The Influence of a Health-Related Fitness Training Program on Motor Performance as Well as Hematological and Biochemical Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
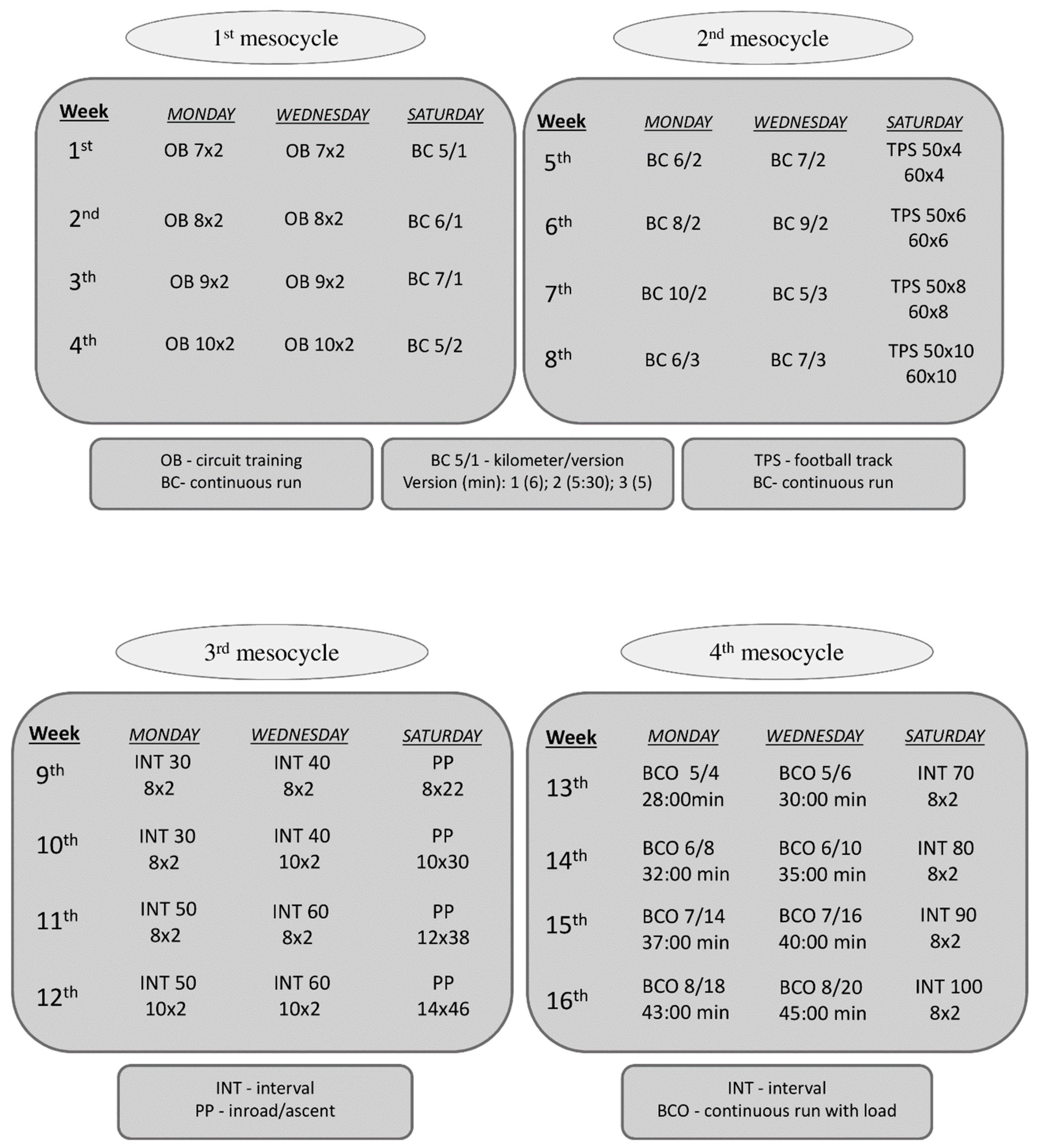
2.2. Training Program
2.3. Methods
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. The Impact of the Training Program on Exercise Performance and Body Composition
4.2. The Impact of the Training Program on Biochemical Plasma Profile
4.3. The Impact of the Training Program on Red and White Blood Cell Morphology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E. For 2018 physical activity guidelines advisory committee. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Lukács, A.; Sasvári, P.; Kiss-Tóth, E. Physical activity and physical fitness as protective factors of adolescent health. Int. J. Adolesc. Med. Health 2018. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.A.; Esmaeilzadeh, S. Association between academic achievement and physical status including physical activity, aerobic and muscular fitness tests in adolescent boys. Environ. Health Prev. Med. 2016, 21, 27–33. [Google Scholar] [CrossRef]
- Kostrzewa-Nowak, D.; Nowak, R.; Jastrzębski, Z.; Zarębska, A.; Bichowska, M.; Drobnik-Kozakiewicz, I.; Radzimiński, Ł.; Leońska-Duniec, A.; Ficek, K.; Cięszczyk, P. Effect of 12-week-long aerobic training programme on body composition, aerobic capacity, complete blood count and blood lipid profile among young women. Biochem. Med. (Zagreb) 2015, 25, 103–113. [Google Scholar] [CrossRef]
- Sawczyn, S.; Mishchenko, V.; Moska, W.; Sawczyn, M.; Jagiełło, M.; Kuehne, T.; Kostrzewa-Nowak, D.; Nowak, R.; Cięszczyk, P. Strength and aerobic training in overweight females in Gdańsk, Poland. Open Med. (Wars.) 2014, 10, 152–162. [Google Scholar] [CrossRef]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar]
- Chamera, T.; Spieszny, M.; Klocek, T.; Kostrzewa-Nowak, D.; Nowak, R.; Lachowicz, M.; Buryta, R.; Cięszczyk, P. Could biochemical liver profile help to assess metabolic response to aerobic effort in athletes? J. Strength Cond. Res. 2014, 28, 2180–2186. [Google Scholar] [CrossRef]
- Galan-Lopez, P.; Sánchez-Oliver, A.J.; Ries, F.; González-Jurado, J.A. Mediterranean Diet, Physical Fitness and Body Composition in Sevillian Adolescents: A Healthy Lifestyle. Nutrients 2019, 11, 2009. [Google Scholar] [CrossRef]
