Analysis of the Relationship between Asthma and Coffee/Green Tea/Soda Intake
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Participant Selection
2.3. Survey
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Soriano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Aichour, M.T.E.; Alam, K. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Sohn, K.-H.; Shin, J.E.; Park, M.; Lim, J.; Lee, J.Y.; Yang, M.-S. Changing patterns of adult asthma incidence: Results from the National Health Insurance Service–National Sample Cohort (NHIS-NSC) database in Korea. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-Y.; Park, H.-W.; Ko, S.-K.; Chang, S.-I.; Moon, H.-B.; Kim, Y.-Y.; Cho, S.-H. The financial burden of asthma: A nationwide comprehensive survey conducted in the republic of Korea. Allergy Asthma Immunol. Res. 2011, 3, 34–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.; Kim, J. Green tea, coffee, and caffeine consumption are inversely associated with self-report lifetime depression in the Korean population. Nutrients 2018, 10, 1201. [Google Scholar] [CrossRef]
- Choi, H.K.; Curhan, G. Coffee, tea, and caffeine consumption and serum uric acid level: The third national health and nutrition examination survey. Arthritis Care Res. Off. J. Am. Coll. Rheumatol. 2007, 57, 816–821. [Google Scholar] [CrossRef]
- Torquati, L.; Peeters, G.; Brown, W.J.; Skinner, T.L. A Daily Cup of Tea or Coffee May Keep You Moving: Association between Tea and Coffee Consumption and Physical Activity. Int. J. Environ. Res. Public Health 2018, 15, 1812. [Google Scholar] [CrossRef]
- Giovannucci, E. Meta-analysis of coffee consumption and risk of colorectal cancer. Am. J. Epidemiol. 1998, 147, 1043–1052. [Google Scholar] [CrossRef]
- Alfaro, T.M.; Monteiro, R.A.; Cunha, R.A.; Cordeiro, C.R. Chronic coffee consumption and respiratory disease: A systematic review. Clin. Respir. J. 2018, 12, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Pagano, R.; Negri, E.; Decarli, A.; La Vecchia, C. Coffee drinking and prevalence of bronchial asthma. Chest 1988, 94, 386–389. [Google Scholar] [CrossRef]
- Schwartz, J.; Weiss, S.T. Caffeine intake and asthma symptoms. Ann. Epidemiol. 1992, 2, 627–635. [Google Scholar] [CrossRef]
- Annesi, I.; Kauffmann, F.; Oryszczyn, M.P.; Neukirch, F.; Doré, M.F. Coffee drinking and prevalence of bronchial asthma. Chest 1990, 97, 1268–1269. [Google Scholar] [CrossRef] [PubMed]
- Al-Zalabani, A.H.; Noor Elahi, I.; Katib, A.; Alamri, A.G.; Halawani, A.; Alsindi, N.M.; Almatrafi, M.; Wesselius, A.; Stewart, K. Association between soft drinks consumption and asthma: A systematic review and meta-analysis. BMJ Open 2019, 9, e029046. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Han, B.-G.; Group, K. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- World Health Organization/International Association for the Study of Obesity/International Obesity TaskForce. The Asia-Pacific Perspective: Redefining Obesity and its Treatment; Health Communications Australia Pty Ltd.: Sydney, Australia, 2000. [Google Scholar]
- Ahn, Y.; Kwon, E.; Shim, J.; Park, M.; Joo, Y.; Kimm, K.; Park, C.; Kim, D. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Sanchez, J.M. Methylxanthine content in commonly consumed foods in Spain and determination of its intake during consumption. Foods 2017, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Onatibia-Astibia, A.; Martínez-Pinilla, E.; Franco, R. The potential of methylxanthine-based therapies in pediatric respiratory tract diseases. Respir. Med. 2016, 112, 1–9. [Google Scholar] [CrossRef]
- Becker, A.B.; Simons, K.J.; Gillespie, C.A.; Simons, F.E.R. The bronchodilator effects and pharmacokinetics of caffeine in asthma. N. Engl. J. Med. 1984, 310, 743–746. [Google Scholar] [CrossRef]
- Tilley, S.L.; Boucher, R.C. A 1 antagonism in asthma: Better than coffee? J. Clin. Investig. 2005, 115, 13–16. [Google Scholar] [CrossRef]
- León-Carmona, J.R.; Galano, A. Is caffeine a good scavenger of oxygenated free radicals? J. Phys. Chem. B 2011, 115, 4538–4546. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med Sci. Monit. 2003, 9, BR325–BR330. [Google Scholar]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, Ö. Oxidative stress in asthma: Part of the puzzle. Pediatric Allergy Immunol. 2018, 29, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, L. How Much Caffeine Is in Coffee, Tea, Cola, and Other Drinks? Available online: https://www.thespruceeats.com/caffeine-in-coffee-tea-cola-765276 (accessed on 20 April 2020).
