Misalignment of Stakeholder Incentives in the Opioid Crisis
Abstract
1. Introduction
- Regulations on reimbursement policies, such as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, which partially rewards healthcare systems and providers when patients score high on pain management experience of care, thus making providers more inclined towards opioid analgesics [4];
- Lack of a comprehensive multi-modal pain management strategy [5];
- Barriers in adopting treatments for opioid /substance use disorders [6];
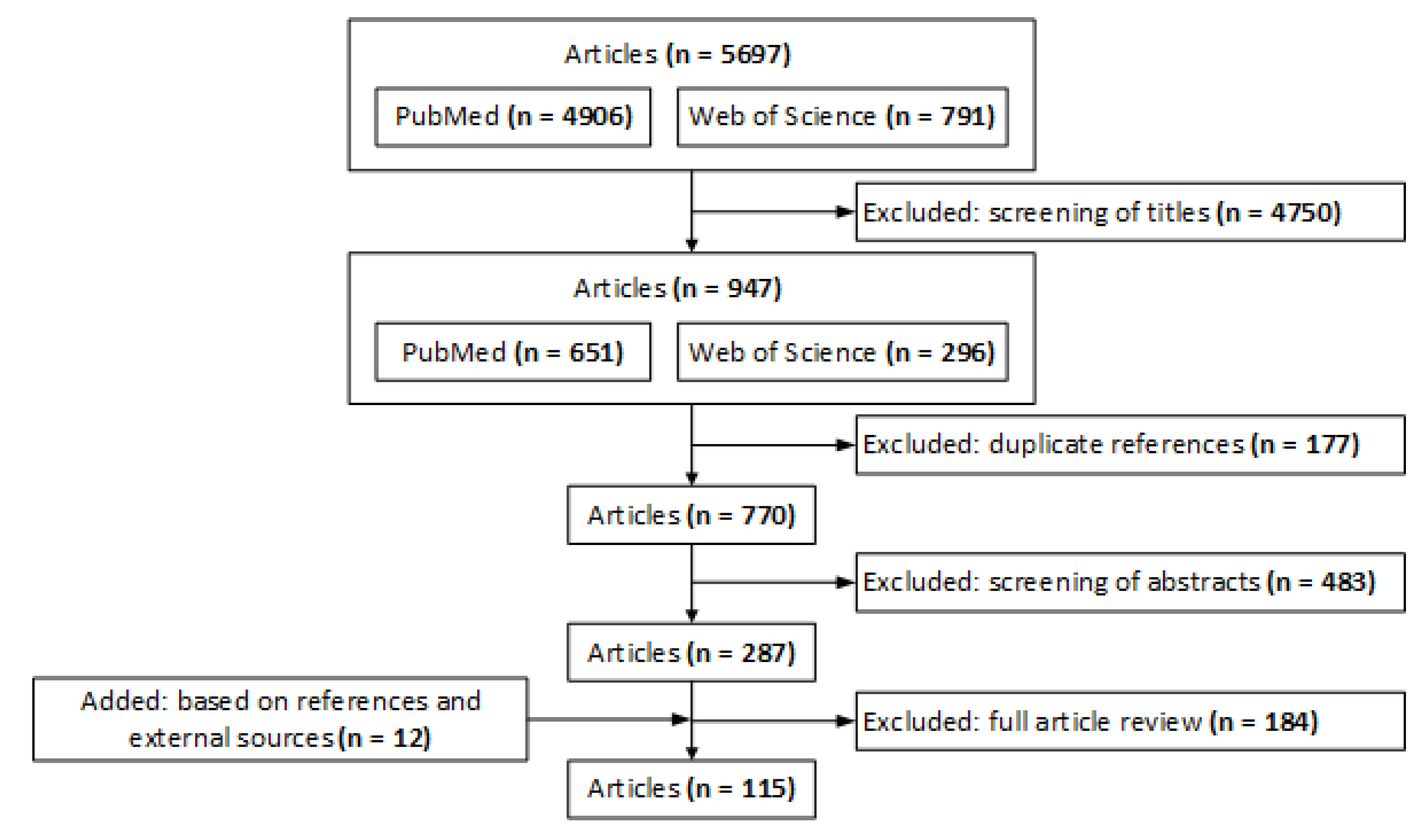
2. Methods
- Category 1: “opioid”.
- Category 2: “alignment”, “misalignment”, “align”, “misaligned”, and “incentive”.
- Category 3: “stakeholder”, (“societal planner”, “payer”, “insurer”, “insurance”, or “coverage”), (“payment”, “reimbursement”, “fee-for-service”, “capitation”, “capitated”, “pay-for-performance”, “bundled payment”, “accountable care”, or “value-based”), (“provider”, “physician”, or “hospital”), “patient”, (“employer” or “employment”), (“pharmaceutical” or “drug”), (“pharmacologic”, “non-pharmacologic”, or “nonpharmacologic”), (“barrier” or “facilitator”), and (“contingent” or “contingency”).
3. Results
3.1. Stakeholder Misalignment Before Onset of OUD/SUD: A Prevention Perspective
3.1.1. Payment Mechanisms, Reimbursement Schemes, and Incentives
3.1.2. Practice Guidelines and Healthcare System Structures
- Providers do not have a clear idea about how to easily implement these guidelines in their practices or there exist uncertainties surrounding the impact of the recommendations on patient pain levels, particularly in the presence of comorbidities [27].
- Across different specialties/medical conditions, (i) there is no consensus among providers in selecting optimal treatments, and (ii) there are various perspectives on how opioids are deemed appropriate, resulting in many of the providers not aligning with the guidelines and/or significant variations among them in opioid prescription [28,29,30]. On a similar note, emergency departments (EDs) are shown to be more aligned with the CDC guidelines than non-EDs [31].
3.1.3. Multi-Modal Pain Management
3.1.4. Initiatives for Opioid Prescription/Side Effects Reduction
3.1.5. Physician-Patient Shared Decision Making
3.2. Stakeholder Misalignment After Onset of OUD/SUD: An Intervention Perspective
3.2.1. Barriers in Adopting Treatments
3.2.2. Facilitators to Adopting Treatments
4. Discussion
- Payers, providers, and patients due to conventional payment mechanisms such as FFS and capitation, lack of proper insurance coverage for multi-modal pain management, and system structures such as dual drug benefit programs for VA and Medicare Part D enrollees resulting in care fragmentation;
- Policy makers and providers due to guidelines that are not easily translatable for implementation in practice;
- Providers and patients due to lack of shared decision making on treatments, which is also common in the intervention stage.
- Payers, providers, and patients due to lack of proper insurance coverage for OUD/SUD treatments, the limited number of providers for prescribing treatments, and lack of effective incentives and reimbursements for providers;
- Pharmaceutical companies, payers, and patients due to the high cost of medications;
- Providers (PCPs and specialists) due to care fragmentation and lack of proper guidelines to streamline pathways for patients.
- (1)
- The co-occurrence of OUD/SUD and chronic pain can impose pressure on providers due to multi-layered and complex treatment requirements, lack of patient improvement for either condition, and care fragmentation caused by ineffective pain management referrals [6].