- Galan-Lopez, P.; Ries, F.; Gisladottir, T.; Domínguez, R.; Sánchez-Oliver, A.J. Healthy Lifestyle: Relationship between Mediterranean Diet, Body Composition and Physical Fitness in 13 to 16-Years Old Icelandic Students. Int. J. Environ. Res. Public Health 2018, 15, 2632. [Google Scholar] [CrossRef]
- Britton, Ú.; Belton, S.; Issartel, J. Small fish, big pond: The role of health-related fitness and perceived athletic competence in mediating the physical activity-motor competence relationship during the transition from primary to secondary school. J. Sports Sci. 2019, 37, 2538–2548. [Google Scholar] [CrossRef]
- Baceviciene, M.; Jankauskiene, R.; Emeljanovas, A. Self-perception of physical activity and fitness is related to lower psychosomatic health symptoms in adolescents with unhealthy lifestyles. BMC Public Health 2019, 19, 980. [Google Scholar] [CrossRef]
- Fallon, K.E. The clinical utility of screening of biochemical parameters in elite athletes: Analysis of 100 cases. Br. J. Sports Med. 2008, 42, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, R.; Russo-Volpe, S.; Cervato, G.; Cestaro, B. Biochemical assessments of oxidative stress, erythrocyte membrane fluidity and antioxidant status in professional soccer players and sedentary controls. Eur. J. Clin. Investig. 2003, 33, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Gravina, L.; Ruiz, F.; Lekue, J.A.; Irazusta, J.; Gil, S.M. Metabolic impact of a soccer match on female players. J. Sports Sci. 2011, 29, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Cadefau, J.; Casademont, J.; Grau, J.M.; Fernández, J.; Balaguer, A.; Vernet, M.; Cussó, R.; Urbano-Márquez, A. Biochemical and histochemical adaptation to sprint training in young athletes. Acta Physiol. Scand. 1990, 140, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Meister, S. Routine blood parameters in elite soccer players. Int. J. Sports Med. 2011, 32, 875–881. [Google Scholar] [CrossRef]
- Wiacek, M.; Andrzejewski, M.; Chmura, J.; Zubrzycki, I.Z. The changes of the specific physiological parameters in response to 12-week individualized training of young soccer players. J. Strength Cond. Res. 2011, 25, 1514–1521. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Craig, C.L.; Thyfault, J.P.; Spence, J.C. A step-defined sedentary lifestyle index: <5000 steps/day. Appl. Physiol. Nutr. Metab. 2012, 38, 100–114. [Google Scholar] [CrossRef]
- IDF Consensus Worldwide Definition of the Metabolic Syndrome. Available online: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome (accessed on 26 July 2019).
