The Quality of Counselling for Oral Emergency Contraceptive Pills—A Simulated Patient Study in German Community Pharmacies
Abstract
1. Introduction
2. Methods
2.1. Design
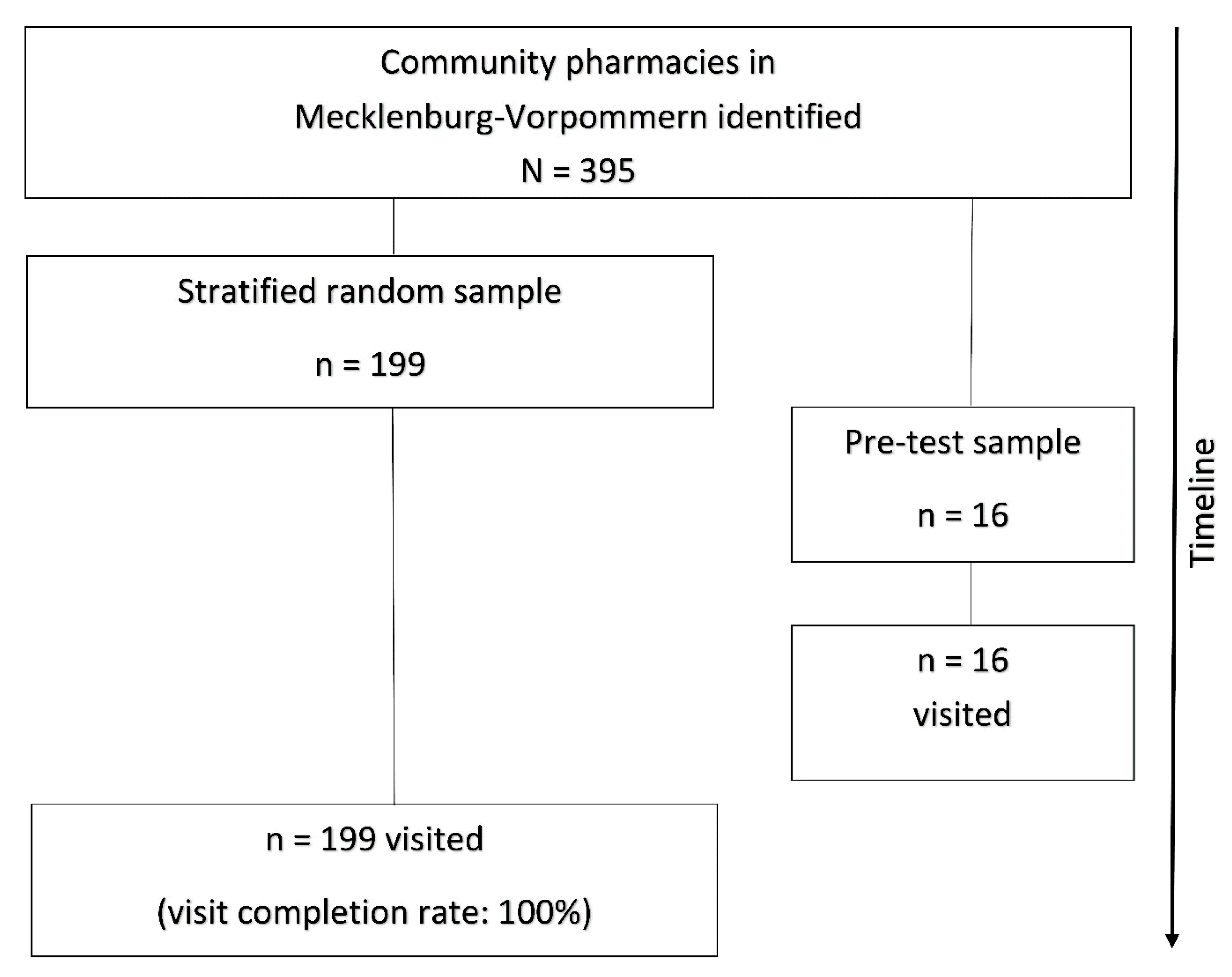
2.2. Setting and Participation
2.3. Scenario and Assessment
2.4. Data Collection
2.5. Ethical Approval
2.6. Data Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matyanga, C.M.J.; Dzingirai, B. Clinical pharmacology of hormonal emergency contraceptive pills. Int. J. Reprod. Med. 2018, 2018, 2785839. [Google Scholar] [CrossRef]
- Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN). Emergency contraception. J. Obs. Gynecol. Neonatal. Nurs. 2017, 46, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Helfferich, C.; Klindworth, H.; Heine, Y.; Wlosnewski, I. Frauen leben 3—Familienplanung im Lebenslauf von Frauen. Schwerpunkt: Ungewollte Schwangerschaften. Studie im Auftrag des Federal Centre for Health Education (BZgA). 2016. Available online: https://service.bzga.de/pdf.php?id=34c03dbb54311efa0d4aa9ad5bb9bd9c (accessed on 29 March 2020).
