Arsenic Concentrations and Dietary Exposure in Rice-Based Infant Food in Australia
Abstract
1. Introduction
- to determine the tAs and iAs concentrations in rice-based infant foods for sale in Australia;
- to investigate how the characteristics of rice-based products (i.e., rice content, rice texture and origin) are related to arsenic concentrations;
- to calculate the dietary intake and exposure of infants to examine the potential As exposure risk to Australian infants.
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation and Chemical Analysis
2.3. Quality Control
2.4. Dietary Exposure Estimations
- DI = dietary intake (μg person−1 day−1),
- C = concentration of contaminants (μg kg−1) and
- CR = food consumption rate (kg person−1 day−1).
- DE = dietary exposure (μg kg−1 b. wt. day−1)
- DI = dietary intake (μg person−1 day−1) and
- BW = mean individual body weight (kg)
2.5. Reference Values for Comparison
2.6. Statistical Analysis
3. Results and Discussion
3.1. As Occurrence in Australia Rice-Based Food
3.2. The Relationship between As Concentrations and Rice Properties and Other Factors
3.2.1. The Relationship between tAs Concentration and Rice Content
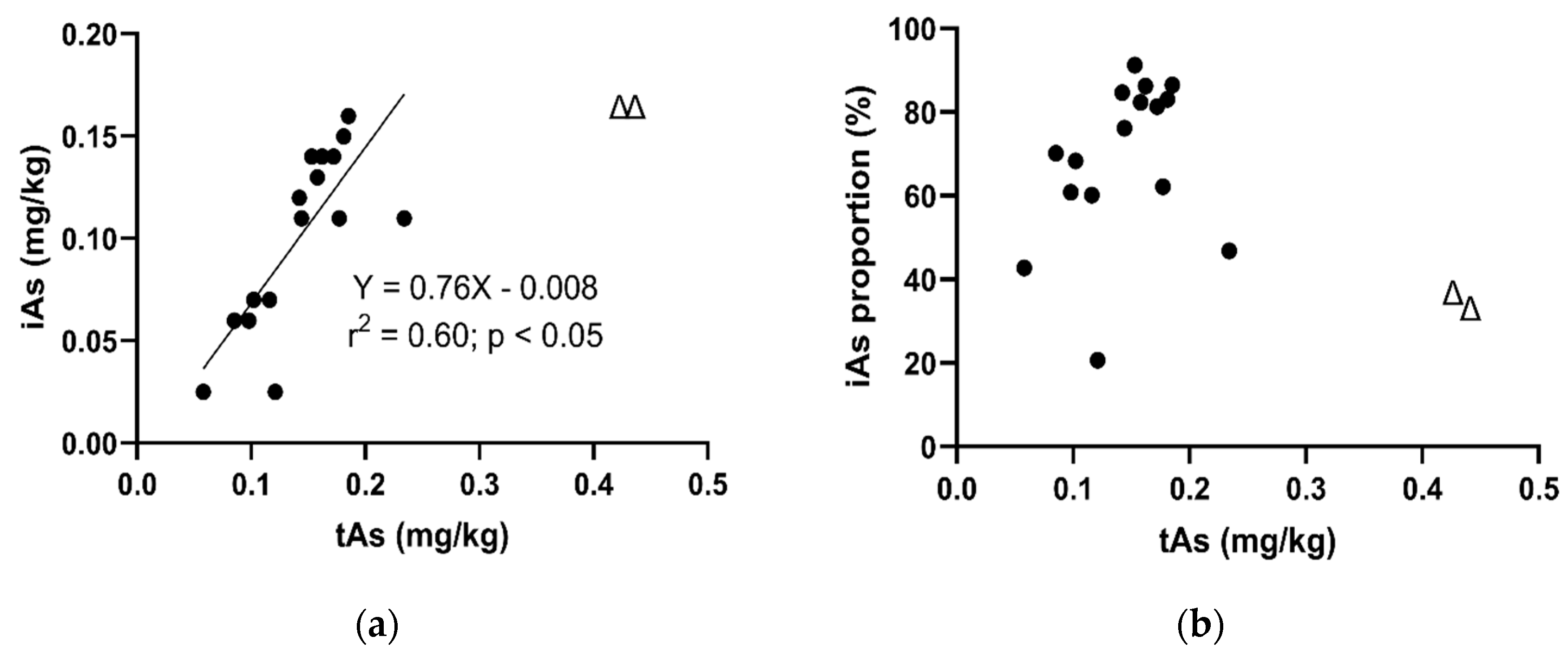
3.2.2. The Relationship between iAs and tAs Concentrations
3.2.3. The Relationship between tAs Concentration and Rice Type
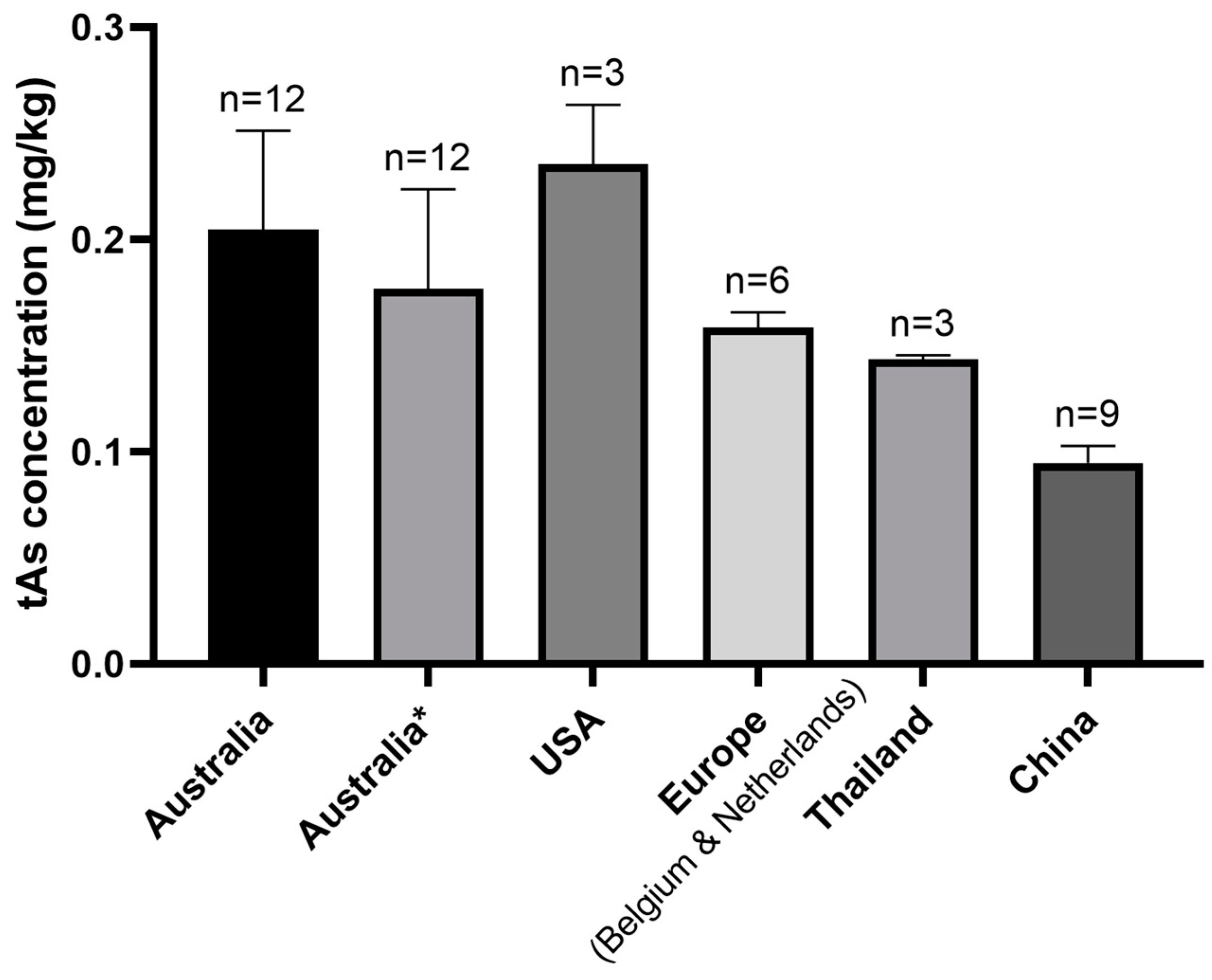
3.2.4. The Relationship between tAs Concentration and Country of Origin of Products
3.3. Dietary Exposure Estimation
Dietary Exposure Based on Australian Mean Food Consumption Rate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Some Drinking-water Disinfectants and Contaminants, including Arsenic; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 84; International Agency for Research on Cancer: Lyon, France, 2004. [Google Scholar]
- Christen, K. The arsenic threat worsens.(vietnamese wells are found to be contaminated) (statistical data included). Environ. Sci. Technol. 2001, 286A. [Google Scholar] [CrossRef] [PubMed]