- WHO. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/ (accessed on 26 July 2019).
- Léger, L.A.; Lambert, J. A maximal multistage 20-m shuttle run test to predict VO2 max. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 49, 1–12. [Google Scholar] [CrossRef]
- Metsios, G.S.; Flouris, A.D.; Koutedakis, Y.; Nevill, A. Criterion-related validity and test-retest reliability of the 20m square shuttle test. J. Sci. Med. Sport 2008, 11, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.F.; Turner, A.N.; Miller, S.C. Reliability, factorial validity, and interrelationships of five commonly used change of direction speed tests. Scand. J. Med. Sci. Sports 2014, 24, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Biddle, S.J.; Asare, M. Physical activity and mental health in children and adolescents: A review of reviews. Br. J. Sports Med. 2011, 45, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Hötting, K.; Röder, B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013, 37, 2243–2257. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Cui, J.; Chen, L.Z.; Wang, X.; Fan, M.; Wei, G.X. Fitness-Dependent Effect of Acute Aerobic Exercise on Executive Function. Front. Physiol. 2019, 10, 902. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Henriksen, T.; Saugstad, O.D.; Tonstad, S. Physical activity and the risk of gestational diabetes mellitus: A systematic review and dose-response meta-analysis of epidemiological studies. Eur. J. Epidemiol. 2016, 31, 967–997. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Kiely, J. Exercise Response Efficiency: A Novel Way to Enhance Population Health? Lifestyle Genom. 2018, 11, 129–135. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Westerterp, K.R. Exercise, energy balance and body composition. Eur. J. Clin. Nutr. 2018, 72, 1246–1250. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, W.R.; Poterucha, J.J. Evaluation of elevated liver enzymes. Clin. Liver Dis. 2012, 16, 183–198. [Google Scholar] [CrossRef]
- Banfi, G.; Morelli, P. Relation between body mass index and serum aminotransferases concentrations in professional athletes. J. Sports Med. Phys. Fit. 2008, 48, 197–200. [Google Scholar]
- Nagel, D.; Seiler, D.; Franz, H.; Jung, K. Ultra-long-distance running and the liver. Int. J. Sports Med. 1990, 11, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, A.; Kostrzewa-Nowak, D.; Buryta, R.; Nowak, R. Blood Biomarkers of Recovery Efficiency in Soccer Players. Int. J. Environ. Res. Public Health 2019, 16, 3279. [Google Scholar] [CrossRef] [PubMed]
- Chamera, T.; Spieszny, M.; Klocek, T.; Kostrzewa-Nowak, D.; Nowak, R.; Lachowicz, M.; Buryta, R.; Ficek, K.; Eider, J.; Moska, W.; et al. Post-Effort Changes in Activity of Traditional Diagnostic Enzymatic Markers in Football Players’ Blood. J. Med. Biochem. 2015, 34, 179–190. [Google Scholar] [CrossRef]
- Lucia, A.; Morán, M.; Pérez, M.; Saborido, A.; Díaz, E.; Megias, A.; Chicharro, J.L. Short-term effects of marathon running in master runners: No evidence of myocardial injury. Int. J. Sports Med. 1999, 20, 482–486. [Google Scholar] [CrossRef]
