Chest CT Computerized Aided Quantification of PNEUMONIA Lesions in COVID-19 Infection: A Comparison among Three Commercial Software
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. CT Technique
2.3. CT Post-Processing
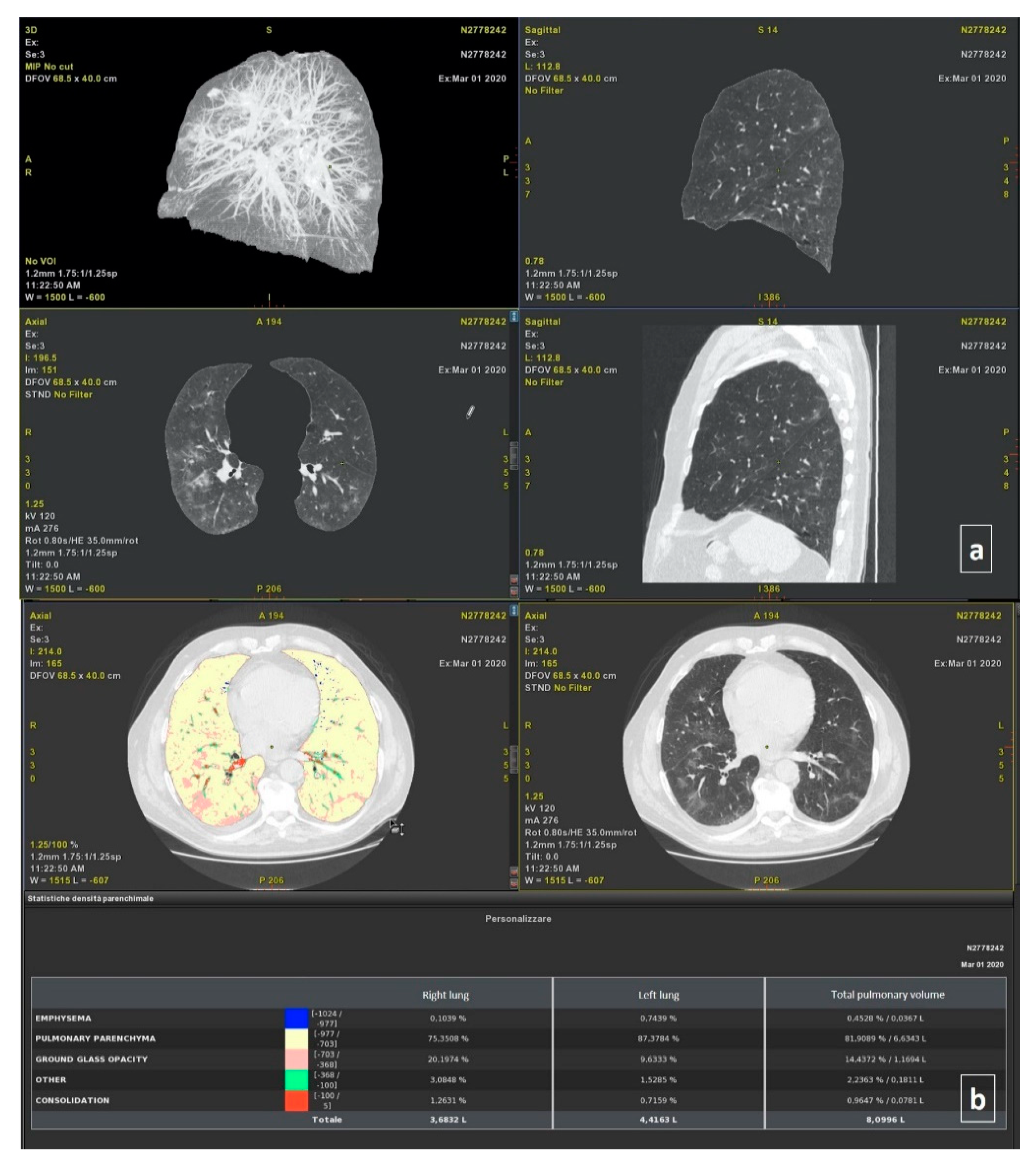
2.3.1. Post-Processing with Thoracic VCAR Software
2.3.2. Post-Processing with Myrian Software
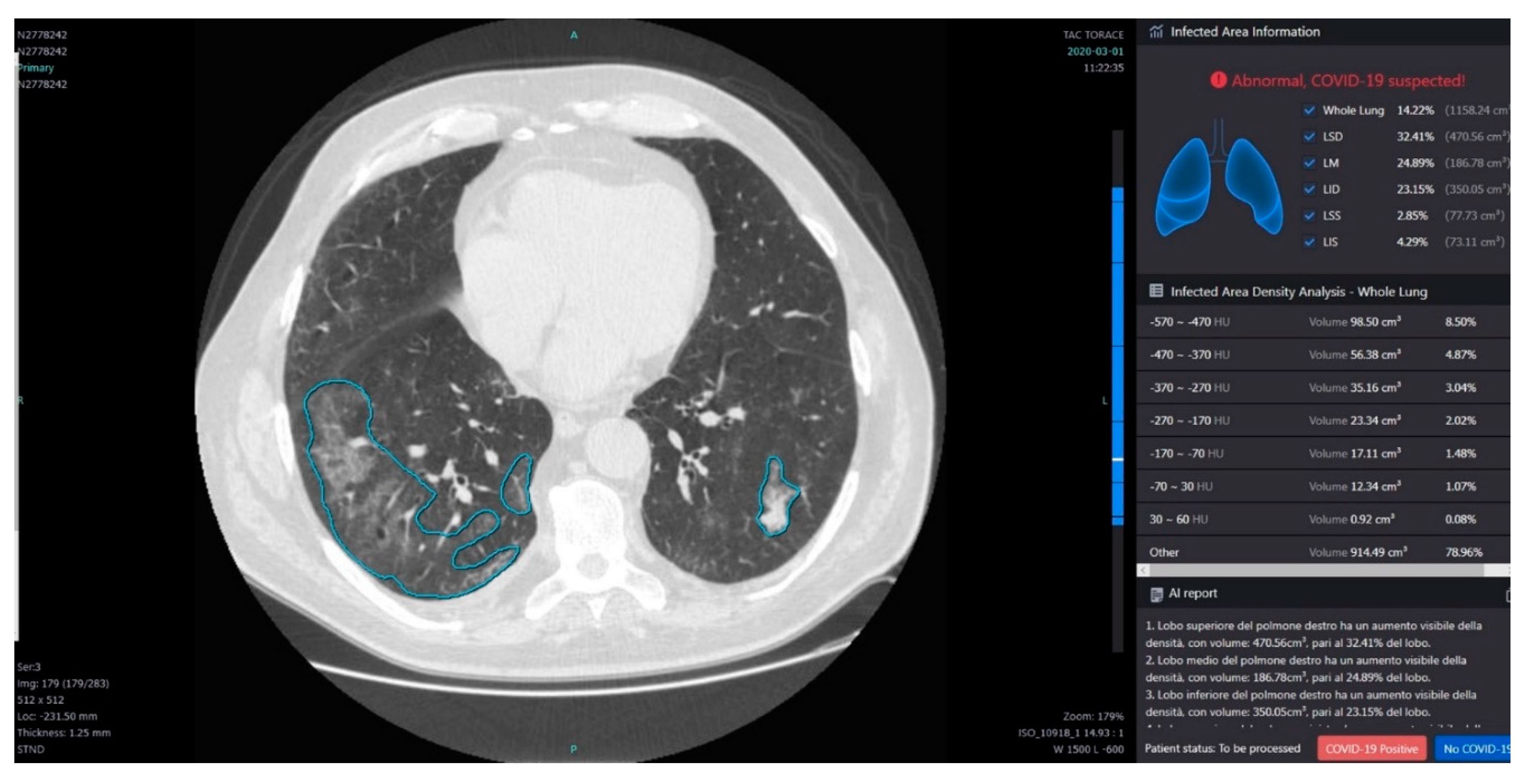
2.3.3. Post Processing with InferRead Software
2.4. Radiologists Analysis
2.5. Statistical Analysis
3. Results
4. Discussions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization Website. Naming the Coronavirus Disease (COVID-2019) and the Virus that Causes it. Available online: www.who.int/emergencies/diseases/ (accessed on 21 March 2020).
- Wuhan Coronavirus (2019-nCoV) Global Cases (by Johns Hopkins CSSE). Case Dashboard. Available online: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed on 21 March 2020).