- Wolrd Health Organization. Exposure to Arsenic: A Major Public Health Concern. Preventing Disease Through Healthy Environments Public Health and Environment; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Williams, P.N.; Price, A.H.; Raab, A.; Hossain, S.A.; Feldmann, J.; Meharg, A.A. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ. Sci. Technol. 2005, 39, 5531–5540. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Zhao, F.-J. Arsenic and Rice; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Zhao, F.-J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Y.; Ago, Y.; Liu, W.-J.; Mitani, N.; Feldmann, J.; McGrath, S.P.; Ma, J.F.; Zhao, F.-J. Rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009, 150, 2071–2080. [Google Scholar] [CrossRef]
- Abedin, M.J.; Cresser, M.S.; Meharg, A.A.; Feldmann, J.; Cotter-Howells, J. Arsenic accumulation and metabolism in rice (Oryza sativa L.). Environ. Sci. Technol. 2002, 36, 962. [Google Scholar] [CrossRef]
- Llorente-Mirandes, T.; Calderón, J.; Centrich, F.; Rubio, R. A fully validated method for the determination of arsenic species in rice and infant cereal products. Pure Appl. Chem. 2012, 84, 225–238. [Google Scholar] [CrossRef]
- Meharg, A.A.; Hartley-Whitaker, J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 2002, 154, 29–43. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Carey, M.; Meharg, A.A. Inorganic arsenic in rice-based products for infants and young children. Food Chem. 2016, 191, 128–134. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Arsenic in Food; European Food Safety Authority: Parma, Italy, 2009. [Google Scholar]
- Rahman, M.; Reichman, S.; Lim, R.; Naidu, R. Arsenic speciation in australian-grown and imported rice on sale in australia: Implications for human health risk. J. Agric. Food Chem. 2014, 62, 6016–6024. [Google Scholar] [CrossRef]
- Sun, G.X.; Williams, P.N.; Carey, A.M.; Zhu, Y.G.; Deacon, C.; Raab, A.; Feldmann, J.; Islam, R.M.; Meharg, A.A. Inorganic arsenic in rice bran and its products are an order of magnitude higher than in bulk grain. Environ. Sci. Technol. 2008, 42, 7542–7546. [Google Scholar] [CrossRef]