- Diaz, E.; Ruiz, F.; Hoyos, I.; Zubero, J.; Gravina, L.; Gil, J.; Irazusta, J.; Gil, S.M. Cell damage, antioxidant status, and cortisol levels related to nutrition in ski mountaineering during a two-day race. J. Sports Sci. Med. 2010, 9, 338–346. [Google Scholar]
- Lippi, G.; Schena, F.; Montagnana, M.; Salvagno, G.L.; Banfi, G.; Guidi, G.C. Significant variation of traditional markers of liver injury after a half-marathon run. Eur. J. Intern. Med. 2011, 22, 36–38. [Google Scholar] [CrossRef]
- Brancaccio, P.; Limongelli, F.M.; Maffulli, N. Monitoring of serum enzymes in sport. Br. J. Sports Med. 2006, 40, 96–97. [Google Scholar] [CrossRef]
- Klapcińska, B.; Iskra, J.; Poprzecki, S.; Grzesiok, K. The effects of sprint (300 m) running on plasma lactate, uric acid, creatine kinase and lactate dehydrogenase in competitive hurdlers and untrained men. J. Sports Med. Phys. Fit. 2001, 41, 306–311. [Google Scholar]
- Iida, T.; Harada, T.; Ishizaki, F.; Nitta, Y.; Aoi, S.; Ikeda, H.; Chikamura, C.; Shiokawa, M.; Nitta, K. Changes in bone mineral density and metabolism in women: Evaluation of bodily characteristics, bone metabolic markers and bone mineral density. Hiroshima J. Med. Sci. 2013, 62, 49–53. [Google Scholar]
- Bayhan, I.; Dogan, N.U.; Ozaksit, G.; Uygur, D.; Ugurlu, N.; Ugur, M. Effect of strontium ranelate on serum leptin and bone turnover markers in women with established postmenopausal osteoporosis. J. Reprod. Med. 2013, 58, 319–323. [Google Scholar] [PubMed]
- Ford, E.S.; Giles, W.H.; Mokdad, A.H.; Myers, G.L. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin. Chem. 2004, 50, 574–581. [Google Scholar] [CrossRef]
- Aronson, D.; Sheikh-Ahmad, M.; Avizohar, O.; Kerner, A.; Sella, R.; Bartha, P.; Markiewicz, W.; Levy, Y.; Brook, G.J. C-Reactive protein is inversely related to physical fitness in middle-aged subjects. Atherosclerosis 2004, 176, 173–179. [Google Scholar] [CrossRef]
- Maffulli, N.; Testa, V.; Capasso, G. Post-viral fatigue syndrome. A longitudinal assessment in varsity athletes. J. Sports Med. Phys. Fit. 1993, 33, 392–399. [Google Scholar]
- Puglisi, M.J.; Fernandez, M.L. Modulation of C-reactive protein, tumor necrosis factor-alpha, and adiponectin by diet, exercise, and weight loss. J. Nutr. 2008, 138, 2293–2296. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; Pyne, D.B.; Gleeson, M.; Callister, R. Relationship between C-reactive protein concentration and cytokine responses to exercise in healthy and illness-prone runners. Eur. J. Appl. Physiol. 2009, 107, 611–614. [Google Scholar] [CrossRef]
- Czarkowska-Paczek, B.; Bartlomiejczyk, I.; Gabrys, T.; Przybylski, J.; Nowak, M.; Paczek, L. Lack of relationship between interleukin-6 and CRP levels in healthy male athletes. Immunol. Lett. 2005, 99, 136–140. [Google Scholar] [CrossRef]
- Strachan, A.F.; Noakes, T.D.; Kotzenberg, G.; Nel, A.E.; De Beer, F.C. C reactive protein concentrations during long distance running. Br. Med. J. (Clin. Res. Ed.) 1984, 289, 1249–1251. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Charchar, F.J.; Przybycin, M.; Crawford, L.; Wallace, A.M.; Gosek, K.; Lowe, G.D.; Zukowska-Szczechowska, E.; Grzeszczak, W.; Sattar, N.; et al. Strikingly low circulating CRP concentrations in ultramarathon runners independent of markers of adiposity. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1640–1644. [Google Scholar] [CrossRef]