- Bearak, J.; Popinchalk, A.; Alkema, L.; Sedgh, G. Global, regional, and subregional trends in unintended pregnancy and its outcomes from 1990 to 2014: Estimates from a Bayesian hierarchical model. Lancet Glob. Health 2018, 6, e380–e389. [Google Scholar] [CrossRef]
- Cameron, S.T.; Li, H.; Gemzell-Danielsson, K. Current controversies with oral emergency contraception. BJOG 2017, 124, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Haeger, K.O.; Lamme, J.; Cleland, K. State of emergency contraception in the U.S., 2018. Contracept. Reprod. Med. 2018, 3, 20. [Google Scholar] [CrossRef]
- Rabe, T.; Goeckenjan, M.; Ahrendt, H.J.; Ludwig, M.; Merkle, E.; König, K.; Merki Feld, G.; Albring, C. Postkoitale Kontrazeption—Gemeinsame Stellungnahme der Deutschen Gesellschaft für Gynäkologische Endokrinologie und Fortpflanzungsmedizin (DGGEF) e.V. und des Berufsverbands der Frauenärzte (BVF) e.V. J. Reprod. Endokrinol. 2011, 8, 390–414. Available online: https://www.kup.at/kup/pdf/10330.pdf (accessed on 29 March 2020).
- International Consortium on Emergency Contraception. EC Status and Availability: Germany. Available online: https://www.cecinfo.org/country-by-country-information/status-availability-database/countries/germany/ (accessed on 29 March 2020).
- Rosato, E.; Farris, M.; Bastianelli, C. Mechanism of action of ulipristal acetate for emergency contraception: A systematic review. Front. Pharm. 2016, 6, 315. [Google Scholar] [CrossRef]
- Black, K.I.; Hussainy, S.Y. Emergency contraception: Oral and intrauterine options. Aust. Fam. Physician 2017, 46, 722–726. Available online: https://www.racgp.org.au/download/Documents/AFP/2017/October/V2/AFP-2017-10-Focus-Emergency-Contraception.pdf (accessed on 29 March 2020).
- Creinin, M.D.; Schlaff, W.; Archer, D.F.; Wan, L.; Frezieres, R.; Thomas, M.; Rosenberg, M.; Higgins, J. Progesterone receptor modulator for emergency contraception: A randomized controlled trial. Obs. Gynecol. 2006, 108, 1089–1097. [Google Scholar] [CrossRef]
- Glasier, A.F.; Cameron, S.T.; Fine, P.M.; Logan, S.J.; Casale, W.; Van Horn, J.; Sogor, L.; Blithe, D.L.; Scherrer, B.; Mathe, H.; et al. Ulipristal acetate versus levonorgestrel for emergency contraception: A randomised non-inferiority trial and meta-analysis. Lancet 2010, 375, 555–562. [Google Scholar] [CrossRef]
- Shen, J.; Che, Y.; Showell, E.; Chen, K.; Cheng, L. Interventions for emergency contraception. Cochrane Database Syst. Rev. 2019, 1, CD001324. [Google Scholar] [CrossRef]
- Rabe, T.; Albring, C. Arbeitskreis “Postkoitale Kontrazeption”: Ahrendt HJ, Mueck A, Merkle E, König K, Merki, G. Notfallkontrazeption—Ein Update: Gemeinsame Steellungnahme der Deutschen Gesellschaft für Gynäkologische Endokrinologie und Fortpflanzungsmedizin (DGGEF) e.V. und des Berufsverbands der Frauenärzte (BVF) e.V. Gynäkologische Endokrinol. 2013, 11, 197–202. [Google Scholar] [CrossRef]
- Bundesapothekerkammer (BAK). Rezeptfreie Abgabe von oralen Notfallkontrazeptiva (“Pille danach”). Handlungsempfehlungen der BAK. 2018. Available online: https://www.abda.de/fileadmin/assets/Praktische_Hilfen/Leitlinien/Selbstmedikation/BAK_Handlungsempfehlungen-Checkliste-NFK_20180228.pdf (accessed on 29 March 2019).
- International Consortium on Emergency Contraception. EC Status and Availability: Countries with Non-prescription Access to EC. Available online: https://www.cecinfo.org/country-by-country-information/status-availability-database/countries-with-non-prescription-access-to-ec/ (accessed on 29 March 2020).