- Li, Y.; Xia, L. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. Am. J. Roentgenol. 2020, 214, 1280–1286. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 15, 497–506. [Google Scholar] [CrossRef]
- Lei, J.; Li, J.; Li, X.; Qi, X. CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology 2020, 295, 18. [Google Scholar] [CrossRef]
- American College of Radiology. ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. Available online: https://psnet.ahrq.gov/issue/acr-recommendations-use-chest-radiography-and-computed-tomography-ct-suspected-covid-19 (accessed on 1 March 2020).
- The Royal Australian and New Zealand College of Radiologist. COVID-19 Updates. Available online: https://www.ranzcr.com/our-work/coronavirus (accessed on 13 September 2020).
- The Royal College of Radiologists. RCR Position on the Role of CT in Patients Suspected with COVID-19 Infection. Available online: https://www.rcr.ac.uk/college/coronavirus-covid-19-what-rcr-doing/clinical-information/role-ct-chest/role-ct-patients (accessed on 13 September 2020).
- Canadian Association of Radiologists. Canadian Society of Thoracic Radiology and Canadian Association of Radiologists’ Statement on COVID-19. Available online: https://car.ca/ (accessed on 13 September 2020).
- Mossa-Basha, M.; Meltzer, C.C.; Kim, D.; Tuite, M.J.; Kolli, K.P.; Tan, B.-S. Radiology Department Preparedness for COVID-19: Radiology Scientific Expert Panel. Radiology 2020, 200988. [Google Scholar] [CrossRef] [PubMed]
- Zu, Z.Y.; Di Jiang, M.; Xu, P.P.; Chen, W.; Ni, Q.Q.; Lu, G.; Zhang, L.J. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology 2020, 296, E15–E25. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Urraro, F.; Grassi, R.; Giacobbe, G.; Patelli, G.; Cappabianca, S.; Reginelli, A. Artificial intelligence to codify lung CT in Covid-19 patients. Radiol. Med. 2020, 125, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Neri, E.; Miele, V.; Coppola, F.; Grassi, R. Use of CT and artificial intelligence in suspected or COVID-19 positive patients: Statement of the Italian Society of Medical and Interventional Radiology. Radiol. Med. 2020, 125, 505–508. [Google Scholar] [CrossRef]
- Laghi, A.; Grassi, R. Italian Radiology’s Response to the COVID-19 Outbreak. J. Am. Coll. Radiol. 2020, 17, 699–700. [Google Scholar] [CrossRef]
- Brady, A.P.; Neri, E. Artificial Intelligence in Radiology-Ethical Considerations. Diagnostics 2020, 10, 231. [Google Scholar] [CrossRef]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Artificial Intelligence Distinguishes COVID-19 from Community Acquired Pneumonia on Chest CT. Radiology 2020, 19, 200905. [Google Scholar] [CrossRef] [PubMed]
- Tárnok, A. Machine Learning, COVID-19 (2019-nCoV), and multi-OMICS. Cytometry 2020, 97, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Gozes, O.; Frid-Adar, M.; Greenspan, H.; Browning, P.; Zhang, H.; Ji, W.; Bernheim, A.; Siegel, E. Rapid AI Development Cycle for the Coronavirus (COVID-19) Pandemic: Initial Results for Automated Detection & Patient Monitoring using Deep Learning CT Image Analysis. arXiv 2020, arXiv:2003.05037. [Google Scholar]
- Wang, S.; Kang, B.; Ma, J.; Zeng, X.; Xiao, M.; Guo, J.; Cai, M.; Yang, J.; Li, Y.; Meng, C.; et al. A deep learning algorithm using CT images to screen for Corona Virus Disease (COVID-19). MedRxiv 2020. [Google Scholar] [CrossRef]
- Grassi, R.; Fusco, R.; Belfiore, M.P.; Montanelli, A.; Patelli, G.; Urraro, F.; Petrillo, A.; Granata, V.; Sacco, P.; Mazzei, M.A.; et al. Coronavirus Disease 2019 (COVID-19) in Italy: Features on Chest Computed Tomography using a structured report system. Sci. Rep. 2020. [Google Scholar] [CrossRef]
- COVID-19 Structured Report. Available online: https://www.sirm.org/wp-content/uploads/2020/03/Covid19-Structured-Report-Short-EN.pdf (accessed on 1 March 2020).