- Nieman, D.C. Immune response to heavy exertion. J. Appl. Physiol. (1985) 1997, 82, 1385–1394. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Labilloy, D. The influence of work intensity on postexercise proteinuria. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 57, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Chaouachi, A.; Coutts, A.J.; del Wong, P.; Roky, R.; Mbazaa, A.; Amri, M.; Chamari, K. Haematological, inflammatory, and immunological responses in elite judo athletes maintaining high training loads during Ramadan. Appl. Physiol. Nutr. Metab. 2009, 34, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Nowak, D.; Nowak, R.; Chamera, T.; Buryta, R.; Moska, W.; Cięszczyk, P. Post-effort chances in C-reactive protein level among soccer players at the end of the training season. J. Strength Cond. Res. 2015, 29, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Mairbäurl, H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 2013, 4, 332. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, N.M.; Swift, D.L.; Johnson, W.D.; Dixit, V.D.; Earnest, C.P.; Blair, S.N.; Church, T.S. Effect of different doses of aerobic exercise on total white blood cell (WBC) and WBC subfraction number in postmenopausal women: Results from DREW. PLoS ONE 2012, 7, e31319. [Google Scholar] [CrossRef]
- Kostrzewa-Nowak, D.; Buryta, R.; Nowak, R. Comparison of selected CD45+ cell subsets’ response and cytokine levels on exhaustive effort among soccer players. J. Med. Biochem. 2019, 38, 256–267. [Google Scholar] [CrossRef]
- Kostrzewa-Nowak, D.; Nowak, R. Analysis of selected T cell subsets in peripheral blood after exhaustive effort among elite soccer players. Biochem. Med. (Zagreb) 2018, 28, 030707. [Google Scholar] [CrossRef]
- Nowak, R.; Kostrzewa-Nowak, D. Assessment of selected exercise-induced CD3+ cell subsets and cell death parameters among soccer players. J. Med. Biochem. 2019, 38, 437–444. [Google Scholar] [CrossRef]
- Polat, H.; Gulpinar, M.T.; Sarıca, M.A.; Benlioglu, C. Relationship between mean platelet volume, platelet distribution width, plateletcrit and varicocele. Andrologia 2017, 49. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Danese, E.; Skafidas, S.; Tarperi, C.; Guidi, G.C.; Schena, F. Mean platelet volume (MPV) predicts middle distance running performance. PLoS ONE 2014, 9, e112892. [Google Scholar] [CrossRef][Green Version]
- Alis, R.; Sanchis-Gomar, F.; Risso-Ballester, J.; Blesa, J.R.; Romagnoli, M. Effect of training status on the changes in platelet parameters induced by short-duration exhaustive exercise. Platelets 2016, 27, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Maffulli, N. Biological influence of physical exercise on hemostasis. Semin. Thromb. Hemost. 2009, 35, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.; Ernst, E. Exercise and thrombosis. Coron. Artery Dis. 2000, 11, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Imhof, A.; Koenig, W. Exercise and thrombosis. Cardiol. Clin. 2001, 19, 389–400. [Google Scholar] [CrossRef]