- (2)
- Guidelines that promote curbing the supply of opioids may have unintended consequences such as the increase in the number of deaths caused by fentanyl misuse. In the presence of conflicting interests, one can investigate how facilitating aligning incentives can contribute to remedying such effects.
- (3)
- Stigma and discrimination against people with concurrent OUD/SUD and mental health disorders can stymie an effective care delivery process [132].
- (4)
- Although incurred medical expenditures for OUD/SUD would be higher than that for under-treated pain [133], employers’ cost of lost productivity would not be much different, because their employees could miss work due to both OUD/SUD and unrelieved pain [134,135]. Hence, the role of employers should not be limited to expanding access to OUD/SUD treatments. Indeed, employers’ contribution to employment-based insurance coverage would impact the availability of treatment options and the cost of prescription drugs [136], which, in turn, affects pain management outcomes.
- (5)
- Strategies like contingency management, aimed at improving OUD/SUD treatment adherence and retention in opioid substitution programs, have been reported to be effective only in the short term (due to financial/resource limitations), and their efficacy over the long term is yet to be investigated [137].
- (6)
- The timing of initiating OUD/SUD treatments is a deciding factor in their success. However, patients at higher risk may not be always easy to identify. To address this, one can benefit from points of access to patients to potentially initiating treatments. These include ED visit/hospital admission [138,139,140,141,142] and incarceration [143,144,145,146,147,148,149]. Employing techniques like screening, brief intervention, and referral to treatment (SBIRT) can also be helpful in this regard [75].
- (7)
- (8)
- As a result of opioid consumption ramping up during the COVID-19 pandemic [9,10], the long-term rates of OUD/SUD can be impacted as well, which can inevitably aggravate misaligned incentives. In addition to the avenues discussed thus far, this is another stream that warrants further investigation and knowledge production.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- NIDA: National Institute on Drug Abuse. Overdose Death Rates. 2020. Available online: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates (accessed on 10 February 2020).
- Muennig, P.A.; Reynolds, M.; Fink, D.S.; Zafari, Z.; Geronimus, A.T. America’s declining well-being, health, and life expectancy: Not just a white problem. Am. J. Public Health 2018, 108, 1626–1631. [Google Scholar] [CrossRef]
- APHA: American Public Health Association. Suicide, Opioids Tied to Ongoing Fall in US Life Expectancy: Third Year of Drop. 2019. Available online: http://thenationshealth.aphapublications.org/content/49/1/1.2 (accessed on 10 February 2020).
- Zgierska, A.; Rabago, D.; Miller, M.M. Impact of patient satisfaction ratings on physicians and clinical care. Patient Prefer. Adher. 2014, 8, 437–446. [Google Scholar] [CrossRef]
- Bonnie, R.J.; Schumacher, M.A.; Clark, D.J.; Kesselheim, A.S. Pain management and opioid regulation: Continuing public health challenges. Am. J. Public Health 2019, 109, 31–34. [Google Scholar] [CrossRef]
- Beitel, M.; Oberleitner, L.; Kahn, M.; Kerns, R.D.; Liong, C.; Madden, L.M.; Ginn, J.; Barry, D.T. Drug counselor responses to patients’ pain reports: A qualitative investigation of barriers and facilitators to treating patients with chronic pain in methadone maintenance treatment. Pain Med. 2017, 18, 2152–2161. [Google Scholar] [CrossRef]
- Van Zee, A. The promotion and marketing of oxycontin: Commercial triumph, public health tragedy. Am. J. Public Health 2009, 99, 221–227. [Google Scholar] [CrossRef]
- Scher, C.; Meador, L.; Van Cleave, J.H.; Reid, M.C. Moving beyond pain as the fifth vital sign and patient satisfaction scores to improve pain care in the 21st century. Pain Manag. Nurs. 2018, 19, 125–129. [Google Scholar] [CrossRef]
- Silva, M.J.; Kelly, Z. The Escalation of the Opioid Epidemic due to COVID-19 and Resulting Lessons about Treatment Alternatives. 2020. Available online: https://ajmc.s3.amazonaws.com/_media/_pdf/AJMC_07_2020_Silva%20final.pdf (accessed on 10 July 2020).
- Academia, E.C.; Gabriel, C.J.; Mueller, A.; Schwarz, K.A.; Bartels, K.; Valuck, R.J.; Reynolds, P.M. Opioid Prescribing after Discharge in a Previously Mechanically Ventilated, Opioid-Naive Cohort. Ann. Pharmacother. 2020, 54, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. Jama 2016, 315, 1624–1645. [Google Scholar] [CrossRef] [PubMed]
- HCAHPS: Hospital Consumer Assessment of Healthcare Providers and Systems. What’s New. 2020. Available online: https://hcahpsonline.org/en/whats-new/#CommAboutPain (accessed on 17 February 2020).
- Kertesz, S.G.; Satel, S.L.; DeMicco, J.; Dart, R.C.; Alford, D.P. Opioid discontinuation as an institutional mandate: Questions and answers on why we wrote to the Centers for Disease Control and Prevention. Subst. Abus. 2019, 40, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Fink, A. Conducting Research Literature Reviews: From the Internet to Paper; Sage Publications: New York, NY, USA, 2019. [Google Scholar]
- Jayawardhana, J.; Abraham, A.J.; Young, H.N.; Perri, M., III. Opioids in Georgia Medicaid: Gender and insurance disparities in utilization and potential inappropriate prescribing practices. J. Pharmaceut. Health Serv. Res. 2018, 9, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.L.; Ye, X.; Stevens, V.; Oderda, G.M. The prevalence and cost of Medicare beneficiaries diagnosed and at risk for opioid abuse, dependence, and poisoning. J. Manag. Care Spec. Pharm. 2019, 25, 18–27. [Google Scholar] [CrossRef] [PubMed]
- IPRCC: Interagency Pain Research Coordinating Committee. National Pain Strategy. A Comprehensive Population Health-Level Strategy for Pain. 2015. Available online: https://www.iprcc.nih.gov/sites/default/files/HHSNational_Pain_Strategy_508C.pdf (accessed on 10 February 2020).
- The U.S. Department of Health and Human Services. National Pain Strategy: A comprehensive population health-level strategy for pain. In Interagency Pain Research Coordinating Committee and Others; United States Department of Health and Human Services (HHS): Washington, DC, USA, 2016. [Google Scholar]
- Sinnenberg, L.E.; Wanner, K.J.; Perrone, J.; Barg, F.K.; Rhodes, K.V.; Meisel, Z.F. What factors affect physicians’ decisions to prescribe opioids in emergency departments? MDM Policy Pract. 2017, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schulman, M.; O’Brien, J.; Pierre-Wright, M.; Thomas-Henkel, C. Exploring Value-Based Payment to Encourage Substance Use Disorder Treatment in Primary Care. 2018. Available online: https://www.chcs.org/media/VBP-for-SUD_Final_June-2018.pdf (accessed on 20 March 2020).