- Bundesrat. Beschluss des Bundesrates. Vierzehnte Verordnung zur Änderung der Arzneimittelverschreibungsverordnung. 2015. Available online: https://www.bundesrat.de/SharedDocs/drucksachen/2015/0001-0100/28-15(B).pdf?__blob=publicationFile&v=4 (accessed on 29 March 2020).
- Berufsverband der Frauenärzte (BVF) und Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (DGGG). Stellungnahme des Berufsverbandes der Frauenärzte (BVF) und der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG) zu den Anträgen: Abgeordnete Mechthild Rawert et al., und die Fraktion der SPD, Rezeptfreiheit von Notfallkontrazeptiva Pille danach gewährleisten‘ Drucksache 17/11039 und Abgeordnete Yvonne Ploetz et al., und die Fraktion DIE LINKE ‚Die Pille danach rezeptfrei machen‘ Drucksache 17/12102 vom 17.04.2013. Available online: https://www.dggg.de/fileadmin/documents/stellungnahmen/aktuell/2013/180_Rezeptfreiheit_von_Notfallkontrazeptiva_Pille_danach_gewaehrleisten.pdf (accessed on 5 February 2020).
- Bundesärztekammer. Stellungnahme der Bundesärztekammer zum Entwurf einer Vierzehnten Verordnung zur Änderung der Arzneimittelverschreibungsverordnung vom 14.01.2015. 2015. Available online: https://www.bundesaerztekammer.de/fileadmin/user_upload/downloads/BAeK-Stn_und_AkdAe_AMVV-AendVO_15.01.2015.pdf (accessed on 5 February 2020).
- Schulz, M.; Goebel, R.; Schumann, C.; Zagermann-Muncke, P. Non-prescription dispensing of emergency oral contraceptives: Recommendations from the German Federal Chamber of Pharmacists [Bundesapothekerkammer]. Pharm. Pr. 2016, 14, 828. [Google Scholar] [CrossRef]
- Queddeng, K.; Chaar, B.; Williams, K. Emergency contraception in Australian community pharmacies: A simulated patient study. Contraception 2011, 83, 176–182. [Google Scholar] [CrossRef]
- Seubert, L.J.; Whitelaw, K.; Boeni, F.; Hattingh, L.; Watson, M.C.; Clifford, R.M. Barriers and facilitators for information exchange during over-the-counter consultations in community pharmacy: A focus group study. Pharmacy 2017, 5, 65. [Google Scholar] [CrossRef]
- Haag, M.; Gudka, S.; Hersberger, K.E.; Arnet, I. Do Swiss community pharmacists address the risk of sexually transmitted infections during a consultation on emergency contraception? A simulated patient study. Eur. J. Contracept. Reprod. Health Care 2019, 24, 407–412. [Google Scholar] [CrossRef]
- Khojah, H.M.J. Privacy level in private community pharmacies in Saudi Arabia: A simulated client survey. Pharm. Pharm. 2019, 10, 445–455. [Google Scholar] [CrossRef]
- Ordinance on the Operation of Pharmacies. Available online: https://www.abda.de/fileadmin/assets/Gesetze/ApBetrO_engl_Stand-2016-12.pdf (accessed on 11 May 2020).
- Said, A.; Ganso, M.; Freudewald, L.; Schulz, M. Trends in dispensing oral emergency contraceptives and safety issues: A survey of German community pharmacists. Int. J. Clin. Pharm. 2019, 41, 1499–1506. [Google Scholar] [CrossRef]
- Bruhns, C. Ergebnisse einer bundesweiten Befragung zur aktuellen Abgabepraxis der Pille danach. Rezeptfreie Pille danach—Abgabepraxis und Information. Pro Fam. Dok. 2015, 14–20. Available online: https://www.profamilia.de/fileadmin/publikationen/Fachpublikationen/doku_pille__danach-2016_web.pdf (accessed on 12 April 2020).