| Variable | Participant No. | Baseline | Post-Training | PC Ratio |
|---|---|---|---|---|
| Time of outdoor run on 1 km distance (min) | 1 | 3.57 | 3.32 | 0.93 |
| 2 | 4.12 | 3.58 | 0.87 | |
| 3 | 4.32 | 3.51 | 0.81 | |
| 4 | 3.15 | 2.51 | 0.79 | |
| 5 | 3.38 | 3.05 | 0.90 | |
| 6 | 3.52 | 3.02 | 0.86 | |
| VO2 max (mL/kg/min) | 1 | 43.06 | 48.86 | 1.13 |
| 2 | 40.18 | 42.86 | 1.07 | |
| 3 | 36.86 | 44.33 | 1.20 | |
| 4 | 53.74 | 62.47 | 1.16 | |
| 5 | 47.34 | 57.07 | 1.20 | |
| 6 | 44.11 | 58.15 | 1.32 | |
| Pace (s) | 1 | 4.23 | 4.06 | 0.96 |
| 2 | 3.67 | 3.63 | 0.99 | |
| 3 | 4.21 | 4.01 | 0.95 | |
| 4 | 3.76 | 3.68 | 0.98 | |
| 5 | 4.02 | 3.89 | 0.97 | |
| 6 | 4.24 | 4.13 | 0.97 | |
| Agility (s) | 1 | 17.86 | 16.82 | 0.94 |
| 2 | 15.77 | 15.32 | 0.97 | |
| 3 | 16.68 | 15.47 | 0.93 | |
| 4 | 14.84 | 14.23 | 0.96 | |
| 5 | 14.85 | 14.52 | 0.98 | |
| 6 | 15.44 | 14.79 | 0.96 |
| Variable | t0 | t1 | t2 | t3 | t4 | pANOVA |
|---|---|---|---|---|---|---|
| Weight (kg) | 82.5 | 84.6 | 85.4 | 87.1 | 91.6 | NS |
| (75.6–98.5) | (77.0–96.6) | (75.3–98.1) | (79.2–97.5) | (81.9–96.3) | ||
| BMI (kg/m2) | 25.6 | 25.5 | 25.0 | 26.9 | 27.8 | NS |
| (23.0–28.8) | (23.4–28.9) | (23.2–28.7) | (24.4–28.5) | (25.3–28.4) | ||
| BMR (kJ) | 9150 | 9238 | 9199 | 8908 | 9253 | NS |
| (8602–10,309) | (8506–9560) | (8468–10,125) | (8782–878) | (9247–9782) | ||
| Fat (%) | 14.9 | 16.4 | 12.7 | 17.6 | 17.0 | NS |
| (7.0–21.5) | (8.7–22.7) | (7.1–18.5) | (9.8–18.5) | (15.9–20.9) | ||
| Fat mass (kg) | 12.2 | 13.6 | 11.1 | 15.3 | 15.8 | NS |
| (5.3–20.1) | (7.4–21.7) | (6.0–16.7) | (7.8–18.0) | (14.1–19.7) | ||
| FFM (kg) | 73.5 | 74 | 74.4 | 71.8 | 74.6 | NS |
| (69.8–102.0) | (69.1–77.2) | (69.1–81.4) | (71.4–79.5) | (74.5–78.8) | ||
| TBW (kg) | 53.8 | 54.2 | 54.5 | 52.6 | 54.6 | NS |
| (51.1–60.7) | (50.6–56.5) | (50.6–59.6) | (52.3–58.2) | (54.5–57.7) |
| Variable | t0 | t1 | t2 | t3 | t4 | pANOVA | ppost-hoc |
|---|---|---|---|---|---|---|---|
| Amylase (U/L) | 53.2 | 62.8 | 56.8 | 96.1 | 63.8 | NS | - |
| (35.7–108.9) | (35.8–104.4) | (39.7–112.4) | (35.0–116.2) | (32.4–106.0) | |||
| AST (U/L) | 42.1 | 29.1 | 30.5 | 31.2 | 53.9 | 0.0242 | 0.0590 (t4 vs. t1) |
| (36.1–58.9) | (25.5–37.4) | (25.7–53.8) | (26.1–67.5) | (34.0–73.3) | |||
| ALT (U/L) | 16.9 | 23.2 | 20.8 | 36.5 | 31.1 | 0.0156 | 0.0263 (t3 vs. t0) |
| (14.7–26.8) | (15.7–25.6) | (17.7–75.4) | (22.3–75.3) | (25.7–35.4) | |||
| CK (U/L) | 275 | 131 | 191 | 124 | 280 | NS | - |
| (109–485) | (87.8–196.7) | (172–562) | (114–845) | (145–732) | |||
| GGT (U/L) | 16.0 | 20.2 | 17.1 | 16.5 | 18.2 | NS | - |
| (12.0–24.9) | (14.1–36.1) | (13.2–27.8) | (13.3–27.2) | (16.6–31.9) | |||
| LDH (U/L) | 280 | 314 | 356 | 290 | 383 | 0.0285 | 0.0399 (t4 vs. t0) |
| (212–338) | (257–424) | (340–465) | (272–420) | (358–520) | |||
| ALP (U/L) | 112.3 | 88.2 | 81.6 | 61.2 | 78.0 | 0.0486 | 0.0348 (t3 vs. t0) |
| (73.7–135.0) | (51.9–104.3) | (61.5–98.9) | (61–96.2) | (58.3–83) |
| Variable | t0 | t1 | t2 | t3 | t4 | pANOVA | ppost-hoc |