- Caffeine Chart. Available online: https://cspinet.org/eating-healthy/ingredients-of-concern/caffeine-chart (accessed on 20 April 2020).
- Welsh, E.J.; Bara, A.; Barley, E.; Cates, C.J. Caffeine for asthma. Cochrane Database Syst. Rev. 2010, 2010, CD001112. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Akinbami, L.J.; McGuire, L.C.; Blanck, H.M. Association of sugar-sweetened beverage intake frequency and asthma among US adults, 2013. Prev. Med. 2016, 91, 58–61. [Google Scholar] [CrossRef] [PubMed]
- DeChristopher, L.R.; Tucker, K.L. Excess free fructose, high-fructose corn syrup and adult asthma: The Framingham Offspring Cohort. Br. J. Nutr. 2018, 119, 1157–1167. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Brix, T.H.; Kyvik, K.O.; Brøsen, K. The interindividual differences in the 3-demthylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharm. Genom. 2002, 12, 473–478. [Google Scholar]
- Zaigler, M.; Rietbrock, S.; Szymanski, J.; Dericks-Tan, J.; Staib, A.; Fuhr, U. Variation of CYP1A2-dependent caffeine metabolism during menstrual cycle in healthy women. Int. J. Clin. Pharmacol. Ther. 2000, 38, 235–244. [Google Scholar] [CrossRef]
- Temple, J.L.; Ziegler, A.M.; Graczyk, A.; Bendlin, A.; Sion, T.; Vattana, K. Cardiovascular responses to caffeine by gender and pubertal stage. Pediatrics 2014, 134, e112–e119. [Google Scholar] [CrossRef]
- Schliep, K.C.; Schisterman, E.F.; Mumford, S.L.; Pollack, A.Z.; Zhang, C.; Ye, A.; Stanford, J.B.; Hammoud, A.O.; Porucznik, C.A.; Wactawski-Wende, J. Caffeinated beverage intake and reproductive hormones among premenopausal women in the BioCycle Study. Am. J. Clin. Nutr. 2012, 95, 488–497. [Google Scholar] [CrossRef]
- Dillon, P.; Kelpin, S.; Kendler, K.; Thacker, L.; Dick, D.; Svikis, D. Gender Differences in Any-Source Caffeine and Energy Drink Use and Associated Adverse Health Behaviors. J. Caffeine Adenosine Res. 2019, 9, 12–19. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Service, U.F.A. Coffee Market Brief Update. Available online: https://www.fas.usda.gov/data/south-korea-coffee-market-brief-update (accessed on 23 April 2020).