- Dekker, A.B.E.; Kleiss, I.; Batra, N.; Seghers, M.; Schipper, I.B.; Ring, D.; Claborn, K. Patient and clinician incentives and barriers for opioid use for musculoskeletal disorders a qualitative study on opioid use in musculoskeletal setting. J. Orthopaed. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tefera, L.; Lehrman, W.G.; Goldstein, E.G.; Agrawal, S. A special contribution from the Centers for Medicare and Medicaid Services: Valuing patient experience while addressing the prescription opioid epidemic. Ann. Emerg. Med. 2016, 69, 181–183. [Google Scholar] [CrossRef]
- Carrico, J.A.; Mahoney, K.; Raymond, K.M.; Mims, L.; Smith, P.C.; Sakai, J.T.; Mikulich-Gilbertson, S.K.; Hopfer, C.J.; Bartels, K. The association of patient satisfaction-based incentives with primary care physician opioid prescribing. J. Am. Board Fam. Med. 2018, 31, 941–943. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Shallcross, M.L.; Schäfer, W.L.; Buckley, B.A.; Stulberg, J.J.; Holl, J.L.; Bilimoria, K.Y.; Johnson, J.K. Minimizing opioid prescribing in surgery (MOPiS) initiative: An analysis of implementation barriers. J. Surg. Res. 2019, 239, 309–319. [Google Scholar] [CrossRef]
- DiRocco, D.; Day, S. A better approach to opioid prescribing in primary care. J. Fam. Pract. 2014, 63, E1–E8. [Google Scholar]
- Tenney, L.; McKenzie, L.M.; Matus, B.; Mueller, K.; Newman, L.S. Effect of an opioid management program for Colorado workers’ compensation providers on adherence to treatment guidelines for chronic pain. Am. J. Ind. Med. 2019, 62, 21–29. [Google Scholar] [CrossRef]
- Robinson-Papp, J.; Aberg, J.; Benn, E.K.; Bryan, A.; Cedillo, G.; Chikamoto, Y.; George, M.C.; Horn, B.; Kamler, A.; Navis, A.; et al. Decreasing risk among HIV patients on opioid therapy for chronic pain: Development of the TOWER intervention for HIV care providers. Contemp. Clin. Trials Commun. 2019, 16, 100468. [Google Scholar] [CrossRef]
- Ringwalt, C.; Gugelmann, H.; Garrettson, M.; Dasgupta, N.; Chung, A.E.; Proescholdbell, S.K.; Skinner, A.C. Differential prescribing of opioid analgesics according to physician specialty for Medicaid patients with chronic noncancer pain diagnoses. Pain Res. Manag. 2014, 19. [Google Scholar] [CrossRef]
- Hannon, C.P.; Keating, T.C.; Lange, J.K.; Ricciardi, B.F.; Waddell, B.S.; Della Valle, C.J. Anesthesia and analgesia practices in total joint arthroplasty: A survey of the American association of hip and knee surgeons membership. J. Arthroplast. 2019, 34, 2872–2877. [Google Scholar] [CrossRef] [PubMed]
- Mikosz, C.A.; Zhang, K.; Haegerich, T.; Xu, L.; Losby, J.L.; Greenspan, A.; Baldwin, G.; Dowell, D. Indication-Specific Opioid Prescribing for US Patients with Medicaid or Private Insurance, 2017. JAMA Netw. Open 2020, 3, e204514. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, M.M.; Hooten, W.M.; Hess, E.P.; Meara, E.R.; Ross, J.S.; Henk, H.J.; Borgundvaag, B.; Shah, N.D.; Bellolio, M.F. Opioid prescribing for opioid-naive patients in emergency departments and other settings: Characteristics of prescriptions and association with long-term use. Ann. Emerg. Med. 2018, 71, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.K.; Huang, Y.; Meisel, Z.; Hennessy, S.; Yokell, M.; Polsky, D.; Perrone, J. National variation in opioid prescribing and risk of prolonged use for opioid-naive patients treated in the emergency department for ankle sprains. Ann. Emerg. Med. 2018, 72, 389–400. [Google Scholar] [CrossRef]
- Linnaus, M.E.; Sheaffer, W.W.; Ali-Mucheru, M.N.; Velazco, C.S.; Neville, M.; Gray, R.J. The opioid crisis and surgeons: National survey of prescribing patterns and the influence of motivators, experience, and gender. Am. J. Surg. 2019, 217, 1116–1120. [Google Scholar] [CrossRef]
- Moyo, P.; Zhao, X.; Thorpe, C.T.; Thorpe, J.M.; Sileanu, F.E.; Cashy, J.P.; Hale, J.A.; Mor, M.K.; Radomski, T.R.; Donohue, J.M.; et al. Dual receipt of prescription opioids from the department of Veterans Affairs and Medicare Part D and prescription opioid overdose death among Veterans: A nested case–control study. Ann. Intern. Med. 2019, 170, 433–442. [Google Scholar] [CrossRef]
- Schleiden, L.J.; Thorpe, C.T.; Cashy, J.P.; Gellad, W.F.; Good, C.B.; Hanlon, J.T.; Mor, M.K.; Niznik, J.D.; Pleis, J.R.; Van Houtven, C.H.; et al. Characteristics of dual drug benefit use among veterans with dementia enrolled in the Veterans Health Administration and Medicare Part D. Res. Soc. Adm. Pharm. 2019, 15, 701–709. [Google Scholar] [CrossRef]
- Greenwald, M.K.; Steinmiller, C.L. Behavioral economic analysis of opioid consumption in heroin-dependent individuals: Effects of alternative reinforcer magnitude and post-session drug supply. Drug Alcohol Depend. 2009, 104, 84–93. [Google Scholar] [CrossRef]
- Weeks, W.B.; Goertz, C.M. Cross-sectional analysis of per capita supply of doctors of chiropractic and opioid use in younger Medicare beneficiaries. J. Manip. Physiol. Ther. 2016, 39, 263–266. [Google Scholar] [CrossRef][Green Version]
- Maiers, M.; Agaoglu, M.; Brown, R.; Cassirer, C.; DaSilva, K.; Lystad, R.P.; Mohammad, S.; Wong, J.J. Chiropractic in Global Health and wellbeing: A white paper describing the public health agenda of the World Federation of Chiropractic. Chiropr. Man. Ther. 2018, 26, 26. [Google Scholar] [CrossRef]
- Karmali, R.N.; Skinner, A.C.; Trogdon, J.G.; Weinberger, M.; George, S.Z.; Hassmiller, L.K. The association between the supply of nonpharmacologic providers, use of nonpharmacologic pain treatments, and high-risk opioid prescription patterns among Medicare beneficiaries With persistent musculoskeletal pain. Med. Care 2020, 58, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Edelen, C.; Perlow, M. A comparison of the effectiveness of an opioid analgesic and a nonpharmacologic intervention to improve incentive spirometry volumes. Pain Manag. Nurs. 2002, 3, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.H.; Pock, A.; Ling, C.G.; Kwon, K.N.; Vaughan, M. Battlefield acupuncture: Opening the door for acupuncture in Department of Defense/Veteran’s Administration health care. Nurs. Outlook 2016, 64, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kayhan, G.E.; Sanli, M.; Ozgul, U.; Kirteke, R.; Yologlu, S. Comparison of intravenous ibuprofen and acetaminophen for postoperative multimodal pain management in bariatric surgery: A randomized controlled trial. J. Clin. Anesth. 2018, 50, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Bao, Y.; Wallace, M.; Grant, I.; Shi, Y. Medical cannabis legalization and opioid prescriptions: Evidence on US Medicaid enrollees during 1993–2014. Addiction 2018, 113, 2060–2070. [Google Scholar] [CrossRef]