|---|---|---|---|---|---|---|---|
| Cr (μmol/L) | 101.1 | 110.0 | 111.0 | 110.0 | 105.5 | NS | - |
| (96.0–116.0) | (92.0–123.0) | (91.0–124.0) | (99.0–136.0) | (91.0–128.0) | |||
| U (mmol/L) | 8.4 | 6.7 | 7.3 | 7.2 | 6.4 | NS | - |
| (6.5–12.1) | (5.1–7.1) | (4.9–9.5) | (3.7–8.3) | (5.3–6.7) | |||
| UA (μmol/L) | 291 | 270 | 329 | 293 | 297 | NS | - |
| (191–402) | (247–386) | (214–406) | (249–306) | (269–351) | |||
| DBIL (μmol/L) | 6.02 | 5.54 | 5.43 | 5.37 | 6.85 | NS | - |
| (4.49–7.31) | (2.37–6.87) | (3.65–9.78) | (2.17–8.29) | (1.36–10.75) | |||
| TBIL (μmol/L) | 6.74 | 12.42 | 11.82 | 11.23 | 12.10 | NS | - |
| (4.05–9.98) | (7.87–14.2) | (8.42–15.25) | (8.10–13.36) | (4.98–16.82) | |||
| total protein (g/L) | 67.6 | 67.3 | 67.0 | 63.2 | 65.8 | NS | - |
| (63.4–76.2) | (64.6–74.8) | (63.7–71.0) | (61.1–69.1) | (60.3–70.1) | |||
| albumin (g/L) | 49.6 | 48.2 | 47.4 | 46.7 | 46.3 | NS | - |
| (44,4–50.7) | (45.2–50.6) | (46.9–49.2) | (43.7–47.5) | (44.5–49.2) | |||
| CRP (mg/L) | 0.0 | 0.6 | 2.3 | 8.05 | 8.13 | 0.005 | 0.0387 (t3 vs. t0) 0.0126 (t4 vs. t0) |
| (0.0) | (0.0–1.6) | (0.0–3.3) | (0.84–18.9) | (1.77–14.12) | |||
| TC (mmol/L) | 3.82 | 4.39 | 4.09 | 4.50 | 4.01 | NS | - |
| (3.27–4.45) | (3.54–4.86) | (3.46–4.74) | (3.29–4.64) | (3.55–4.74) | |||
| LDL-C (mmol/L) | 2.02 | 2.24 | 2.01 | 2.40 | 2.13 | NS | - |
| (1.11–2.60) | (1.14–2.61) | (1.43–2.61) | (1.12–2.55) | (1.05–2.44) | |||
| HDL-C (mmol/L) | 1.40 | 1.80 | 1.66 | 1.84 | 1.75 | NS | - |
| (1.15–1.84) | (1.33–2.13) | (1.45–1.76) | (1.19–1.92) | (1.42–2.22) | |||
| TG (mmol/L) | 0.83 | 0.93 | 0.97 | 0.59 | 0.90 | NS | - |
| (0.50–1.53) | (0.43–1.77) | (0.50–1.51) | (0.54–2.00) | (0.60–1.04) | |||
| ferritin (ng/mL) | 144.5 | 143.2 | 141.7 | 144.7 | 143.0 | NS | - |
| (140.9–146.2) | (141.6–147.1) | (139.9–146.3) | (143.4–147.7) | (140–147) | |||
| Fe (μmol/L) | 19.7 | 21.1 | 19.7 | 18.9 | 15.6 | NS | - |
| (12.7–31.1) | (18.8–28.2) | (17.9–24.2) | (18.5–20.8) | (11.6–31.5) | |||
| Mg (mmol/L) | 0.85 | 0.8 | 0.79 | 0.86 | 0.84 | NS | - |
| (0.81–0.91) | (0.74–0.89) | (0.78–0.82) | (0.83–0.87) | (0.78–0.87) | |||
| P (mmol/L) | 1.53 | 1.36 | 1.21 | 1.19 | 1.26 | NS | - |
| (1.36–1.55) | (0.81–1.64) | (1.05–1.41) | (0.00–1.42) | (0.87–1.52) | |||
| Ca (mmol/L) | 2.58 | 2.53 | 2.56 | 2.40 | NS | - | |
| (2.52–2.74) | (2.44–2.54) | (2.42–2.62) | (2.37–2.48) |
| Variable | t0 | t1 | t2 | t3 | t4 | pANOVA | ppost-hoc |
|---|---|---|---|---|---|---|---|
| RBC (109/L) | 4.92 | 4.87 | 5.08 | 4.84 | 5.12 | NS | - |
| (4.13–5.07) | (4.36–5.22) | (4.98–5.17) | (4.57–5.17) | (4.48–5.93) | |||
| HGB (mmol/L) | 8.60 | 8.40 | 8.45 | 8.60 | 8.85 | NS | - |
| (7.40–9.40) | (7.60–9.00) | (8.40–8.50) | (7.7–9.1) | (7.50–10.3) | |||
| HTC (L/L) | 0.46 | 0.46 | 0.47 | 0.47 | 0.49 | NS | - |
| (0.39–048) | (0.41–0.49) | (0.46–0.48) | (0.41–0.49) | (0.42–0.57) | |||
| MCV (fL) | 95 | 95 | 93 | 95 | 96 | NS | - |
| (92–95) | (92–96) | (90–96) | (91–97) | (93–97) | |||
| MCH (fmol) | 1.80 | 1.75 | 1.66 | 1.76 | 1.72 | 0.0176 | 0.013 (t2 vs. t0) |
| (1.74–1.85) | (1.68–1.76) | (1.62–1.70) | (1.68–1.78) | (1.67–1.77) | |||