- Joeres, R.; Klinker, H.; Heusler, H.; Epping, J.; Zilly, W.; Richter, E. Influence of smoking on caffeine elimination in healthy volunteers and in patients with alcoholic liver cirrhosis. Hepatology 1988, 8, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.G.; Peacock, J.L.; Feyerabend, C.; Carey, I.M.; Jarvis, M.J.; Anderson, H.R.; Bland, J.M. Relation of caffeine intake and blood caffeine concentrations during pregnancy to fetal growth: Prospective population based study. BMJ 1996, 313, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |


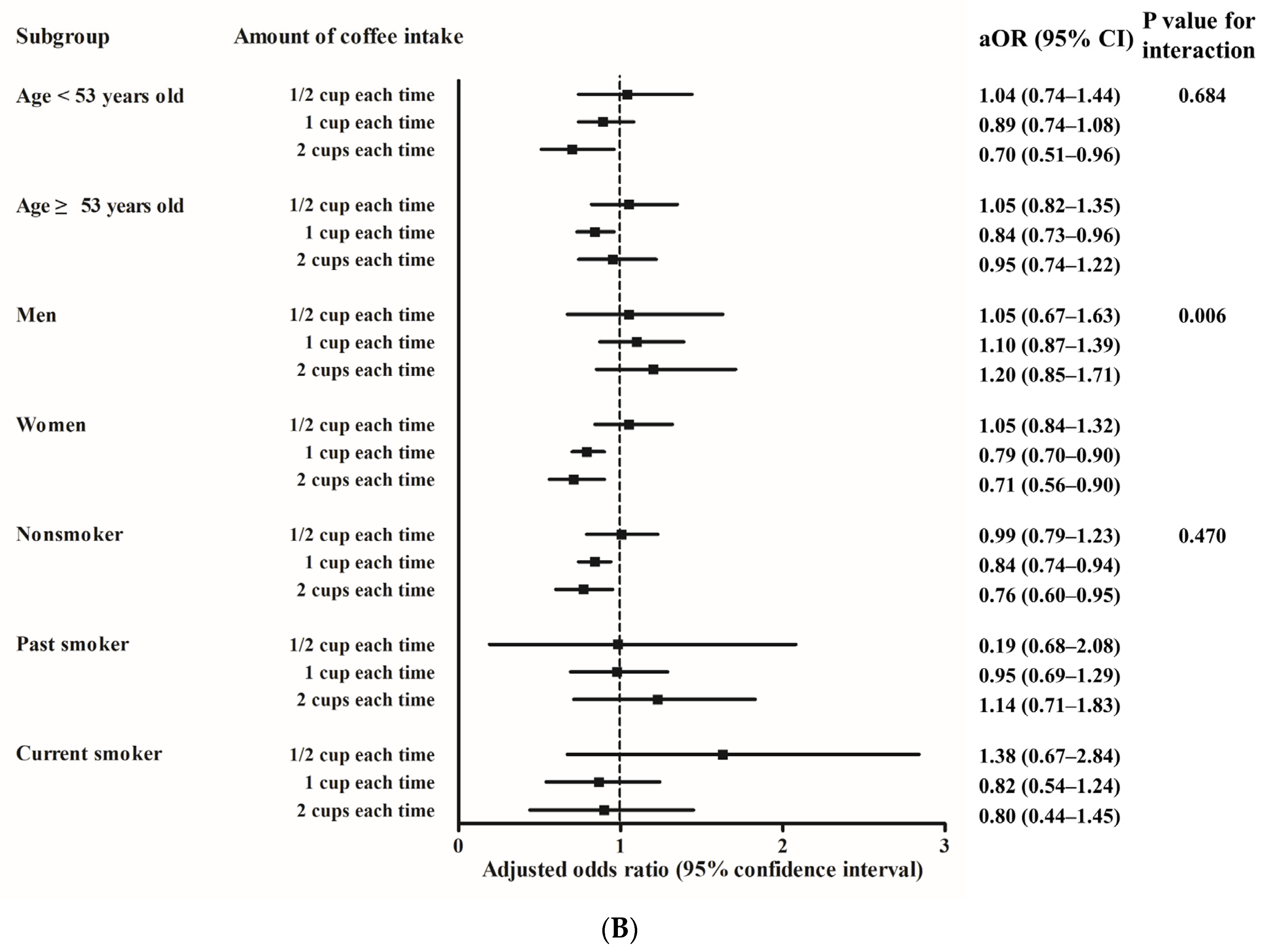
| Characteristics | Total Participants | p-Value | |
|---|---|---|---|
| Asthma | Non-Asthma | ||
| Age (mean, SD, y) | 55.9 (8.7) | 53.2 (8.4) | <0.001 1 |
| Sex (n, %) | <0.001 1 | ||
| Men | 946 (30.1) | 54,613 (34.4) | |
| Women | 2200 (69.9) | 104,289 (65.6) | |
| Obesity (n, %) | <0.001 1 | ||
| Underweight (BMI < 18.5 kg/m2) | 63 (2.0) | 2838 (1.8) | |
| Normal (18.5 kg/m2 ≤ BMI < 23 kg/m2) | 1023 (32.5) | 59,608 (37.5) | |
| Overweight (23 kg/m2 ≤ BMI < 25 kg/m2) | 846 (26.9) | 44,162 (27.8) | |
| Obese (25 kg/m2 ≤ BMI) | 1214 (38.6) | 52,294 (32.9) | |
| Income (n, %) | <0.001 1 | ||
| Missing, no response | 467 (14.8) | 20,194 (12.7) | |
| Lowest | 1121 (35.6) | 44,817 (28.2) | |
| Middle | 993 (37.4) | 59,491 (37.4) | |
| Highest | 565 (21.6) | 34,400 (21.6) | |
| Smoking status (n, %) | <0.001 1 | ||
| Nonsmoker | 2337 (74.3) | 115,848 (72.9) | |
| Past smoker | 505 (16.1) | 23,256 (14.6) | |
| Current smoker | 304 (9.7) | 19,798 (12.5) | |
| Alcohol consumption (n, %) | <0.001 1 | ||
| Nondrinker | 1794 (57.0) | 80,746 (50.8) | |
| Past drinker | 175 (5.6) | 6070 (3.8) | |
| Current drinker | 1177 (37.4) | 72,086 (45.4) | |
| Nutritional intake (mean, SD) | |||
| Total calories (kcal/d) | 1722.4 (574.2) | 1755.3 (581.9) | 0.002 1 |
| Protein (g/d) | 57.8 (25.4) | 59.7 (26.8) | <0.001 1 |
| Fat (g/d) | 26.5 (17.3) | 28.1 (18.4) | <0.001 1 |
| Carbohydrate (g/d) | 309.1 (96.4) | 311.8 (95.2) | 0.114 |
| Frequency of coffee (n, %) | <0.001 1 | ||
| None | 643 (20.4) | 26,464 (16.7) | |
| 1 time (m) through 6 times (w) | 779 (24.8) | 33,799 (21.3) | |
| 1–2 times (d) | 1201 (38.2) | 67,273 (42.3) | |
| ≥3 times (d) | 523 (16.6) | 31,366 (19.7) | |
| Amount of coffee (n, %) | <0.001 1 | ||
| None | 643 (20.4) | 26,464 (16.7) | |
| 1/2 cup each time | 142 (4.5) | 6118 (3.9) | |
| 1 cup each time | 2213 (70.3) | 117,884 (74.2) | |
| 2 cups each time | 148 (4.7) | 8436 (5.3) | |
| Frequency of green tea (n, %) | <0.001 1 | ||