- Dierolf, V.; Freytag, S. Zugang zur Pille danach in den Apotheken nach der Rezeptfreigabe. Verhütungsberatung. Pro Fam. Mag. 2017, 4, 9–12. [Google Scholar]
- Callegaro, M. Social desirability. Encycl. Surv. Res. Methods 2008, 825–826. [Google Scholar] [CrossRef]
- Saxena, P.; Mishra, A.; Nigam, A. Evaluation of pharmacists’ services for dispensing emergency contraceptive pills in Delhi, India: A mystery shopper study. Indian J. Community Med. 2016, 41, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Converse, L.; Barrett, K.; Rich, E.; Reschovsky, J. Methods of observing variations in physicians’ decisions: The opportunities of clinical vignettes. J. Gen. Intern. Med. 2015, 30, S586–S594. [Google Scholar] [CrossRef]
- Bardage, C.; Westerlund, T.; Barzi, S.; Bernsten, C. Non-prescription medicines for pain and fever—A comparison of recommendations and counseling from staff in pharmacy and general sales stores. Health Policy 2013, 110, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; de Almeida Neto, A.C.; Moles, R.J. A systematic review of simulated-patient methods used in community pharmacy to assess the provision of non-prescription medicines. Int. J. Pharm. Pract. 2012, 20, 307–319. [Google Scholar] [CrossRef]
- Obare, F.; Liambila, W. The provision of emergency contraceptives in private sector pharmacies in urban Kenya: Experiences of mystery clients. Afr. Popul. Stud. 2013, 24, 42–52. [Google Scholar] [CrossRef][Green Version]
- Schneider, C.R.; Gudka, S.; Fleischer, L.; Clifford, R.M. The use of a written assessment checklist for the provision of emergency contraception via community pharmacies: A simulated patient study. Pharm. Pract. 2013, 11, 127–131. [Google Scholar] [CrossRef][Green Version]
- Collins, J.C.; Schneider, C.R.; Moles, R.J. Emergency contraception supply in Australian pharmacies after the introduction of ulipristal acetate: A mystery shopping mixed-methods study. Contraception 2018, 98, 243–246. [Google Scholar] [CrossRef]
- Cleland, K.; Bass, J.; Doci, F.; Foster, A.M. Access to emergency contraception in the over-the-counter era. Womens Health Issues 2016, 26, 622–627. [Google Scholar] [CrossRef]
- Hernandez, J.H.; Mbadu, M.F.; Garcia, M.; Glover, A. The provision of emergency contraception in Kinshasa’s private sector pharmacies: Experiences of mystery clients. Contraception 2018, 97, 57–61. [Google Scholar] [CrossRef]
- Bell, D.L.; Camacho, E.J.; Velasquez, A.B. Male access to emergency contraception in pharmacies: A mystery shopper survey. Contraception 2014, 90, 413–415. [Google Scholar] [CrossRef]
- Smith, H.; Whyte, S.; Chan, H.F.; Kyle, G.; Lau, E.T.L.; Nissen, L.M.; Torgler, B.; Dulleck, U. Pharmacist compliance with therapeutic guidelines on diagnosis and treatment provision. JAMA Netw. Open 2019, 2, e197168. [Google Scholar] [CrossRef]
- Uzun, G.D.; Sancar, M.; Okuyan, B. Evaluation of knowledge and attitude of pharmacist and pharmacy technicians on emergency contraception method in Istanbul, Turkey: A simulated patient study. J. Res. Pharm. 2018, 23, 395–402. [Google Scholar] [CrossRef]
- Kagashe, G.A.B.; Maregesi, S.M.; Mashaka, A. Availability, awareness, attitude and knowledge of emergency contraceptives in Dar Es Salaam. J. Pharm. Sci. Res. 2013, 5, 216–219. Available online: https://www.jpsr.pharmainfo.in/Documents/Volumes/vol5issue11/jpsr05111302.pdf (accessed on 12 April 2020).
- Higgins, S.J.; Hattingh, H.L. Requests for emergency contraception in community pharmacy: An evaluation of services provided to mystery patients. Res. Soc. Adm. Pharm. 2013, 9, 114–119. [Google Scholar] [CrossRef]
- Tavares, M.P.; Foster, A.M. Emergency contraception in a public health emergency: Exploring pharmacy availability in Brazil. Contraception 2016, 94, 109–114. [Google Scholar] [CrossRef]
- Glasier, A.; Manners, R.; Loudon, J.C.; Muir, A. Community pharmacists providing emergency contraception give little advice about future contraceptive use: A mystery shopper study. Contraception 2010, 82, 538–542. [Google Scholar] [CrossRef]
- Berger, K.; Eickhoff, C.; Schulz, M. Counselling quality in community pharmacies: Implementation of the pseudo customer methodology in Germany. J. Clin. Pharm. 2005, 30, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Alte, D.; Weitschies, W.; Ritter, C.A. Evaluation of consultation in community pharmacies with mystery shoppers. Ann. Pharm. 2007, 41, 1023–1030. [Google Scholar] [CrossRef]
- Langer, B.; Bull, E.; Burgsthaler, T.; Glawe, J.; Schwobeda, M.; Simon, K. Assessment of counselling for acute diarrhoea in German pharmacies: A simulated patient study. Int. J. Pharm. Pr. 2018, 26, 310–317. [Google Scholar] [CrossRef]
- Langer, B.; Kieper, M.; Laube, S.; Schramm, J.; Weber, S.; Werwath, A. Assessment of counselling for acute diarrhoea in North-Eastern German pharmacies—A follow-up study using the simulated patient methodology. Pharm. Pharm. 2018, 9, 257–269. [Google Scholar] [CrossRef]
- Langer, B.; Kunow, C. Medication dispensing, additional therapeutic recommendations, and pricing practices for acute diarrhoea by community pharmacies in Germany: A simulated patient study. Pharm. Pract. 2019, 17, 1579. [Google Scholar] [CrossRef]
- Langer, B.; Kunow, C. Do north-eastern German pharmacies recommend a necessary medical consultation for acute diarrhoea? Magnitude and determinants using a simulated patient approach. F1000Research 2020, 8, 1841. [Google Scholar] [CrossRef]
- STROBE Statement—Checklist of Items That Should Be Included in Reports of Cross-Sectional Studies. Available online: https://www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf (accessed on 8 February 2020).