- Meharg, A.A.; Lombi, E.; Williams, P.N.; Scheckel, K.G.; Feldmann, J.; Raab, A.; Zhu, Y.; Islam, R. Speciation and localization of arsenic in white and brown rice grains. Environ. Sci. Technol. 2008, 42, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Arsenic in Rice: Full Analytical Result from Rice/Rice Product Sampling-September; U.S. FDA: Silver Spring, MD, USA, 2013.
- Meharg, A.A.; Sun, G.; Williams, P.N.; Adomako, E.; Deacon, C.; Zhu, Y.-G.; Feldmann, J.; Raab, A. Inorganic arsenic levels in baby rice are of concern. Environ. Pollut. 2008, 152, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Rintala, E.-M.; Ekholm, P.; Koivisto, P.; Peltonen, K.; Venäläinen, E.-R. The intake of inorganic arsenic from long grain rice and rice-based baby food in finland—Low safety margin warrants follow up. Food Chem. 2014, 150, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Munera-Picazo, S.; Ramírez-Gandolfo, A.; Burló, F.; Carbonell-Barrachina, Á.A. Inorganic and total arsenic contents in rice-based foods for children with celiac disease. J. Food Sci. 2014, 79, T122–T128. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Meng, C.; Umoren, J.; West, H. Risk Assessment of Arsenic in Rice Cereal and Other Dietary Sources for Infants and Toddlers in the U.S. Int. J. Environ. Res. Public Health 2016, 13, 361. [Google Scholar] [CrossRef]
- Rothenberg, S.E.; Jackson, B.P.; McCalla, G.C.; Donohue, A.; Emmons, A.M. Co-exposure to methylmercury and inorganic arsenic in baby rice cereals and rice-containing teething biscuits. Environ. Res. 2017, 159, 639–647. [Google Scholar] [CrossRef]
- The European Commission. Amending Regulation (EC) no 1881/2006 as Regards Maximum Levels of Inorganic Arsenic in Foodstuffs; The European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Islam, S.; Rahman, M.M.; Rahman, M.A.; Naidu, R. Inorganic arsenic in rice and rice-based diets: Health risk assessment. Food Control 2017, 82, 196–202. [Google Scholar] [CrossRef]
- Australia New Zealand Food Standards. Australia New Zealand Food Standards Code—Schedule 19—Maximum Levels of Contaminants and Natural Toxicants; Australia New Zealand Food Standards: Canberra, Australia, 2017.