| None | 1421 (45.2) | 68,171 (42.9) | |
| 1 time (m) through 6 times (w) | 589 (18.7) | 25,616 (16.1) | |
| 1–2 times (d) | 803 (25.5) | 45,069 (28.4) | |
| ≥3 times (d) | 333 (10.6) | 20,046 (12.6) | |
| Amount of green tea (n, %) | 0.057 | ||
| None | 1421 (45.2) | 68,171 (42.9) | |
| 1/2 cup each time | 504 (16.0) | 26,672 (16.8) | |
| 1 cup each time | 1168 (37.1) | 61,640 (38.8) | |
| 2 cups each time | 53 (1.7) | 2419 (1.5) | |
| Frequency of soda drinks (n, %) | 0.004 1 | ||
| None | 1290 (41.0) | 63,960 (40.3) | |
| 1 time (m) through 6 times (w) | 1399 (45.5) | 68,108 (42.9) | |
| 1–2 times (d) | 350 (11.1) | 21,097 (13.3) | |
| ≥3 times (d) | 107 (3.4) | 5737 (3.6) | |
| Amount of soda drinks (n, %) | 0.866 | ||
| None | 1290 (41.0) | 63,960 (40.3) | |
| 1/2 cup each time | 107 (3.4) | 5472 (3.4) | |
| 1 cup each time | 1667 (53.0) | 85,257 (53.7) | |
| 2 cups each time | 82 (2.6) | 4213 (2.7) | |
| Characteristics | Odds Ratios for Asthma | |||||
|---|---|---|---|---|---|---|
| Crude | p-Value | Model 1 2 | p-Value | Model 2 3 | p-Value | |
| Coffee | ||||||
| None | 1.00 | 1.00 | 1.00 | |||
| 1 time (m) through 6 times (w) | 0.95 (0.85–1.05) | 0.328 | 1.03 (0.93–1.15) | 0.565 | 0.98 (0.85–1.12) | 0.743 |
| 1–2 times (d) | 0.74 (0.67–0.81) | <0.001 1 | 0.81 (0.74–0.90) | <0.001 1 | 0.82 (0.73–0.93) | 0.002 1 |
| ≥3 times (d) | 0.69 (0.61–0.77) | <0.001 1 | 0.85 (0.75–0.96) | 0.010 1 | 0.86 (0.73–1.02) | 0.086 |
| Green tea | ||||||
| None | 1.00 | 1.00 | 1.00 | |||
| 1 time (m) through 6 times (w) | 1.10 (1.00–1.22) | 0.048 1 | 1.11 (1.01–1.23) | 0.031 1 | 1.06 (0.94–1.21) | 0.331 |
| 1–2 times (d) | 0.86 (0.78–0.93) | <0.001 1 | 0.87 (0.80–0.95) | 0.002 1 | 0.98 (0.88–1.11) | 0.790 |
| ≥3 times (d) | 0.80 (0.71–0.90) | <0.001 1 | 0.91 (0.80–1.03) | 0.149 | 0.98 (0.81–1.17) | 0.790 |
| Soda drinks | ||||||
| None | 1.00 | 1.00 | 1.00 | |||
| 1 time (m) through 6 times (w) | 1.02 (0.94–1.10) | 0.639 | 1.09 (1.01–1.18) | 0.022 1 | 1.07 (0.99–1.15) | 0.106 |
| 1–2 times (d) | 0.82 (0.73–0.93) | 0.001 1 | 0.92 (0.81–1.04) | 0.185 | 0.94 (0.83–1.06) | 0.344 |
| ≥3 times (d) | 0.93 (0.76–1.13) | 0.441 | 1.07 (0.87–1.31) | 0.528 | 1.07 (0.87–1.31) | 0.563 |
| Characteristics | Odds Ratios for Asthma | |||||
|---|---|---|---|---|---|---|
| Crude | p-Value | Model 1 2 | p-Value | Model 2 3 | p-Value | |