- Watson, M.C.; Norris, P.; Granas, A.G. A systematic review of the use of simulated patients and pharmacy practice research. Int. J. Pharm. Pr. 2006, 14, 83–93. [Google Scholar] [CrossRef]
- Björnsdottir, I.; Granas, A.G.; Bradley, A.; Norris, P. A systematic review of the use of simulated patient methodology in pharmacy practice research from 2006 to 2016. Int. J. Pharm. Pr. 2020, 28, 13–25. [Google Scholar] [CrossRef]
- da Costa, F.A. Covert and overt observations in pharmacy practice. In Pharmacy Practice Research Methods; Babar, Z.U.D., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Statistisches Amt Mecklenburg-Vorpommern. Statistisches Jahrbuch Mecklenburg-Vorpommern 2019. 2019. Available online: https://www.laiv-mv.de/static/LAIV/Statistik/Dateien/Publikationen/Statistisches%20Jahrbuch/Z011%202019%2000.pdf (accessed on 24 April 2020).
- Apotheken Umschau. Apothekenfinder. 2019. Available online: https://www.apotheken-umschau.de/Apothekenfinder (accessed on 11 April 2019).
- ABDA—Bundesvereinigung Deutscher Apothekerverbände. Die Apotheke—Zahlen, Daten, Fakten 2019. Berlin: ABDA. 2019. Available online: https://www.abda.de/fileadmin/user_upload/assets/ZDF/ZDF_2019/ABDA_ZDF_2019_Brosch.pdf (accessed on 30 June 2019).
- Israel, G.D. Determining Sample Size; University of Florida: Gainesville, FL, USA, 2012; Available online: http://www.psycholosphere.com/Determining%20sample%20size%20by%20Glen%20Israel.pdf (accessed on 4 April 2019).
- Bundesapothekerkammer (BAK). Rezeptfreie Abgabe von oralen Notfallkontrazeptiva (“Pille danach”). Handlungsempfehlungen der BAK. 2018. Anhang 1. Available online: https://www.abda.de/fileadmin/assets/Praktische_Hilfen/Leitlinien/Selbstmedikation/Anhang_1_Vergleich-NFK_20180228.pdf (accessed on 28 April 2019).
- Federal Centre for Health Education (BZgA). Contraceptive Behaviour of Adults 2011. 2011. Available online: https://www.bzga.de/infomaterialien/sexualaufklaerung/sexualaufklaerung/contraceptive-behaviour-of-adults-2011/ (accessed on 28 April 2020).
- German Pharmacies Act. Available online: https://www.abda.de/fileadmin/assets/Gesetze/Apothekengesetz_engl-Stand_2012-10-26.pdf (accessed on 11 May 2020).