- Sohler, N.L.; Starrels, J.L.; Khalid, L.; Bachhuber, M.A.; Arnsten, J.H.; Nahvi, S.; Jost, J.; Cunningham, C.O. Cannabis use is associated with lower odds of prescription opioid analgesic use among HIV-infected individuals with chronic pain. Subst. Use Misuse 2018, 53, 1602–1607. [Google Scholar] [CrossRef]
- Wen, H.; Hockenberry, J.M. Association of medical and adult-use marijuana laws with opioid prescribing for Medicaid enrollees. JAMA Intern. Med. 2018, 178, 673–679. [Google Scholar] [CrossRef]
- Chihuri, S.; Li, G. State marijuana laws and opioid overdose mortality. Inj. Epidemiol. 2019, 6, 38. [Google Scholar] [CrossRef]
- Clem, S.N.; Bigand, T.L.; Wilson, M. Cannabis use motivations among adults prescribed opioids for pain versus opioid addiction. Pain Manag. Nurs. 2020, 21, 43–47. [Google Scholar] [CrossRef]
- Scala, E.; Decosterd, I.; Faouzi, M.; Burnand, B.; Rodondi, P.Y. Level of readiness of chronic pain patients to practise active self-care. Eur. J. Pain 2018, 22, 1800–1812. [Google Scholar] [CrossRef]
- Mattocks, K.; Rosen, M.I.; Sellinger, J.; Ngo, T.; Brummett, B.; Higgins, D.M.; Reznik, T.E.; Holtzheimer, P.; Semiatin, A.M.; Stapley, T.; et al. Pain Care in the Department of Veterans Affairs: Understanding How a Cultural Shift in Pain Care Impacts Provider Decisions and Collaboration. Pain Med. 2020, 21, 970–977. [Google Scholar] [CrossRef]
- Becker, W.C.; Dorflinger, L.; Edmond, S.N.; Islam, L.; Heapy, A.A.; Fraenkel, L. Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Fam. Pract. 2017, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Bonnie, R.J.; Ford, M.A.; Phillips, J.K. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Heyward, J.; Jones, C.M.; Compton, W.M.; Lin, D.H.; Losby, J.L.; Murimi, I.B.; Baldwin, G.T.; Ballreich, J.M.; Thomas, D.A.; Bicket, M.C.; et al. Coverage of nonpharmacologic treatments for low back pain among US public and private insurers. JAMA Netw. Open. 2018, 1, e183044. [Google Scholar] [CrossRef] [PubMed]
- Ankawi, B.; Kerns, R.D.; Edmond, S.N. Enhancing motivation for change in the management of chronic painful conditions: A review of recent literature. Curr. Pain Headache Rep. 2019, 23, 75. [Google Scholar] [CrossRef]
- Katz, N.P.; Birnbaum, H.; Brennan, M.J.; Freedman, J.D.; Gilmore, G.P.; Jay, D.; Kenna, G.A.; Madras, B.K.; McElhaney, L.; Weiss, R.D.; et al. Prescription opioid abuse: Challenges and opportunities for payers. Am. J. Manag. Care 2013, 19, 295. [Google Scholar]
- Moyo, P.; Simoni-Wastila, L.; Griffin, B.A.; Onukwugha, E.; Harrington, D.; Alexer, G.C.; Palumbo, F. Impact of prescription drug monitoring programs (PDMPs) on opioid utilization among Medicare beneficiaries in 10 US States. Addiction 2017, 112, 1784–1796. [Google Scholar] [CrossRef] [PubMed]
- Hartung, D.M.; Alley, L.; Leichtling, G.; Korthuis, P.T.; Hildebran, C. A statewide effort to reduce high-dose opioid prescribing through coordinated care organizations. Addict. Behav. 2018, 86, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Keast, S.L.; Kim, H.; Deyo, R.A.; Middleton, L.; McConnell, K.J.; Zhang, K.; Ahmed, S.M.; Nesser, N.; Hartung, D.M. Effects of a prior authorization policy for extended-release/long-acting opioids on utilization and outcomes in a state Medicaid program. Addiction 2018, 113, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Mospan, G.; Gillette, C.; Chaplin, M.; Bush, M. Do more opioid policies reduce opioid dispensing in traditional medicaid?: A national analysis. Res. Soc. Adm. Pharm. 2019, 15, 1000–1006. [Google Scholar] [CrossRef]
- Davis, M.T.; Bateman, B.; Avorn, J. Educational outreach to opioid prescribers: The case for academic detailing. Pain Phys. 2017, 20, S147–S151. [Google Scholar] [CrossRef]
- Kunstler, B.E.; Lennox, A.; Bragge, P. Changing prescribing behaviours with educational outreach: An overview of evidence and practice. BMC Med. Educ. 2019, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Sceats, L.A.; Ayakta, N.; Merrell, S.B.; Kin, C. Drivers, beliefs, and barriers surrounding surgical opioid prescribing: A qualitative study of surgeons’ opioid prescribing habits. J. Surg. Res. 2020, 247, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.L.; Lerner, J.; McGirt, M.J. Effect of minimally invasive technique on return to work and narcotic use following transforaminal lumbar inter-body fusion: A review. Prof. Case Manag. 2012, 17, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, R.S.; Hirschi, M.; Alford, B.; Evans, T.; Kiluk, J.V.; Patel, S.Y. Enhanced revenue after surgery? A cost-standardized enhanced recovery pathway for mastectomy decreases length of stay. World J. Surg. 2019, 43, 839–845. [Google Scholar] [CrossRef]
- Tadrous, M.; Greaves, S.; Martins, D.; Nadeem, K.; Singh, S.; Mamdani, M.M.; Juurlink, D.N.; Gomes, T. Evaluation of the fentanyl patch-for-patch program in Ontario, Canada. Int. J. Drug Policy 2019, 66, 82–86. [Google Scholar] [CrossRef]
- Pardo, D.; Miller, L.; Chiulli, D. Implementation of a pharmacy consult to reduce co-prescribing of opioids and benzodiazepines in a veteran population. Subst. Abus. 2017, 38, 157–160. [Google Scholar] [CrossRef]
- Zhuang, T.; Shapiro, L.M.; Ring, D.; Akelman, E.; Richard, M.J.; Ladd, A.; Blazar, P.; Yao, J.; Kakar, S.; Harris, A.H.; et al. Quality measures to reduce opioid use after common soft tissue hand and wrist procedures. J. Hand Surg. 2020. [Google Scholar] [CrossRef]
- Lo-Ciganic, W.H.; Huang, J.L.; Zhang, H.H.; Weiss, J.C.; Wu, Y.; Kwoh, C.K.; Donohue, J.M.; Cochran, G.; Gordon, A.J.; Malone, D.C.; et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among medicare beneficiaries with opioid prescriptions. JAMA Netw. Open 2019, 2, e190968. [Google Scholar] [CrossRef]
- Tran, S.; Lavitas, P.; Stevens, K.; Greenwood, B.C.; Clements, K.; Alper, C.J.; Lenz, K.; Price, M.; Hydery, T.; Arnold, J.L.; et al. The effect of a federal controlled substance act schedule change on hydrocodone combination products claims in a Medicaid population. J. Manag. Care Spec. Pharm. 2017, 23, 532–539. [Google Scholar] [CrossRef]
