with increased kidney graft loss caused by chronic rejection in male patients with previous acute rejection. Transplantation 2000, 70, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.L.; Langewisch, E.; Ojo, A. Metabolic syndrome and new onset diabetes after transplantation in kidney transplant recipients. Clin. Transplant. 2010, 24, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Dedinska, I.; Palkoci, B.; Miklusica, J.; Osinova, D.; Galajda, P.; Mokan, M. Metabolic syndrome and new onset diabetes after kidney transplantation. Diabetes Metab. Syndr. 2017, 11, 211–214. [Google Scholar] [CrossRef]
- De Vries, A.P.; Bakker, S.J.; van Son, W.J.; van der Heide, J.J.; Ploeg, R.J.; The, H.T.; de Jong, P.E.; Gans, R.O. Metabolic syndrome is associated with impaired long-term renal allograft function; not all component criteria contribute equally. Am. J. Transplant. 2004, 4, 1675–1683. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef]
- Hsu, B.G.; Chen, Y.C.; Lee, R.P.; Lee, C.C.; Lee, C.J.; Wang, J.H. Fasting serum level of fatty-acid-binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ. J. 2010, 74, 327–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Ryu, D.R.; Kim, S.J.; Choi, K.B.; Kang, D.H. Clinical implication of metabolic syndrome on chronic kidney disease depends on gender and menopausal status: Results from the Korean National Health and Nutrition Examination Survey. Nephrol. Dial. Transplant. 2010, 25, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Chu, C.H.; Hsu, C.H.; Hsieh, P.C.; Chung, T.C.; Bai, C.H.; You, S.L.; Hwang, L.C.; Lin, C.M.; Sun, C.A. Impact of metabolic syndrome on the incidence of chronic kidney disease: A Chinese cohort study. Nephrology 2012, 17, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Pedrollo, E.F.; Correa, C.; Nicoletto, B.B.; Manfro, R.C.; Leitao, C.B.; Souza, G.C.; Gonçalves, L.F. Effects of metabolic syndrome on kidney transplantation outcomes: A systematic review and meta-analysis. Transpl. Int. 2016, 29, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaGuardia, H.; Zhang, R. Obesity and metabolic syndrome in kidney transplantation. Curr. Hypertens. Rep. 2013, 15, 215–223. [Google Scholar] [CrossRef]
- Khalil, M.A.M.; Tan, J. Cigarette smoking and its hazards in kidney transplantation. Adv. Med. 2017, 2017, 6213814. [Google Scholar]
| Variables | All Participants | Low A-FABP Group | High A-FABP Group | p Value |
|---|---|---|---|---|
| (n = 72) | (n = 36) | (n = 36) | ||
| Age (years) | 52.15 ± 9.64 | 51.17 ± 9.87 | 53.14 ± 9.44 | 0.389 |
| KT duration (months) | 72.90 ± 43.27 | 74.39 ± 49.76 | 71.42 ± 36.29 | 0.773 |
| Height (cm) | 162.31 ± 8.23 | 164.42 ± 7.46 | 160.19 ± 8.52 | 0.029 * |
| Body weight (kg) | 62.85 ± 12.40 | 61.75 ± 10.66 | 63.94 ± 13.99 | 0.456 |
| Body mass index (kg/m2) | 23.81 ± 4.20 | 22.77 ± 3.18 | 24.84 ± 4.85 | 0.036 * |
| SBP (mmHg) | 133.79 ± 10.43 | 133.47 ± 15.90 | 144.72 ± 15.68 | 0.003 * |
| DBP (mmHg) | 86.21 ± 11.01 | 84.92 ± 11.13 | 87.50 ± 10.89 | 0.323 |
| Albumin (mg/dL) | 4.14 ± 0.48 | 4.24 ± 0.38 | 4.04 ± 0.55 | 0.082 |
| Total cholesterol (mg/dL) | 195.81 ± 45.47 | 182.81 ± 31.63 | 208.81 ± 53.38 | 0.014 * |
| Triglyceride (mg/dL) | 114.50 (80.25–167.00) | 95.50 (72.00–148.00) | 133.50 (81.50–209.75) | 0.040 * |
| HDL-C (mg/dL) | 51.19 ± 16.09 | 52.36 ± 13.86 | 50.03 ± 18.17 | 0.542 |
| LDL-C (mg/dL) | 108.56 ± 39.43 | 109.32 ± 44.21 | 107.81 ± 34.63 | 0.872 |
| Fasting glucose (mg/dL) | 94.50 (86.50–110.00) | 93.00 (85.25–99.00) | 97.50 (88.00–134.75) | 0.199 |
| Blood urea nitrogen (mg/dL) | 16.00 (17.00–34.75) | 18.00 (14.25–25.75) | 26.00 (19.50–47.00) | 0.001 * |
| Creatinine (mg/dL) | 1.60 (1.230–2.10) | 1.50 (1.13–1.90) | 1.85 (1.43–2.60) | 0.032 * |
| eGFR (mL/min) | 43. 74 ± 21.81 | 50.50 ± 21.24 | 36.97 ± 20.49 | 0.008 * |
| A-FABP (ng/mL) | 40.53 ± 31.37 | 15.91 ± 7.07 | 65.16 ± 26.43 | <0.001 * |
| Female, n (%) | 33 (45.8) | 12 (33.3) | 21 (58.3) | 0.033 * |