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020. [Google Scholar] [CrossRef]
- Neri, E.; Coppola, F.; Miele, V.; Bibbolino, C.; Grassi, R. Artificial intelligence: Who is responsible for the diagnosis? Radiol. Med. 2020. [Google Scholar] [CrossRef]
- Allam, Z.; Jones, D.S. On the coronavirus (COVID-19) outbreak and the smart city network: Universal data sharing standards coupled with artificial intelligence (AI) to benefit urban health monitoring and management. Healthcare 2020. [Google Scholar] [CrossRef]
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, N.W.; Volpi, A.; Yim, J.J.; Martin, I.B.K.; et al. The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic: A Multinational Consensus Statement from the Fleischner Society. Radiology 2020, 296, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Grassi, R.; Miele, V.; Giovagnoni, A. Artificial intelligence: A challenge for third millennium radiologist. Radiol. Med. 2019, 124, 241–242. [Google Scholar] [CrossRef]
- Sverzellati, N.; Odone, A.; Silva, M.; Polverosi, R.; Florio, C.; Cardinale, L.; Cortese, G.; Addonisio, G.; Zompatori, M.; Dalpiaz, G.; et al. Italian Structured Report on Fibrosing Lung Disease Consort. Structured reporting for fibrosing lung disease: A model shared by radiologist and pulmonologist. Radiol. Med. 2018, 123, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Hoesein, F.A.; de Hoop, B.; Zanen, P.; Gietema, H.; Kruitwagen, C.L.; van Ginneken, B.; Isgum, I.; Mol, C.; van Klaveren, R.J.; Dijkstra, A.E.; et al. CT-quantified emphysema in male heavy smokers: Association with lung function decline. Thorax 2011, 66, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, F.; Moua, T.; Rajagopalan, S.; Karwoski, R.A.; Raghunath, S.; Decker, P.A.; Hartman, T.E.; Bartholmai, B.J.; Robb, R.A.; Ryu, J.H. Automated quantification of radiological patterns predicts survival in idiopathic pulmonary fibrosis. Eur. Respir. J. 2014, 43, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, X.; Liu, H.; Zhen, Y.; Zhang, X.; Xiong, Q.; Luo, Y.; Gao, C.; Zeng, W. Chest CT Severity Score: An Imaging Tool for Assessing Severe COVID-19. Radiology 2020, 2, e200047. [Google Scholar] [CrossRef]
- Colombi, D.; Bodini, F.C.; Petrini, M.; Maffi, G.; Morelli, N.; Milanese, G.; Silva, M.; Sverzellati, S.; Michieletti, E. Well-aerated Lung on Admitting Chest CT to Predict Adverse Outcome in COVID-19 Pneumonia. Radiology 2020. [Google Scholar] [CrossRef]

| Functionalities | Thoracic VCAR | Myriam | InferRead |
|---|---|---|---|
| Quantitative data automatically divided by lobes | no | no | yes |
| Quantitative data automatically divided by lung | yes | yes | yes |
| Total quantitative data | yes | yes | yes |
| Ability to segment manually | yes | no | no |
| Preliminary possibility of excluding automatically vascular structures | no | no | no |
| Preliminary possibility of excluding automatically airways | yes | yes | no |
| Possibility to create as many threshold windows as desired | yes | no | no |
| Possibility to modify the HU values in the threshold windows | yes | yes | no |
| Possibility to change the colors of the threshold windows | yes | yes | no |
| Possibility of 3D reconstruction | yes | yes | no |
| CE marking for lung study for COVID-19 | no | yes | no |
| CE marking for lung study | yes | yes | no |