- Meara, E.; Horwitz, J.R.; Powell, W.; McClell, L.; Zhou, W.; O’malley, A.J.; Morden, N.E. State legal restrictions and prescription-opioid use among disabled adults. N. Engl. J. Med. 2016, 375, 44–53. [Google Scholar] [CrossRef]
- Henry, S.G.; Bell, R.A.; Fenton, J.J.; Kravitz, R.L. Goals of chronic pain management: Do patients and primary care physicians agree and does it matter? Clin. J. Pain 2017, 33, 955. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.G.; Matthias, M.S. Patient-clinician communication about pain: A conceptual model and narrative review. Pain Med. 2018, 19, 2154–2165. [Google Scholar] [CrossRef] [PubMed]
- Connock, M.; Juarez-Garcia, A.; Jowett, S.; Frew, E.; Liu, Z.; Taylor, R.J.; Fry-Smith, A.; Day, E.; Lintzeris, N.; Roberts, T.; et al. Methadone and buprenorphine for the management of opioid dependence: A systematic review and economic evaluation. In NIHR Health Technology Assessment Programme: Executive Summaries; NIHR Journals Library: Southampton, UK, 2007. [Google Scholar]
- Notley, C.; Blyth, A.; Maskrey, V.; Pinto, H.; Holland, R. Exploring the concepts of abstinence and recovery through the experiences of long-term opiate substitution clients. Subst. Abus. 2015, 36, 232–239. [Google Scholar] [CrossRef]
- Kidorf, M.; King, V.L.; Peirce, J.; Kolodner, K.; Brooner, R.K. An observation of lower rates of drug use over time in community syringe exchangers. Am. J. Addict. 2013, 22, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Agerwala, S.M.; McCance-Katz, E.F. Integrating screening, brief intervention, and referral to treatment (SBIRT) into clinical practice settings: A brief review. J. Psychoact. Drugs 2019, 44, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Rhee, T.G.; Rosenheck, R.A. Use of drug treatment services among adults with opioid use disorder: Rates, patterns, and correlates. Psychiat. Serv. 2019, 70, 992–999. [Google Scholar] [CrossRef]
- Barry, D.T.; Irwin, K.S.; Jones, E.S.; Becker, W.C.; Tetrault, J.M.; Sullivan, L.E.; Hansen, H.; O’Connor, P.G.; Schottenfeld, R.S.; Fiellin, D.A. Integrating buprenorphine treatment into office-based practice: A qualitative study. J. Gen. Intern. Med. 2009, 24, 218–225. [Google Scholar] [CrossRef]
- Haffajee, R.L.; Bohnert, A.S.; Lagisetty, P.A. Policy pathways to address provider workforce barriers to buprenorphine treatment. Am. J. Prev. Med. 2018, 54, S230–S242. [Google Scholar] [CrossRef]
- NIDA: National Institute on Drug Abuse. How Much Does Opioid Treatment Cost? 2018. Available online: https://www.drugabuse.gov/publications/research-reports/medications-to-treat-opioid-addiction/how-much-does-opioid-treatment-cost (accessed on 10 February 2020).
- Samples, H.; Williams, A.R.; Olfson, M.; Crystal, S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J. Subst. Abus. Treat. 2018, 95, 9–17. [Google Scholar] [CrossRef]
- Jones, C.M.; McCance-Katz, E.F. Characteristics and prescribing practices of clinicians recently waivered to prescribe buprenorphine for the treatment of opioid use disorder. Addiction 2019, 114, 471–482. [Google Scholar] [CrossRef]
- McLean, K.; Kavanaugh, P.R. “They’re making it so hard for people to get help:” Motivations for non-prescribed buprenorphine use in a time of treatment expansion. Int. J. Drug Policy 2019, 71, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Proctor, S.L.; Herschman, P.L.; Lee, R.; Kopak, A.M. The role of patient payment method in premature discharge from methadone maintenance treatment. Subst. Use Misuse 2019, 54, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Do, V.; Behar, E.; Turner, C.; Geier, M.; Coffin, P. Acceptability of naloxone dispensing among pharmacists. J. Pharm. Pract. 2020, 33, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.; Goodyear-Smith, F.; Butler, R.; Wheeler, A.; Gohns, A. Barriers to, and incentives for, the transfer of opioid-dependent people on methadone maintenance treatment from secondary care to primary health care. Drug Alcohol Rev. 2008, 27, 178–184. [Google Scholar] [CrossRef]
- Chu, C.; Galang, A. Hospital nurses’ attitudes toward patients with a history of illicit drug use. Can. Nurse 2013, 109, 29. [Google Scholar]
- Larsen, T.; Sagvaag, H. Empowerment and pathologization: A case study in Norwegian mental health and substance abuse services. Health Expect. 2018, 21, 1231–1240. [Google Scholar] [CrossRef]
- Russell, C.; Neufeld, M.; Sabioni, P.; Varatharajan, T.; Ali, F.; Miles, S.; Henderson, J.; Fischer, B.; Rehm, J. Assessing service and treatment needs and barriers of youth who use illicit and non-medical prescription drugs in Northern Ontario, Canada. PLoS ONE 2019, 14, e0225548. [Google Scholar] [CrossRef]
- Andrews, C.M.; D’Aunno, T.A.; Pollack, H.A.; Friedmann, P.D. Adoption of evidence-based clinical innovations: The case of buprenorphine use by opioid treatment programs. Med. Care Res. Rev. 2014, 71, 43–60. [Google Scholar] [CrossRef]
- Berends, L.; Larner, A.; Lubman, D.I. Delivering opioid maintenance treatment in rural and remote settings. Aust. J. Rural Health 2015, 23, 201–206. [Google Scholar] [CrossRef]
- Thomas, C.P. Addressing Workforce Needs for Medication Treatment of Opioid Use Disorder. J. Addict. Med. 2019, 13, 1–2. [Google Scholar] [CrossRef]
- SAMHSA-HRSA Center for Integrated Health Solutions (CIHS). Expanding the Use of Medications to Treat Individuals with Substance Use Disorders. 2014. Available online: https://www.thenationalcouncil.org/wp-content/uploads/2020/01/Expanding_the_Use_of_Medications_to_Treat_Individuals_with_SU_Disorders_in_Safety_Net_Settings.pdf?daf=375ateTbd56 (accessed on 10 February 2020).
- Office of National Drug Control Policy, U.S. Department of Agriculture (USDA). Federal Resources for Rural Communities to Help Address Substance Use Disorder and Opioid Misuse. 2020. Available online: https://www.ruralcommunitytoolbox.org/assets/3664-15201/federal-rural-resource-guide.pdf (accessed on 10 February 2020).
- CVS Health. CVS Health Expands Efforts to Educate Patients about Naloxone. 2018. Available online: https://cvshealth.com/news-and-insights/press-releases/cvs-health-expands-efforts-to-educate-patients-about-naloxone (accessed on 10 February 2020).