- Joint FAO/WHO Expert Committee on Food Additives. Joint Fao/Who Food Standards Programme Codex Alimentarius Commission: Report of the Sixth Session of the Codex Committee on Contaminants in Foods; World Health Organization: Rome, Italy, 2012. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Joint Fao/Who Food Standards Programme Codex Alimentarius Commission 37th Session: Report of the Eighth Session of the Codex Committe on Contaminants in Foods; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Thirty-third Report of the Joint Fao/Who Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 1989. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Seventy-Fourth Report of the Joint Fao/Who Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Fransisca, Y.; Small, D.; Morrison, P.; Spencer, M.; Ball, A.; Jones, O. Assessment of arsenic in australian grown and imported rice varieties on sale in australia and potential links with irrigation practises and soil geochemistry. Chemosphere 2015, 138, 1008–1013. [Google Scholar] [CrossRef]
- Holak, W.; Specchio, J.J. Determination of total arsenic, as (iii) and as (v), in foods by atomic abosorption spectrophotometry. At. Spectrosc. 1991, 12, 105–108. [Google Scholar]
- Joint FAO/WHO Food Contamination Monitoring Programme. Guidelines for the Study of Dietary Intakes of Chemical Contaminants; World Health Organization: Geneva, Switzerland, 1985. [Google Scholar]
- Food Standards Australia New Zealand. The 23rd Australian Total Diet Study; Food Standards Australia New Zealand: Canberra, Australia, 2011.
- Mikkonen, H.G.; Clarke, B.O.; Dasika, R.; Wallis, C.J.; Reichman, S.M. Evaluation of methods for managing censored results when calculating the geometric mean. Chemosphere 2018, 191, 412–416. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, M.; Bi, X.; He, Y.; Ren, L.; Xiang, W.; Qiao, S.; Yan, S.; Li, Z.; Ma, Z. Occurrence of arsenic in brown rice and its relationship to soil properties from Hainan Island, China. Environ. Pollut. 2011, 159, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Guillod-Magnin, R.; Brüschweiler, B.J.; Aubert, R.; Haldimann, M. Arsenic species in rice and rice-based products consumed by toddlers in switzerland. Food Addit. Contam. A 2018, 35, 1164–1178. [Google Scholar] [CrossRef]
- USDA Foreign Agricultural Service. China, Peoples Republic of Fairs Product Specific. Maximum Levels of Contaminants in Foods; United States Department of Agriculture Foreign Agricultural Service: Washington, DC, USA, 2011.
- United States Environmental Protection Agency. Technical Fact Sheet: Final Rule for Arsenic in Drinking Water; U.S. EPA: Washington, DC, USA, 2001.
- Potera, C. Food Safety: U.S. rice serves up arsenic. Environ. Health Perspect. 2007, 115, A296–A299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- U.S. Food and Drug Administratioin. Inorganic Arsenic in Rice Cereals for Infants: Action Level; Draft Guidance for Industry; U.S.FDA: Rockvile, MD, USA, 2014.
- Maher, W.; Duncan, E.; Martin, H.; Snell, P.; Krikowa, F.; Jagtap, R.; Foster, S.; Ezaz, T.; Ellwood, M.J. Arsenic concentrations and speciation in Australian and imported rice and commercial rice products. Environ. Chem. 2018, 15, 387–402. [Google Scholar] [CrossRef]
- Cummins, A.G.; Roberts-Thomson, I.C. Prevalence of celiac disease in the asia-pacific region. J. Gastroenterol. Hepatol. 2009, 24, 1347–1351. [Google Scholar] [CrossRef]


| Year of Implementation | Name | Concentration | Jurisdiction | Reference |
|---|---|---|---|---|
| As Concentration in Food (mg kg−1) | ||||
| 2017 | Permissible limit of tAs in rice and rice cereal | 1.0 | Australia | [24] |
| 2012 | Proposed maximum levels for tAs in rice | 0.3 | FAO/WHO | [25] |