| Evaluation of emphysematous areas distinct from GGO, consolidation and residual parenchyma | yes | no | no |
| Possibility to evaluate GGO areas distinct from others | yes | yes | no |
| Possibility to evaluate consolidation areas distinct from others | yes | yes | no |
| Possibility to evaluate healthy parenchyma areas distinct from others | yes | yes | yes |
| Evaluation separately pleural effusion | no | no | no |
| Combined structured report | no | yes | no |
| Ability to export values to an unstructured report | yes | yes | yes |
| Automatic comparison of the previous exam with the current one in the follow-up | no | yes | yes |
| Variability | ICC | Lower Bound | Upper Bound |
|---|---|---|---|
| Total LHP (%) | 0.17 | 0.05 | 0.31 |
| Total GGO (%) | 0.51 | 0.30 | 0.67 |
| Total Consolidation (%) | 0.20 | 0.04 | 0.37 |
| ThoracicVCAR Total LHP (%) | ThoracicVCAR Total GGO (%) | ThoracicVCAR Total Consolidation (%) | Myrian Total LHP (%) | Myrian Total GGO (%) | Myrian Total Consolidation (%) | Infervision Total LHP (%) | Infervision Total GGO (%) | Infervision Total Consolidation (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ThoracicVCAR Total LHP (%) | Spearman Correlation Coefficient | 1.00 | −0.964 ** | −0.722 ** | 0.753 ** | −0.677 ** | −0.767 ** | 0.10 | −0.499 ** | −0.400 ** |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.39 | 0.00 | 0.00 | ||
| ThoracicVCAR Total GGO (%) | Spearman Correlation Coefficient | −0.964 ** | 1.00 | 0.619 ** | −0.780 ** | 0.741 ** | 0.748 ** | −0.06 | 0.539 ** | 0.343 ** |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.60 | 0.00 | 0.00 | ||
| ThoracicVCAR Total Consolidation (%) | Spearman Correlation Coefficient | −0.722 ** | 0.619 ** | 1.00 | −0.536 ** | 0.557 ** | 0.559 ** | −0.13 | 0.333 ** | 0.421 ** |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.26 | 0.00 | 0.00 | ||
| Myrian Total LHP (%) | Spearman Correlation Coefficient | 0.753 ** | −0.780 ** | −0.536 ** | 1.00 | −0.935 ** | −0.870 ** | 0.14 | −0.570 ** | −0.371 ** |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 | 0.00 | 0.00 | ||
| Myrian Total GGO (%) | Spearman Correlation Coefficient | −0.677 ** | 0.741 ** | 0.557 ** | −0.935 ** | 1.00 | 0.749 ** | −0.10 | 0.568 ** | 0.314 ** |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.39 | 0.00 | 0.00 | ||
| Myrian Total Consolidation (%) | Spearman Correlation Coefficient | −0.767 ** | 0.748 ** | 0.559 ** | −0.870 ** | 0.749 ** | 1.00 | −0.232 * | 0.613 ** | 0.492 ** |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | ||
| Infervision Total LHP (%) | Spearman Correlation Coefficient | 0.10 | −0.06 | −0.13 | 0.14 | −0.10 | −0.232 * | 1.00 | −0.462 ** | −0.462 ** |
| p-value | 0.39 | 0.60 | 0.26 | 0.20 | 0.39 | 0.04 | 0.00 | 0.00 | ||
| Infervision Total GGO (%) | Spearman Correlation Coefficient | −0.499 ** | 0.539 ** | 0.333 ** | −0.570 ** | 0.568 ** | 0.613 ** | −0.462 ** | 1.00 | 0.601 ** |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Infervision Total Consolidation (%) | Spearman Correlation Coefficient | −0.400 ** | 0.343 ** | 0.421 ** | −0.371 ** | 0.314 ** | 0.492 ** | −0.462 ** | 0.601 ** | 1.00 |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| ThoracicVCAR LHP DX (%) | ThoracicVCAR LHP SX (%) | ThoracicVCAR Total LHP (%) | ThoracicVCAR GGO DX (%) | ThoracicVCAR GGO SN (%) | ThoracicVCAR Total GGO (%) | ThoracicVCAR Consolidation DX (%) | ThoracicVCAR Consolidation SX (%) | ThoracicVCAR Total Consolidation (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall radiological score ≤ 2 | Median | 86.60 | 84.50 | 85.80 | 10.90 | 12.00 | 10.30 | 0.70 | 0.70 | 0.70 |