- Miele, G.M.; Caton, L.; Freese, T.E.; McGovern, M.; Darfler, K.; Antonini, V.P.; Perez, M.; Rawson, R. Implementation of the hub and spoke model for opioid use disorders in California: Rationale, design and anticipated impact. J. Subst. Abus. Treat. 2020, 108, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kilaru, A.S.; Perrone, J.; Kelley, D.; Siegel, S.; Lubitz, S.F.; Mitra, N.; Meisel, Z.F. Participation in a hospital incentive program for follow-up treatment for opioid use disorder. JAMA Netw. Open 2020, 3, e1918511. [Google Scholar] [CrossRef] [PubMed]
- Felix, C.; Sharfstein, J.M.; Olsen, Y. Help is on the way: Medicare coverage of opioid treatment programs. J. Am. Geriatr. Soc. 2020, 68, 637–640. [Google Scholar] [CrossRef]
- Donohue, J.M.; Barry, C.L.; Stuart, E.A.; Greenfield, S.F.; Song, Z.; Chernew, M.E.; Huskamp, H.A. Effects of global payment and accountable care on medication treatment for alcohol and opioid use disorders. J. Addict. Med. 2018, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Brooner, R.K.; Kidorf, M.S.; King, V.L.; Stoller, K.B.; Peirce, J.M.; Bigelow, G.E.; Kolodner, K. Behavioral contingencies improve counseling attendance in an adaptive treatment model. J. Subst. Abus. Treat. 2004, 27, 223–232. [Google Scholar] [CrossRef]
- Peirce, J.M.; Petry, N.M.; Stitzer, M.L.; Blaine, J.; Kellogg, S.; Satterfield, F.; Schwartz, M.; Krasnansky, J.; Pencer, E.; Silva-Vazquez, L.; et al. Comparing adaptive stepped care and monetary-based voucher interventions for opioid dependence. Drug Alcohol Depend. 2007, 88, S14–S23. [Google Scholar]
- Peirce, J.M.; Petry, N.M.; Stitzer, M.L.; Blaine, J.; Kellogg, S.; Satterfield, F.; Schwartz, M.; Krasnansky, J.; Pencer, E.; Silva-Vazquez, L.; et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Arch. Gen. Psychiat. 2006, 63, 201–208. [Google Scholar] [CrossRef]
- Olmstead, T.A.; Petry, N.M. The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine-or opioid-dependent outpatients. Drug Alcohol Depend. 2009, 102, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Hartzler, B.; Rabun, C. Community opioid treatment perspectives on contingency management: Perceived feasibility, effectiveness, and transportability of social and financial incentives. J. Subst. Abus. Treat. 2013, 45, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.R.; Landes, R.D.; Jackson, L.; Marsch, L.A.; Mancino, M.J.; Chopra, M.P.; Bickel, W.K. Adding an internet-delivered treatment to an efficacious treatment package for opioid dependence. J. Consult. Clin. Psych. 2014, 82, 964. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.P.; Holtyn, A.F.; DeFulio, A.; Dunn, K.E.; Everly, J.J.; Leoutsakos, J.M.S.; Umbricht, A.; Fingerhood, M.; Bigelow, G.E.; Silverman, K. Effects of incentives for naltrexone adherence on opiate abstinence in heroin-dependent adults. Addiction 2017, 112, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Quintiliani, L.; Heinz, A.; Johnson, N.L.; Xuan, Z.; Truong, V.; Lasser, K.E. A financial incentive program to improve appointment attendance at a safety-net hospital-based primary care hepatitis C treatment program. PLoS ONE 2020, 15, e0228767. [Google Scholar] [CrossRef]
- Toegel, F.; Holtyn, A.F.; Subramaniam, S.; Silverman, K. Effects of time-based administration of abstinence reinforcement targeting opiate and cocaine use. J. Appl. Behav. Anal. 2020. [Google Scholar] [CrossRef]
- Forster, S.E.; DePhilippis, D.; Forman, S.D. “I’s” on the prize: A systematic review of individual differences in Contingency Management treatment response. J. Subst. Abus. Treat. 2019, 100, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Radley, A.; Van Der Pol, M.; Dillon, J.F. Application of a discrete choice experiment approach to support the design of a hepatitis C testing service in primary care. Int. J. Drug Policy 2019, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ryan-Pettes, S.R.; Devoto, A.; DeFulio, A. Acceptability and willingness to pay for contingency management interventions among parents of young adults with problematic opioid use. Drug Alcohol Depend. 2020, 206, 107687. [Google Scholar] [CrossRef]
- Cicero, T.J.; Ellis, M.S.; Surratt, H.L.; Kurtz, S.P. Factors contributing to the rise of buprenorphine misuse: 2008–2013. Drug Alcohol Depend. 2014, 142, 98–104. [Google Scholar] [CrossRef]
- Johnson, B.; Richert, T. Diversion of methadone and buprenorphine from opioid substitution treatment: Patients who regularly sell or share their medication. J. Addict. Dis. 2015, 34, 1–17. [Google Scholar] [CrossRef]
- Vandrey, R.; Bigelow, G.E.; Stitzer, M.L. Contingency management in cocaine abusers: A dose-effect comparison of goods-based versus cash-based incentives. Exp. Clin. Psychopharm. 2007, 15, 338. [Google Scholar] [CrossRef]
- Ducharme, L.J.; Knudsen, H.K.; Abraham, A.J.; Roman, P.M. Counselor attitudes toward the use of motivational incentives in addiction treatment. Am. J. Addict. 2010, 19, 496–503. [Google Scholar] [CrossRef]
- Kidorf, M.; King, V.L.; Neufeld, K.; Peirce, J.; Kolodner, K.; Brooner, R.K. Improving substance abuse treatment enrollment in community syringe exchangers. Addiction 2009, 104, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Kidorf, M.; King, V.L.; Gandotra, N.; Kolodner, K.; Brooner, R.K. Improving treatment enrollment and re-enrollment rates of syringe exchangers: 12-month outcomes. Drug Alcohol Depend. 2012, 124, 162–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schuman-Olivier, Z.; Borodovsky, J.T.; Steinkamp, J.; Munir, Q.; Butler, K.; Greene, M.A.; Goldblatt, J.; Xie, H.Y.; Marsch, L.A. MySafeRx: A mobile technology platform integrating motivational coaching, adherence monitoring, and electronic pill dispensing for enhancing buprenorphine/naloxone adherence during opioid use disorder treatment: A pilot study. Addict. Sci. Clin. Pract. 2018, 13, 21. [Google Scholar] [CrossRef]
- Godersky, M.E.; Klein, J.W.; Merrill, J.O.; Blalock, K.L.; Saxon, A.J.; Samet, J.H.; Tsui, J.I. Acceptability and feasibility of a mobile health application for video directly observed therapy of buprenorphine for opioid use disorders in an office-based setting. J. Addict. Med. 2020, 14, 319–325. [Google Scholar] [CrossRef]
- Langdon, K.J.; Ramsey, S.; Scherzer, C.; Carey, K.; Ranney, M.L.; Rich, J. Development of an integrated digital health intervention to promote engagement in and adherence to medication for opioid use disorder. Addict. Sci. Clin. Pract. 2020, 15, 1–10. [Google Scholar]