- Zapata-Cachafeiro, M.; Piñeiro-Lamas, M.; Guinovart, M.C.; López-Vázquez, P.; Vázquez-Lago, J.M.; Figueiras, A. Magnitude and determinants of antibiotic dispensing without prescription in Spain: A simulated patient study. J. Antimicrob. Chemother. 2019, 74, 511–514. [Google Scholar] [CrossRef]
- Saba, M.; Diep, J.; Bittoun, R.; Saini, B. Provision of smoking cessation services in Australian community pharmacies: A simulated patient study. Int. J. Clin. Pharm. 2014, 36, 604–614. [Google Scholar] [CrossRef]
- Langer, B.; Bull, E.; Burgsthaler, T.; Glawe, J.; Schwobeda, M.; Simon, K. Die Beratungsqualität von Apotheken bei akutem Durchfall—Eine Analyse unter Verwendung der “Simulated Patient”-Methode [Using the simulated patient methodology to assess counselling for acute diarrhoea—Evidence from Germany]. Z. Evid. Qual. Gesundhwes. 2016, 112, 19–26. [Google Scholar] [CrossRef]
- Kashour, T.S.; Joury, A.; Alotaibi, A.M.; Althagafi, M.; Almufleh, A.S.; Hersi, A.; Thalib, L. Quality of assessment and counselling offered by community pharmacists and medication sale without prescription to patients presenting with acute cardiac symptoms: A simulated client study. Eur. J. Clin. Pharm. 2016, 72, 321–328. [Google Scholar] [CrossRef]
- Collins, J.C.; Schneider, C.R.; Faraj, R.; Wilson, F.; de Almeida Neto, A.C.; Moles, R.J. Management of common ailments requiring referral in the pharmacy: A mystery shopping intervention study. Int. J. Clin. Pharm. 2017, 39, 697–703. [Google Scholar] [CrossRef]
- Kippist, C.; Wong, K.; Bartlett, D.; Saini, B. How do pharmacists respond to complaints of acute insomnia? A simulated patient study. Int. J. Clin. Pharm. 2011, 33, 237–245. [Google Scholar] [CrossRef]
- Collins, J.C.; Schneider, C.R.; Naughtin, C.L.; Wilson, F.; de Almeida Neto, A.C.; Moles, R.J. Mystery shopping and coaching as a form of audit and feedback to improve community pharmacy management of non-prescription medicine requests: An intervention study. BMJ Open 2017, 7, e019462. [Google Scholar] [CrossRef]
- Werner, J.B.; Benrimoj, S.I. Audio taping simulated patient encounters in community pharmacy to enhance the reliability of assessments. Am. J. Pharm. Educ. 2008, 72, 136. [Google Scholar] [CrossRef]
- BVM—Berufsverband Deutscher Markt- und Sozialforscher e.V. Richtlinie für den Einsatz von Mystery Research in der Markt- und Sozialforschung [Guideline for the Use of Mystery Research in Market and Social Research]. 2006. Available online: http://bvm.org/fileadmin/pdf/Recht_Berufskodizes/Richtlinien/RL_2006_Mystery.pdf (accessed on 4 April 2020).
- McCambridge, J.; Witton, J.; Elbourne, D.R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol. 2014, 67, 267–277. [Google Scholar] [CrossRef]
- Rhodes, K.V.; Miller, F.G. Simulated patient studies: An ethical analysis. Milbank Q. 2012, 90, 706–724. [Google Scholar] [CrossRef]
- Fitzpatrick, A.; Tumlinson, K. Strategies for optimal implementation of simulated clients for measuring quality of care in low- and middle-income countries. Glob. Health Sci. Pr. 2017, 5, 108–114. [Google Scholar] [CrossRef]
- Ibrahim, M.I.M.; Awaisu, A.; Palaian, S.; Radoui, A.; Atwa, H. Do community pharmacists in Qatar manage acute respiratory conditions rationally? A simulated client study. J. Pharm. Health Serv. Res. 2018, 9, 33–39. [Google Scholar] [CrossRef]
- Cohen, J. The effect size. In Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New Jersey, NJ, USA, 1988. [Google Scholar]
- Glasier, A.; Baraitser, P.; McDaid, L.; Norrie, J.; Radley, A.; Stephenson, J.M.; Battison, C.; Gilson, R.; Cameron, S. Emergency contraception from the pharmacy 20 years on: A mystery shopper study. BMJ Sex. Reprod Health 2020. [Google Scholar] [CrossRef]
- Bullock, H.; Steele, S.; Kurata, N.; Tschann, M.; Elia, J.; Kaneshiro, B.; Salcedo, J. Pharmacy access to ulipristal acetate in Hawaii: Is a prescription enough? Contraception 2016, 93, 452–454. [Google Scholar] [CrossRef]
- Shigesato, M.; Elia, J.; Tschann, M.; Bullock, H.; Hurwitz, E.; Wu, Y.Y.; Salcedo, J. Pharmacy access to Ulipristal acetate in major cities throughout the United States. Contraception 2018, 97, 264–269. [Google Scholar] [CrossRef]
- Kaur, G.; Fontanilla, T.; Bullock, H.; Tschann, M. ‘The difference between plan b and ella®? They’re basically the same thing’: Results from a mystery client study. Pharmacy 2020, 8, 77. [Google Scholar] [CrossRef]
- Edalat, A. Pro und Kontra: Pflichtfortbildung für Apotheker? DAZ.ONLINE vom 12.07.2018. Available online: https://www.deutsche-apotheker-zeitung.de/news/artikel/2018/07/12/pflichtfortbildung-fuer-apotheker/chapter:all (accessed on 22 April 2020).