| Minimum | 59.70 | 65.40 | 67.90 | 2.10 | 3.10 | 2.90 | 0.10 | 0.20 | 0.20 | |
| Maximum | 96.70 | 95.30 | 95.60 | 30.70 | 28.20 | 25.90 | 3.50 | 3.20 | 2.30 | |
| Overall radiological score 3–4 | Median | 77.40 | 75.10 | 77.10 | 16.60 | 17.70 | 16.40 | 0.90 | 0.90 | 0.90 |
| Minimum | 6.30 | 26.30 | 29.10 | 5.60 | 2.40 | 5.20 | 0.30 | 0.30 | 0.30 | |
| Maximum | 92.70 | 94.30 | 93.60 | 63.30 | 66.60 | 64.10 | 17.30 | 17.10 | 17.20 | |
| Overall radiological score 5–6 | Median | 67.90 | 64.80 | 66.00 | 26.00 | 27.90 | 26.80 | 1.40 | 1.30 | 1.30 |
| Minimum | 35.50 | 42.30 | 42.60 | 6.20 | 5.90 | 6.00 | 0.30 | 0.20 | 0.30 | |
| Maximum | 90.60 | 90.90 | 90.80 | 40.50 | 46.30 | 40.50 | 16.40 | 5.80 | 10.70 | |
| Overall radiological score 7–8 | Median | 50.80 | 53.90 | 55.90 | 39.10 | 27.10 | 33.00 | 1.80 | 1.60 | 1.60 |
| Minimum | 18.50 | 6.40 | 21.90 | 29.00 | 20.10 | 27.80 | 0.50 | 0.60 | 0.50 | |
| Maximum | 63.10 | 76.50 | 66.40 | 62.10 | 62.10 | 59.40 | 7.70 | 11.10 | 5.40 | |
| p-value at Kruskal–Wallis test | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.24 | 0.07 | 0.24 | |
| Spearman Correlation Coefficient | -0.76 | -0.57 | -0.74 | 0.68 | 0.58 | 0.65 | 0.40 | 0.38 | 0.39 | |
| p-value of Spearman Correlation | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Myrian LHP DX (%) | Myrian LHP SX (%) | Myrian Total LHP (%) | Myrian GGO DX (%) | Myrian GGO SN (%) | Myrian Total GGO (%) | Myrian Consolidation DX (%) | Myrian Consolidation SX (%) | Myrian Total Consolidation (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall radiological score ≤ 2 | Median | 69.10 | 66.80 | 67.00 | 26.20 | 27.30 | 26.70 | 4.00 | 4.70 | 4.70 |
| Minimum | 22.70 | 23.90 | 25.80 | 9.10 | 9.50 | 9.30 | 2.10 | 2.10 | 2.20 | |
| Maximum | 88.30 | 88.10 | 88.20 | 61.90 | 71.30 | 62.00 | 14.80 | 15.60 | 13.70 | |
| Overall radiological score 3–4 | Median | 56.80 | 57.10 | 56.90 | 31.40 | 31.30 | 31.80 | 7.00 | 8.00 | 7.60 |
| Minimum | 11.10 | 8.10 | 9.70 | 2.40 | 4.50 | 3.40 | 0.00 | 2.00 | 1.20 | |
| Maximum | 97.60 | 92.70 | 95.30 | 74.50 | 79.20 | 76.80 | 32.80 | 46.80 | 43.60 | |
| Overall radiological score 5–6 | Median | 38.60 | 40.30 | 41.00 | 44.00 | 44.25 | 43.05 | 13.10 | 12.70 | 13.95 |
| Minimum | 13.70 | 13.90 | 16.90 | 18.50 | 19.40 | 18.90 | 2.60 | 2.50 | 2.60 | |
| Maximum | 78.50 | 77.80 | 78.20 | 69.10 | 68.60 | 68.90 | 29.60 | 32.90 | 31.10 | |
| Overall radiological score 7–8 | Median | 24.45 | 31.95 | 27.70 | 45.10 | 48.70 | 47.25 | 23.80 | 14.90 | 23.00 |
| Minimum | 9.80 | 2.00 | 8.00 | 30.00 | 36.00 | 34.30 | 7.40 | 4.10 | 5.80 | |
| Maximum | 59.60 | 59.50 | 54.60 | 69.20 | 63.00 | 61.80 | 44.60 | 49.00 | 36.50 | |
| p value at Kruskal–Wallis test | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Spearman Correlation Coefficient | −0.62 | −0.55 | −0.70 | 0.55 | 0.54 | 0.56 | 0.72 | 0.54 | 0.72 | |