- Widman, M.; Lidz, V.; DiGregorio, G.J.; Platt, A.K.; Robison, L.; Platt, J.J. Health status of employed and unemployed methadone patients. J. Subst. Abus. Treat. 2000, 18, 287–289. [Google Scholar] [CrossRef]
- Silverman, K.; DeFulio, A.; Sigurdsson, S.O. Maintenance of reinforcement to address the chronic nature of drug addiction. Prev. Med. 2012, 55, S46–S53. [Google Scholar] [PubMed]
- Zanis, D.A.; Coviello, D.; Alterman, A.I.; Appling, S.E. A community-based trial of vocational problem-solving to increase employment among methadone patients. J. Subst. Abus. Treat. 2001, 21, 19–26. [Google Scholar] [CrossRef]
- Kidorf, M.; Neufeld, K.; Brooner, R.K. Combining stepped-care approaches with behavioral reinforcement to motivate employment in opioid-dependent outpatients. Subst. Use Misuse 2004, 39, 2215–2238. [Google Scholar]
- Aklin, W.M.; Wong, C.J.; Hampton, J.; Svikis, D.S.; Stitzer, M.L.; Bigelow, G.E.; Silverman, K. A therapeutic workplace for the long-term treatment of drug addiction and unemployment: Eight-year outcomes of a social business intervention. J. Subst. Abus. Treat. 2014, 47, 329–338. [Google Scholar] [CrossRef]
- DeFulio, A.; Everly, J.J.; Leoutsakos, J.M.S.; Umbricht, A.; Fingerhood, M.; Bigelow, G.E.; Silverman, K. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: A randomized controlled trial. Drug Alcohol Depend. 2012, 120, 48–54. [Google Scholar]
- Holtyn, A.F.; Toegel, F.; Subramaniam, S.; Arellano, M.; Leoutsakos, J.M.; Fingerhood, M.; Silverman, K. Financial incentives promote engagement in employment services for unemployed adults in treatment for opioid use disorder. Drug Alcohol Depend. 2020, 107982. [Google Scholar] [CrossRef] [PubMed]
- Claxton, G.; Rae, M.; Damico, A.; Young, G.; McDermott, D.; Whitmore, H. Health benefits in 2019: Premiums inch higher, employers respond to federal policy. Health Affair 2019, 38, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.S.; Roelofs, C.; Punnett, L. Work environment factors and prevention of opioid-related deaths. Am. J. Public Health 2020, 110, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- NCSL: National Conference of State Legislatures. Marketing and Advertising of Pharmaceuticals. 2018. Available online: https://www.ncsl.org/research/health/marketing-and-advertising-of-pharmaceuticals.aspx (accessed on 10 February 2020).
- Piccinin, M.A.; Sayeed, Z.; Kozlowski, R.; Bobba, V.; Knesek, D.; Frush, T. Bundle payment for musculoskeletal care: Current evidence (part 1). Orthop. Clin. 2018, 49, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Meisenberg, B.R.; Grover, J.; Campbell, C.; Korpon, D. Assessment of opioid prescribing practices before and after implementation of a health system intervention to reduce opioid overprescribing. JAMA Netw. Open 2018, 1, e182908. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. In Ending Discrimination Against People With Mental and Substance Use Disorders: The Evidence for Stigma Change; The National Academies Press: Washington, DC, USA, 2016.
- Boloori, A.; Saghafian, S.; Traub, S.J. Management of Opioid Prescriptions: Evidence-Based Personalized Pain Treatment Using Longitudinal Machine Learning; Working Paper; Michigan State University: East Lansing, MI, USA, 2020. [Google Scholar]
- Gaskin, D.J.; Patrick, R. The economic costs of pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef]
- Florence, C.; Luo, F.; Xu, L.; Zhou, C. The economic burden of prescription opioid overdose, abuse and dependence in the United States, 2013. Med. Care 2016, 54, 901–906. [Google Scholar] [CrossRef]
- AMA: American Medical Association. How are Prescription Drug Prices Determined? 2019. Available online: https://www.ama-assn.org/delivering-care/public-health/how-are-prescription-drug-prices-determined (accessed on 10 February 2020).
- Petry, N.M.; Weinstock, J.; Alessi, S.M.; Lewis, M.W.; Dieckhaus, K. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J. Consult. Clin. Psych. 2010, 78, 89. [Google Scholar] [CrossRef]
- Shanahan, C.W.; Beers, D.; Alford, D.P.; Brigandi, E.; Samet, J.H. A transitional opioid program to engage hospitalized drug users. J. Gen. Intern. Med. 2010, 25, 803–808. [Google Scholar] [CrossRef]
- Velez, C.M.; Nicolaidis, C.; Korthuis, P.T.; Englander, H. “It’s been an experience, a life learning experience”: A qualitative study of hospitalized patients with substance use disorders. J. Gen. Intern. Med. 2017, 32, 296–303. [Google Scholar] [CrossRef]
- Fanucchi, L.C.; Lofwall, M.R.; Nuzzo, P.A.; Walsh, S.L. In-hospital illicit drug use, substance use disorders, and acceptance of residential treatment in a prospective pilot needs assessment of hospitalized adults with severe infections from injecting drugs. J. Subst. Abus. Treat. 2018, 92, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.D.; Lee, K.; Edwards, C.; Pelullo, A.P.; Khatri, U.G.; Lowenstein, M.; Perrone, J. Providing incentive for emergency physician X-waiver training: An evaluation of program success and postintervention buprenorphine prescribing. Ann. Emerg. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Holl, W.C.; Nath, B.; Li, F.; Maciejewski, K.; Paek, H.; Dziura, J.; Rajeevan, H.; Lu, C.C.; Katsovich, L.; D’Onofrio, G.; et al. Interrupted time series of user-centered clinical decision support implementation for emergency department–initiated buprenorphine for opioid use disorder. Acad. Emerg. Med. 2020. [Google Scholar]
- Fox, A.D.; Maradiaga, J.; Weiss, L.; Sanchez, J.; Starrels, J.L.; Cunningham, C.O. Release from incarceration, relapse to opioid use and the potential for buprenorphine maintenance treatment: A qualitative study of the perceptions of former inmates with opioid use disorder. Addict. Sci. Clin. Pract. 2015, 10, 2. [Google Scholar] [CrossRef]
- Mukherjee, T.I.; Wickersham, J.A.; Desai, M.M.; Pillai, V.; Kamarulzaman, A.; Altice, F.L. Factors associated with interest in receiving prison-based methadone maintenance therapy in Malaysia. Drug Alcohol Depend. 2016, 164, 120–127. [Google Scholar] [CrossRef]
- Polonsky, M.; Rozanova, J.; Azbel, L.; Bachireddy, C.; Izenberg, J.; Kiriazova, T.; Dvoryak, S.; Altice, F.L. Attitudes toward addiction, methadone treatment, and recovery among HIV-infected Ukrainian prisoners who inject drugs: Incarceration effects and exploration of mediators. AIDS. Behav. 2016, 20, 2950–2960. [Google Scholar] [CrossRef]
- Bunting, A.M.; Oser, C.B.; Staton, M.; Eddens, K.S.; Knudsen, H. Clinician identified barriers to treatment for individuals in Appalachia with opioid use disorder following release from prison: A social ecological approach. Addict. Sci. Clin. Pract. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Christopher, P.P.; Anderson, B.; Stein, M.D. Civil commitment experiences among opioid users. Drug Alcohol Depend. 2018, 193, 137–141. [Google Scholar] [CrossRef]