| Coffee | ||||||
| None | 1.00 | 1.00 | 1.00 | |||
| 1/2 cup each time | 0.96 (0.80–1.15) | 0.626 | 1.06 (0.88–1.28) | 0.515 | 1.06 (0.87–1.29) | 0.572 |
| 1 cup each time | 0.77 (0.71–0.84) | <0.001 1 | 0.88 (0.80–0.96) | 0.005 1 | 0.87 (0.78–0.97) | 0.011 1 |
| 2 cups each time | 0.72 (0.60–0.87) | <0.001 1 | 0.86 (0.71–1.03) | 0.105 | 0.84 (0.69–1.02) | 0.080 |
| Green tea | ||||||
| None | 1.00 | 1.00 | 1.00 | |||
| 1/2 cup each time | 0.91 (0.82–1.00) | 0.061 | 0.99 (0.89–1.10) | 0.806 | 1.03 (0.92–1.15) | 0.601 |
| 1 cup each time | 0.91 (0.84–0.98) | 0.017 1 | 0.94 (0.86–1.01) | 0.111 | 1.00 (0.91–1.10) | 0.962 |
| 2 cups each time | 1.05 (0.80–1.39) | 0.725 | 1.03 (0.78–1.36) | 0.826 | 1.11 (0.82–1.47) | 0.488 |
| Soda drinks | ||||||
| None | 1.00 | 1.00 | 1.00 | |||
| 1/2 cup each time | 0.97 (0.79–1.18) | 0.761 | 1.04 (0.85–1.27) | 0.727 | 0.98 (0.79–1.20) | 0.834 |
| 1 cup each time | 0.97 (0.90–1.04) | 0.407 | 1.06 (0.98–1.14) | 0.152 | 1.06 (0.98–1.14) | 0.130 |
| 2 cups each time | 0.97 (0.77–1.21) | 0.757 | 1.13 (0.90–1.42) | 0.280 | 1.13 (0.90–1.42) | 0.296 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wee, J.H.; Yoo, D.M.; Byun, S.H.; Song, C.M.; Lee, H.-J.; Park, B.; Park, M.W.; Choi, H.G. Analysis of the Relationship between Asthma and Coffee/Green Tea/Soda Intake. Int. J. Environ. Res. Public Health 2020, 17, 7471. https://doi.org/10.3390/ijerph17207471
Wee JH, Yoo DM, Byun SH, Song CM, Lee H-J, Park B, Park MW, Choi HG. Analysis of the Relationship between Asthma and Coffee/Green Tea/Soda Intake. International Journal of Environmental Research and Public Health. 2020; 17(20):7471. https://doi.org/10.3390/ijerph17207471
Chicago/Turabian StyleWee, Jee Hye, Dae Myoung Yoo, Soo Hwan Byun, Chang Myeon Song, Hyo-Jeong Lee, Bumjung Park, Min Woo Park, and Hyo Geun Choi. 2020. "Analysis of the Relationship between Asthma and Coffee/Green Tea/Soda Intake" International Journal of Environmental Research and Public Health 17, no. 20: 7471. https://doi.org/10.3390/ijerph17207471
APA StyleWee, J. H., Yoo, D. M., Byun, S. H., Song, C. M., Lee, H.-J., Park, B., Park, M. W., & Choi, H. G. (2020). Analysis of the Relationship between Asthma and Coffee/Green Tea/Soda Intake. International Journal of Environmental Research and Public Health, 17(20), 7471. https://doi.org/10.3390/ijerph17207471