| p value of Spearman Correlation | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| InferRead Total LHP (%) | InferRead Total GGO (%) | InferRead Total Consolidation (%) | −570/−470 (%) | −470/−370 (%) | −370/−270 (%) | −270/−170 (%) | −170/−70 (%) | −70/30 (%) | 30/60 (%) | OTHER (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall radiological score ≤ 2 | Median | 66.63 | 26.19 | 4.80 | 11.20 | 8.40 | 5.72 | 3.86 | 2.66 | 1.40 | 0.23 | 61.89 |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Maximum | 96.08 | 49.88 | 33.74 | 19.82 | 17.69 | 14.88 | 13.78 | 12.54 | 19.27 | 5.68 | 95.90 | |
| Overall radiological score 3–4 | Median | 60.39 | 28.62 | 5.83 | 13.55 | 9.07 | 6.93 | 4.96 | 3.23 | 2.30 | 0.39 | 53.94 |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Maximum | 87.41 | 47.36 | 40.72 | 20.60 | 17.48 | 15.89 | 11.53 | 9.93 | 21.63 | 11.50 | 86.27 | |
| Overall radiological score 5–6 | Median | 57.44 | 32.39 | 8.09 | 12.90 | 9.99 | 8.07 | 7.02 | 4.78 | 3.56 | 0.48 | 50.08 |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Maximum | 80.98 | 47.40 | 23.74 | 18.63 | 16.93 | 14.63 | 11.88 | 9.31 | 12.38 | 2.05 | 78.96 | |
| Overall radiological score 7–8 | Median | 39.95 | 39.80 | 10.16 | 12.01 | 13.23 | 12.20 | 10.59 | 7.34 | 3.77 | 0.56 | 28.76 |
| Minimum | 32.87 | 10.86 | 1.43 | 6.18 | 2.92 | 1.76 | 0.93 | 0.64 | 0.68 | 0.11 | 11.27 | |
| Maximum | 86.74 | 58.12 | 27.35 | 19.24 | 21.38 | 26.91 | 21.60 | 12.34 | 13.34 | 1.71 | 85.47 | |
| p-value at Kruskal–Wallis test | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.75 | 0.00 | |
| Spearman Correlation Coefficient | −0.08 | 0.38 | 0.37 | 0.26 | 0.38 | 0.40 | 0.41 | 0.38 | 0.36 | 0.32 | −0.08 | |
| p-value of Spearman Correlation | 0.41 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.39 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, R.; Cappabianca, S.; Urraro, F.; Feragalli, B.; Montanelli, A.; Patelli, G.; Granata, V.; Giacobbe, G.; Russo, G.M.; Grillo, A.; et al. Chest CT Computerized Aided Quantification of PNEUMONIA Lesions in COVID-19 Infection: A Comparison among Three Commercial Software. Int. J. Environ. Res. Public Health 2020, 17, 6914. https://doi.org/10.3390/ijerph17186914
Grassi R, Cappabianca S, Urraro F, Feragalli B, Montanelli A, Patelli G, Granata V, Giacobbe G, Russo GM, Grillo A, et al. Chest CT Computerized Aided Quantification of PNEUMONIA Lesions in COVID-19 Infection: A Comparison among Three Commercial Software. International Journal of Environmental Research and Public Health. 2020; 17(18):6914. https://doi.org/10.3390/ijerph17186914
Chicago/Turabian StyleGrassi, Roberto, Salvatore Cappabianca, Fabrizio Urraro, Beatrice Feragalli, Alessandro Montanelli, Gianluigi Patelli, Vincenza Granata, Giuliana Giacobbe, Gaetano Maria Russo, Assunta Grillo, and et al. 2020. "Chest CT Computerized Aided Quantification of PNEUMONIA Lesions in COVID-19 Infection: A Comparison among Three Commercial Software" International Journal of Environmental Research and Public Health 17, no. 18: 6914. https://doi.org/10.3390/ijerph17186914
APA StyleGrassi, R., Cappabianca, S., Urraro, F., Feragalli, B., Montanelli, A., Patelli, G., Granata, V., Giacobbe, G., Russo, G. M., Grillo, A., De Lisio, A., Paura, C., Clemente, A., Gagliardi, G., Magliocchetti, S., Cozzi, D., Fusco, R., Belfiore, M. P., Grassi, R., & Miele, V. (2020). Chest CT Computerized Aided Quantification of PNEUMONIA Lesions in COVID-19 Infection: A Comparison among Three Commercial Software. International Journal of Environmental Research and Public Health, 17(18), 6914. https://doi.org/10.3390/ijerph17186914