- Gabler Wirtschaftslexikon. Leistungskurve. Available online: https://wirtschaftslexikon.gabler.de/definition/leistungskurve-37379/version-260815 (accessed on 22 April 2020).
- Watson, M.C.; Bond, C.M.; Grimshaw, J.; Johnston, M. Factors predicting the guideline compliant supply (or non-supply) of non-prescription medicines in the community pharmacy setting. Qual. Saf. Health Care 2006, 15, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ogbo, P.U.; Aina, B.A.; Aderemi-Williams, R.I. Management of acute diarrhea in children by community pharmacists in Lagos, Nigeria. Pharm. Prac. 2014, 12, 376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Surur, A.S.; Getachew, E.; Teressa, E.; Hailemeskel, B.; Getaw, N.S.; Erku, D.A. Self-reported and actual involvement of community pharmacists in patient counseling: A cross-sectional and simulated patient study in Gondar, Ethiopia. Pharm. Pract. 2017, 15, 890. [Google Scholar] [CrossRef] [PubMed]
| Scenario |
|---|
| The test buyers enter the pharmacy and ask for oral emergency contraception without having a specific product in mind (product-based query). |
When questioned by the pharmacy staff, the following information is provided:
|
| Items | Yes | No |
|---|---|---|
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| 1 | 0 |
| Possible Influencing Factors [Literature Source *] | Time of Data Collection | Type of Data Collection |
|---|---|---|
| Location of the pharmacy [47] as an indicator for urban/rural | Before the test purchase because stratification variable | Precise measurement by allocating the number of pharmacies identified in the particular area |
| SP number [63] | During the test purchase | Exact measurement by assigning a number to each SP |
| Age of the pharmacy staff [64] | During the test purchase | Estimate based on visual impression by SP |
| Gender of the pharmacy staff [64] | During the test purchase | Exact measurement using visual impression of the SP |
| Queue—patients waiting after the SP [65] | During the test purchase | Exact measurement using visual impression of the SP |
| Time of the test purchase [66] | During the test purchase | Exact measurement using the SP’s watch |
| Professional group of the pharmacy staff [67] | During and after the test purchase | Exact measurement based on the name tag and, if necessary, using a telephone query by the SP after completing all the test purchases |
| Pharmacy quality certificate [68] | After the test purchase | Precise measurement using a telephone query by the SP after completing all the test purchases |
| Questioning score [69] | After the test purchase | Precise measurement by summing the dichotomous evaluation of the nine individual questions (minimum possible score of 0 points and maximum possible score of 9 points) |
| Frequency (n) | Percentage (%) | |
|---|---|---|
| All pharmacies | 199 | 100 |
| Location of the pharmacy | ||
| 37 | 18.6 |
| 58 | 29.1 |
| 46 | 23.1 |
| 58 | 29.2 |
| Pharmacy quality certificate | ||
| 125 | 62.8 |
| 51 | 25.6 |
| 23 | 11.6 |
| Age of the pharmacy staff | ||
| 27 | 13.6 |
| 114 | 57.3 |
| 58 | 29.1 |
| Gender of the pharmacy staff | ||
| 25 | 12.6 |
| 174 | 87.4 |
| Professional group of the pharmacy staff | ||
| 75 | 37.7 |
| 81 | 40.7 |
| 43 | 21.6 |
| Yes | |||
|---|---|---|---|
| Frequency (n) | Percentage (%) | 95% CI | |
| 118 | 59.3 | 52.3–66.3 |
| 103 | 51.8 | 45.2–58.8 |
| 186 | 93.5 | 89.9–96.5 |
| 133 | 66.8 | 59.8–73.4 |
| 92 | 46.2 | 39.2–53.3 |
| 94 | 47.2 | 39.7–54.3 |
| 82 | 41.2 | 34.2–48.2 |
| 125 | 62.8 | 55.3–70.4 |
| 110 | 55.3 | 48.2–62.8 |
| 157 | 78.9 | 72.9–84.4 |