| Diabetes, n (%) | 14 (19.4) | 4 (11.1) | 10 (27.8) | 0.074 |
| Hypertension, n (%) | 24 (33.3) | 9 (25.0) | 15 (41.7) | 0.134 |
| Deceased donor KT, n (%) | 63 (87.5) | 34 (94.4) | 29 (80.6) | 0.075 |
| Tacrolimus use, n (%) | 43 (59.7) | 23 (63.9) | 20 (55.6) | 0.471 |
| Mycophenolate mofetil use, n (%) | 53 (73.6) | 26 (72.2) | 27 (75.0) | 0.789 |
| Steroid use, n (%) | 56 (77.8) | 25 (69.4) | 31 (86.1) | 0.089 |
| Rapamycin use, n (%) | 14 (19.4) | 6 (16.7) | 8 (22.2) | 0.551 |
| Cyclosporine use, n (%) | 16 (22.2) | 6 (16.7) | 10 (27.8) | 0.257 |
| Variables | Participants without Hospitalization Events | Participants with Hospitalization Events | p Value |
|---|---|---|---|
| (n = 23) | (n = 49) | ||
| Age (years) | 50.57 ± 8.10 | 52.90 ± 10.27 | 0.342 |
| KT duration (months) | 69.13 ± 40.65 | 74.67 ± 44.74 | 0.616 |
| Height (cm) | 162.83 ± 8.27 | 162.06 ± 8.29 | 0.716 |
| Body weight (kg) | 61.26 ± 11.92 | 63.59 ± 12.66 | 0.461 |
| Body mass index (kg/m2) | 23.00 ± 3.49 | 24.19 ± 4.48 | 0.265 |
| SBP (mmHg) | 131.65 ± 10.03 | 134.80 ± 10.57 | 0.236 |
| DBP (mmHg) | 84.70 ± 10.43 | 86.92 ± 11.30 | 0.428 |
| Albumin (mg/dL) | 4.23 ± 0.34 | 4.10 ± 0.53 | 0.294 |
| Total cholesterol (mg/dL) | 185.13 ± 36.20 | 200.82 ± 48.78 | 0.174 |
| Triglyceride (mg/dL) | 102.00 (77.00–142.00) | 135.00 (80.50–195.00) | 0.040 * |
| HDL-C (mg/dL) | 51.17 ± 12.55 | 51.20 ± 17.63 | 0.994 |
| LDL-C (mg/dL) | 107.00 ± 25.76 | 109.30 ± 44.65 | 0.820 |
| Fasting glucose (mg/dL) | 93.00 (85.00–99.00) | 96.00 (88.00–135.50) | 0.199 |
| Blood urea nitrogen (mg/dL) | 18.00 (14.00–23.00) | 26.00 (18.00–40.50) | 0.012 * |
| Creatinine (mg/dL) | 1.40 (1.00–2.10) | 1.80 (1.35–2.10) | 0.032 * |
| eGFR (mL/min) | 51.04 ± 25.21 | 40.31 ± 19.36 | 0.051 |
| A-FABP (ng/mL) | 26.90 ± 22.69 | 46.93 ± 32.99 | 0.011 * |
| Female, n (%) | 9 (39.1) | 24 (49.0) | 0.434 |
| Diabetes, n (%) | 1 (4.3) | 13 (19.6) | 0.027 * |
| Hypertension, n (%) | 4 (17.4) | 20 (40.8) | 0.049 * |
| Deceased donor KT, n (%) | 19 (82.6) | 44 (89.8) | 0.390 |
| Tacrolimus use, n (%) | 15 (65.2) | 28 (57.1) | 0.515 |
| Mycophenolate mofetil use, n (%) | 19 (82.6) | 34 (69.4) | 0.235 |
| Steroid use, n (%) | 15 (65.2) | 43 (87.8) | 0.024 * |
| Rapamycin use, n (%) | 2 (8.7) | 12 (24.5) | 0.114 |
| Cyclosporine use, n (%) | 6 (26.1) | 10 (20.4) | 0.589 |
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| A-FABP, 1 ng/mL | 1.018 | <0.001 * | 1.019 | 0.001 * | 1.015 | 0.009 * | 1.012 | 0.044 * |
| (1.009–1.021) | (1.008–1.027) | (1.004–1.027) | (1.000–1.025) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-C.; Lee, M.-C.; Chen, M.-C.; Hsu, B.-G. Associations between High Serum Adipocyte Fatty Acid Binding Protein and First Hospitalization in Kidney Transplantation Patients: A 5-Year Follow-up Study. Int. J. Environ. Res. Public Health 2020, 17, 7567. https://doi.org/10.3390/ijerph17207567
Lee W-C, Lee M-C, Chen M-C, Hsu B-G. Associations between High Serum Adipocyte Fatty Acid Binding Protein and First Hospitalization in Kidney Transplantation Patients: A 5-Year Follow-up Study. International Journal of Environmental Research and Public Health. 2020; 17(20):7567. https://doi.org/10.3390/ijerph17207567
Chicago/Turabian StyleLee, Wei-Chen, Ming-Che Lee, Ming-Chun Chen, and Bang-Gee Hsu. 2020. "Associations between High Serum Adipocyte Fatty Acid Binding Protein and First Hospitalization in Kidney Transplantation Patients: A 5-Year Follow-up Study" International Journal of Environmental Research and Public Health 17, no. 20: 7567. https://doi.org/10.3390/ijerph17207567
APA StyleLee, W.-C., Lee, M.-C., Chen, M.-C., & Hsu, B.-G. (2020). Associations between High Serum Adipocyte Fatty Acid Binding Protein and First Hospitalization in Kidney Transplantation Patients: A 5-Year Follow-up Study. International Journal of Environmental Research and Public Health, 17(20), 7567. https://doi.org/10.3390/ijerph17207567