- Streisel, S.E. Intent to refer: Exploring bias toward specific medication-assisted treatments by community corrections employees. Subst. Use Misuse 2018, 53, 2421–2430. [Google Scholar] [CrossRef]
- Tsai, J.; Gu, X. Utilization of addiction treatment among US adults with history of incarceration and substance use disorders. Addict. Sci. Clin. Pract. 2019, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Walley, A.Y.; White, M.C.; Kushel, M.B.; Song, Y.S.; Tulsky, J.P. Knowledge of and interest in hepatitis C treatment at a methadone clinic. J. Subst. Abus. Treat. 2005, 28, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Starrels, J.L.; Peyser, D.; Haughton, L.; Fox, A.; Merlin, J.S.; Arnsten, J.H.; Cunningham, C.O. When human immunodeficiency virus (HIV) treatment goals conflict with guideline-based opioid prescribing: A qualitative study of HIV treatment providers. Subst. Abus. 2016, 37, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Claborn, K.R.; Aston, E.R.; Champion, J.; Guthrie, K.M. Prescribing opioids as an incentive to retain patients in medical care: A qualitative investigation into clinician awareness and perceptions. J. Assoc. Nurse AIDS C 2018, 29, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Moonesinghe, R.; Schieber, L.Z.; Truman, B.I. Opioid-related diagnoses and concurrent claims for HIV, HBV, or HCV among Medicare beneficiaries, United States, 2015. J. Clin. Med. 2019, 8, 1768. [Google Scholar] [CrossRef]
- Ko, J.Y.; Haight, S.C.; Schillie, S.F.; Bohm, M.K.; Dietz, P.M. National trends in hepatitis C infection by opioid use disorder status among pregnant women at delivery hospitalization—United States, 2000–2015. MMWR-Morb. Mortal. Wkly. Rep. 2019, 68, 833. [Google Scholar] [CrossRef]
| Stage | Topic | Studies | Stakeholders | Misalignment Source | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PY | PR | PT | PM | OT | Cost | Quality | Access | |||
| Prevention | Payment Mechanisms, Reimbursement Schemes, and Incentives | [4,15,16,17,18,19,20,21,22,23,24,25,26] (13: 2010–2019) | ✓ | ✓ | ✓ | — | — | ✓ | ✓ | — |
| Practice Guidelines and Healthcare System Structures | [27,28,29,30,31,32,33,34,35] (9: 2010–2019) | — | ✓ | ✓ | ✓ | — | — | ✓ | ✓ | |
| Multi-Modal Pain Management | [21,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] (1: 2000–2009, 18: 2010–2019) | ✓ | ✓ | ✓ | — | — | ✓ | ✓ | ✓ | |
| Initiatives for Opioid Prescription/Side Effects Reduction | [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] (16: 2010–2019) | ✓ | ✓ | ✓ | — | — | ✓ | ✓ | ✓ | |
| Physician-Patient Shared Decision Making | [70,71] (2: 2010–2019) | — | ✓ | ✓ | — | — | ✓ | ✓ | — | |
| Intervention | Barriers in Adopting OUD/SUD Treatments | [72,73,74,75,76,77,78,79,80,81,82,83,84] (2: 2000–2009, 11: 2010–2019) | ✓ | ✓ | ✓ | — | ✓ | ✓ | ✓ | ✓ |
| [77,80,81,82,85,86,87,88] (2: 2000–2009, 6: 2010–2019) | — | ✓ | ✓ | — | — | ✓ | ✓ | ✓ | ||
| Facilitators to Adopting OUD/SUD Treatments | [89,90,91,92,93,94,95,96,97,98] (10: 2010–2019) | ✓ | ✓ | ✓ | ✓ | — | ✓ | ✓ | ✓ | |
| [74,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119] (6: 2000–2009, 16: 2010–2019) | ✓ | ✓ | ✓ | — | — | ✓ | ✓ | ✓ | ||
| [120,121,122,123,124,125,126,127,128] (3: 2000–2009, 6: 2010–2019) | — | — | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Term | Description |
|---|---|
| Stakeholder | An entity who plays a role in navigating a healthcare-related problem, e.g., payer, provider, patient, employer, pharmaceutical company, etc. |
| Incentive | An interest for a stakeholder, e.g., monetary (revenue), health-related (quality of life), political (implications of a proposed healthcare bill), organizational (e.g., integrity and power issues), or behavioral (e.g., psychological factors). |
| Misalignment | A condition caused by competing and/or conflicting interests between two or more stakeholders resulting in either an increase in the cost of care, a reduction in the quality of care, or less access to care. |
| Alignment | A condition where devising mechanisms among stakeholders can either lower the cost, improve the quality, or enhance the access to care. This is a relative notion in that a “complete” alignment may not be attainable in reality. |
| Fee-for-service | A payment mechanism where a provider is separately reimbursed for every service delivered to a patient. |
| Capitation | A payment mechanism where a provider is reimbursed per patient per time period. |
| Pay-for-performance | The general class of payment mechanisms where the provider(s) is reimbursed based on the quality of care delivered to patients. Some examples include “bundled payment” and “accountable care”. |
| Bundled payment | A payment mechanism where a bundled payment is paid to a group of providers per patients per episode of care. |
| Accountable care | A payment mechanism where a group of providers shares benefits/savings (upon high-quality delivery of care) or is penalized in reimbursements otherwise. |
| Managed care | Health insurance plans that provide care for enrollees at lowered cost. Different types include health maintenance organizations, preferred provider organizations, and point of service. |
| Care fragmentation | Care that is delivered to a patient via multiple providers while there is little to no coordination between providers. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boloori, A.; Arnetz, B.B.; Viens, F.; Maiti, T.; Arnetz, J.E. Misalignment of Stakeholder Incentives in the Opioid Crisis. Int. J. Environ. Res. Public Health 2020, 17, 7535. https://doi.org/10.3390/ijerph17207535
Boloori A, Arnetz BB, Viens F, Maiti T, Arnetz JE. Misalignment of Stakeholder Incentives in the Opioid Crisis. International Journal of Environmental Research and Public Health. 2020; 17(20):7535. https://doi.org/10.3390/ijerph17207535
Chicago/Turabian StyleBoloori, Alireza, Bengt B. Arnetz, Frederi Viens, Taps Maiti, and Judith E. Arnetz. 2020. "Misalignment of Stakeholder Incentives in the Opioid Crisis" International Journal of Environmental Research and Public Health 17, no. 20: 7535. https://doi.org/10.3390/ijerph17207535
APA StyleBoloori, A., Arnetz, B. B., Viens, F., Maiti, T., & Arnetz, J. E. (2020). Misalignment of Stakeholder Incentives in the Opioid Crisis. International Journal of Environmental Research and Public Health, 17(20), 7535. https://doi.org/10.3390/ijerph17207535