| MCHC (mol/L) | 19.1 | 18.4 | 17.9 | 18.5 | 18.0 | 0.0018 | 0.0098 (t2 vs. t0) 0.0056 (t4 vs. t0) |
| (18.7–19.4) | (13.3–18.5) | (17.8–18.1) | (18.3–18.6) | (17.9–18.3) | |||
| RDW (%) | 10.8 | 11.4 | 11.4 | 11.3 | 11.6 | 0.0332 | 0.0178 (t4 vs. t0) |
| (10.6–11.3) | (11.0–11.9) | (10.9–1.9) | (11.3–11.6) | (11.4–12.0) | |||
| WBC (109/L) | 8.1 | 7.1 | 6.8 | 7.4 | 6.1 | NS | - |
| (6.0–10.2) | (6.3–9.6) | (6.1–7.4) | (5.1–7.8) | (4.2–6.8) | |||
| LYM (%) | 39.0 | 38.4 | 45.0 | 42.9 | 38.8 | NS | - |
| (27.0–49.0) | (19.6–47.1) | (42.1–47.9) | (39.0–45.4) | (18.7–41.5) | |||
| MON (%) | 3.8 | 4.5 | 4.4 | 4.5 | 4.4 | NS | - |
| (3.1–5.5) | (4.2–5.4) | (4.0–4.8) | (4.2–4.6) | (2.7–5.9) | |||
| GRA (%) | 56.9 | 56.7 | 50.6 | 52.5 | 56.8 | NS | - |
| (47.3–70.4) | (48.4–76.2) | (48.1–53.1) | (50.4–56.5) | (52.6–78.6) | |||
| LYM (109/L) | 2.90 | 3.10 | 3.00 | 3.00 | 2.05 | NS | - |
| (2.10–4.00) | (1.30–3.60) | (2.9–3.1) | (2.3–3.1) | (1.00–2.70) | |||
| MON (109/L) | 0.20 | 0.30 | 0.25 | 0.30 | 0.20 | NS | - |
| (0.20–0.50) | (0.20–0.40) | (0.20–0.30) | (0.20–0.30) | (0.10–0.30) | |||
| GRA (109/L) | 5.30 | 3.80 | 3.50 | 4.00 | 3.8 | NS | - |
| (3.50–5.80) | (3.30–5.60) | (3.00–4.00) | (2.6–4.5) | (2.4–4.6) | |||
| PLT (109/L) | 236 | 259 | 290 | 254 | 234 | NS | - |
| (127–326) | (133–280) | (282–297) | (236–306) | (181–250) | |||
| MPV (fL) | 6.9 | 7.1 | 7.6 | 7.5 | 7.1 | NS | - |
| (6.8–9.5) | (6.5–9.6) | (7.3–7.8) | (6.8–8.1) | (6.5–7.5) | |||
| PCT (10−2L/L) | 0.18 | 0.18 | 0.22 | 0.19 | 0.17 | 0.0426 | 0.049 (t4 vs. t2) |
| (0.12–0.22) | (0.13–0.21) | (0.21–0.23) | (0.17–0.23) | (0.12–0.18) | |||
| PDW (%) | 14.7 | 14.9 | 16.1 | 16.1 | 14.7 | NS | - |
| (14.6–17.9) | (14.0–20.1) | (15.8–16.3) | (15.8–16.5) | (14.2–15.9) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzewa-Nowak, D.; Nowakowska, A.; Zwierko, T.; Rybak, M.; Nowak, R. The Influence of a Health-Related Fitness Training Program on Motor Performance as Well as Hematological and Biochemical Parameters. Int. J. Environ. Res. Public Health 2020, 17, 578. https://doi.org/10.3390/ijerph17020578
Kostrzewa-Nowak D, Nowakowska A, Zwierko T, Rybak M, Nowak R. The Influence of a Health-Related Fitness Training Program on Motor Performance as Well as Hematological and Biochemical Parameters. International Journal of Environmental Research and Public Health. 2020; 17(2):578. https://doi.org/10.3390/ijerph17020578
Chicago/Turabian StyleKostrzewa-Nowak, Dorota, Anna Nowakowska, Teresa Zwierko, Maciej Rybak, and Robert Nowak. 2020. "The Influence of a Health-Related Fitness Training Program on Motor Performance as Well as Hematological and Biochemical Parameters" International Journal of Environmental Research and Public Health 17, no. 2: 578. https://doi.org/10.3390/ijerph17020578
APA StyleKostrzewa-Nowak, D., Nowakowska, A., Zwierko, T., Rybak, M., & Nowak, R. (2020). The Influence of a Health-Related Fitness Training Program on Motor Performance as Well as Hematological and Biochemical Parameters. International Journal of Environmental Research and Public Health, 17(2), 578. https://doi.org/10.3390/ijerph17020578