| 2014 | Maximum levels for iAs in rice | 0.2 | FAO/WHO | [26] |
| 2016 | Maximum levels of iAs in food for infants and young children | 0.1 | EU | [22] |
| As Dietary Exposure (μg kg−1 b. wt.) | ||||
| 1967 | Maximum tolerable daily intake (MTDI) for tAs | 50 | FAO/WHO (Withdrawn) | [27] |
| 2010 | Benchmark dose lower confidence of 0.5% (BMDL0.5) | 3 | FAO/WHO | [28] |
| 2009 | Benchmark dose lower confidence of 1% (BMDL01) | 0.3–8.0 | EU | [12] |
| Product Category | n | Mean As Concentration | Mean iAs % | |
|---|---|---|---|---|
| tAs | iAs | |||
| Rice milk powder | 3 | 0.428 ± 0.002 | 0.160 ± 0 | 37.4 ± 0.43 |
| Non-rice milk powder | 3 | <0.004 | − | − |
| P-value | <0.001 | |||
| Rice pasta | 3 | 0.186 ± 0.003 | 0.155 ± 0.005 | 84.8 ± 8.40 |
| Non-rice pasta | 3 | 0.006 ± 0.002 | − | − |
| P-value | <0.001 | |||
| Rice cereal | 12 | 0.134 ± 0.023 | − | − |
| Rice crackers | 21 | 0.132 ± 0.010 | 0.094 ± 0.012 | 74.3 ± 1.73 |
| Product Category | Sampling Place | n | Mean As Concentration | Reference | |
|---|---|---|---|---|---|
| tAs | iAs | ||||
| Rice milk | Australia | 3 | 0.428 ± 0.002 1/0.020 ± 0 2 | 0.160 ± 0 1/0.008 ± 0 2 | Present study |
| Switzerland | 6 | 0.015 (0.011–0.025) 3 | 0.009 (0.007–0.013) 3 | [35] | |
| Rice pasta | Australia | 3 | 0.186 ± 0.003 | 0.155 ± 0.005 | Present study |
| Spain | 4 | 0.192 (0.132–0.285) | 0.136 (0.794–0.170) | [19] | |
| Rice cereal | Australia | 12 | 0.134 ± 0.023 | – | Present study |
| Australia | 2 | 0.268 ± 0.006 | 0.073 ± 0.006 | [23] | |
| United States | 105 | 0.132 (0.050–0.723) | 0.091 (0.023–0.283) | [16] | |
| United Kingdom | 53 | 0.119 1 (0.042–0.396) | 0.075 (0.008–0.323) | [11] | |
| Switzerland | 7 | 0.278 (0.065–0.630) | 0.204 (0.046–0.331) | [35] | |
| Spain | 9 | 0.309 ± 0.011 | 0.107 ± 0.021 | [9] | |
| Rice crackers | Australia | 21 | 0.132 ± 0.010 | 0.094 ± 0.012 | Present study |
| United States | 199 | 0.121 (0.009–1.931) | 0.079 (0.008–0.273) | [16] | |
| United Kingdom | 97 | 0.141 4 (0.019–0.328) | 0.111 (0.018–0.211) | [11] | |
| Switzerland | 25 | 0.168 (0.047–0.361) | 0.134 (0.040–0.279) | [35] | |
| Food Category | n | 9 Month | 2–5 Years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| tAs | iAs | tAs | iAs | ||||||
| Mean | 90th Percentile | Mean | 90th Percentile | Mean | 90th Percentile | Mean | 90th Percentile | ||
| Rice milk powder | 3 | 0.02 | 0.04 | 0.007 | 0.015 | 0.03 | 0.06 | 0.01 | 0.02 |
| Rice pasta | 3 | 0.18 | 0.36 | 0.15 | 0.30 | 0.29 | 0.58 | 0.24 | 0.48 |
| Rice cereal | 12 | 0.03 | 0.06 | − | − | 0.05 | 0.10 | − | − |
| Rice crackers | 21 | 0.13 | 0.26 | 0.09 | 0.18 | 0.21 | 0.41 | 0.15 | 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; de Silva, S.; Reichman, S.M. Arsenic Concentrations and Dietary Exposure in Rice-Based Infant Food in Australia. Int. J. Environ. Res. Public Health 2020, 17, 415. https://doi.org/10.3390/ijerph17020415
Gu Z, de Silva S, Reichman SM. Arsenic Concentrations and Dietary Exposure in Rice-Based Infant Food in Australia. International Journal of Environmental Research and Public Health. 2020; 17(2):415. https://doi.org/10.3390/ijerph17020415
Chicago/Turabian StyleGu, Zhuyun, Shamali de Silva, and Suzie M. Reichman. 2020. "Arsenic Concentrations and Dietary Exposure in Rice-Based Infant Food in Australia" International Journal of Environmental Research and Public Health 17, no. 2: 415. https://doi.org/10.3390/ijerph17020415
APA StyleGu, Z., de Silva, S., & Reichman, S. M. (2020). Arsenic Concentrations and Dietary Exposure in Rice-Based Infant Food in Australia. International Journal of Environmental Research and Public Health, 17(2), 415. https://doi.org/10.3390/ijerph17020415