| 6 | 3.0 | 1.0–5.5 |
| 97 | 59.5 | 52.0–67.1 |
| 88 | 44.2 | 37.2–51.8 |
| 107 | 53.8 | 46.7–61.3 |
| Possible Influencing Factors and Categories | n (%) Total 199 (100) | n (%) Recommendation 157 (78.9) | n (%) No Recommendation 42 (21.1) | COR (95% CI) | p-Value | AOR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
| Location of the pharmacy | |||||||
| 37 (100) | 27 (73.0) | 10 (27.0) | 1 | |||
| 58 (100) | 49 (84.5) | 9 (15.5) | 2.02 (0.73–5.57) | 0.176 | ||
| 46 (100) | 37 (80.4) | 9 (19.6) | 1.52 (0.55–4.26) | 0.423 | ||
| 58 (100) | 44 (75.9) | 14 (24.1) | 1.16 (0.45–2.99) | 0.752 | ||
| Pharmacy quality certificate | |||||||
| 125 (100) | 102 (81.6) | 23 (18.4) | 1 | |||
| 51 (100) | 40 (78.4) | 11 (21.6) | 0.82 (0.37–1.84) | 0.629 | ||
| 23 (100) | 15 (65.2) | 8 (34.8) | 0.42 (0.16–1.12) | 0.082 | ||
| Age of the pharmacy staff | |||||||
| 27 (100) | 17 (63.0) | 10 (37.0) | 1 | 1 | ||
| 114 (100) | 91 (79.8) | 23 (20.2) | 2.33 (0.94–5.75) | 0.067 | 2.05 (0.74–5.64) | 0.166 |
| 58 (100) | 49 (84.5) | 9 (15.5) | 3.20 (1.11–9.21) | 0.031 | 2.68 (0.84–8.54) | 0.095 |
| Gender of the pharmacy staff | |||||||
| 25 (100) | 18 (72.0) | 7 (28.0) | 1 | |||
| 174 (100) | 139 (79.9) | 35 (20.1) | 1.54 (0.60–3.99) | 0.369 | ||
| Professional group of the pharmacy staff | |||||||
| 75 (100) | 63 (84.0) | 12 (16.0) | 1 | |||
| 81 (100) | 63 (77.8) | 18 (22.2) | 0.67 (0.30–1.50) | 0.326 | ||
| 43 (100) | 31 (72.1) | 12 (27.9) | 0.49 (0.20–1.22) | 0.126 | ||
| Time of the test purchase | |||||||
| 85 (100) | 61 (71.8) | 24 (28.2) | 1 | 1 | ||
| 92 (100) | 78 (84.8) | 14 (15.2) | 2.19 (1.05–4.59) | 0.037 | 2.54 (1.13–5.73) | 0.024 * |
| 22 (100) | 18 (81.8) | 4 (18.2) | 1.77 (0.54–5.77) | 0.343 | 1.51 (0.41–5.56) | 0.533 |
| Queue | |||||||
| 144 (100) | 115 (79.9) | 29 (20.1) | 1 | |||
| 55 (100) | 42 (76.4) | 13 (23.6) | 0.82 (0.39–1.71) | 0.589 | ||
| SP number | |||||||
| 46 (100) | 37 (80.4) | 9 (19.6) | 1 | |||
| 55 (100) | 40 (72.7) | 15 (27.3) | 0.65 (0.25–1.66) | 0.367 | ||
| 49 (100) | 42 (85.7) | 7 (14.3) | 1.46 (0.50–4.31) | 0.493 | ||
| 49 (100) | 38 (77.6) | 11 (22.4) | 0.84 (0.31–2.26) | 0.731 | ||
| Questioning score a | 5.0 (2.0–9.0) | 7.0 (3.0–9.0) | 2.5 (1.0–4.0) | 1.39 (1.21–1.60) | <0.001 | 1.41 (1.22–1.63) | <0.001 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langer, B.; Grimm, S.; Lungfiel, G.; Mandlmeier, F.; Wenig, V. The Quality of Counselling for Oral Emergency Contraceptive Pills—A Simulated Patient Study in German Community Pharmacies. Int. J. Environ. Res. Public Health 2020, 17, 6720. https://doi.org/10.3390/ijerph17186720
Langer B, Grimm S, Lungfiel G, Mandlmeier F, Wenig V. The Quality of Counselling for Oral Emergency Contraceptive Pills—A Simulated Patient Study in German Community Pharmacies. International Journal of Environmental Research and Public Health. 2020; 17(18):6720. https://doi.org/10.3390/ijerph17186720
Chicago/Turabian StyleLanger, Bernhard, Sophia Grimm, Gwenda Lungfiel, Franca Mandlmeier, and Vanessa Wenig. 2020. "The Quality of Counselling for Oral Emergency Contraceptive Pills—A Simulated Patient Study in German Community Pharmacies" International Journal of Environmental Research and Public Health 17, no. 18: 6720. https://doi.org/10.3390/ijerph17186720
APA StyleLanger, B., Grimm, S., Lungfiel, G., Mandlmeier, F., & Wenig, V. (2020). The Quality of Counselling for Oral Emergency Contraceptive Pills—A Simulated Patient Study in German Community Pharmacies. International Journal of Environmental Research and Public Health, 17(18), 6720. https://doi.org/10.3390/ijerph17186720






