Impact of Air Pollution on Asthma Outcomes
Abstract
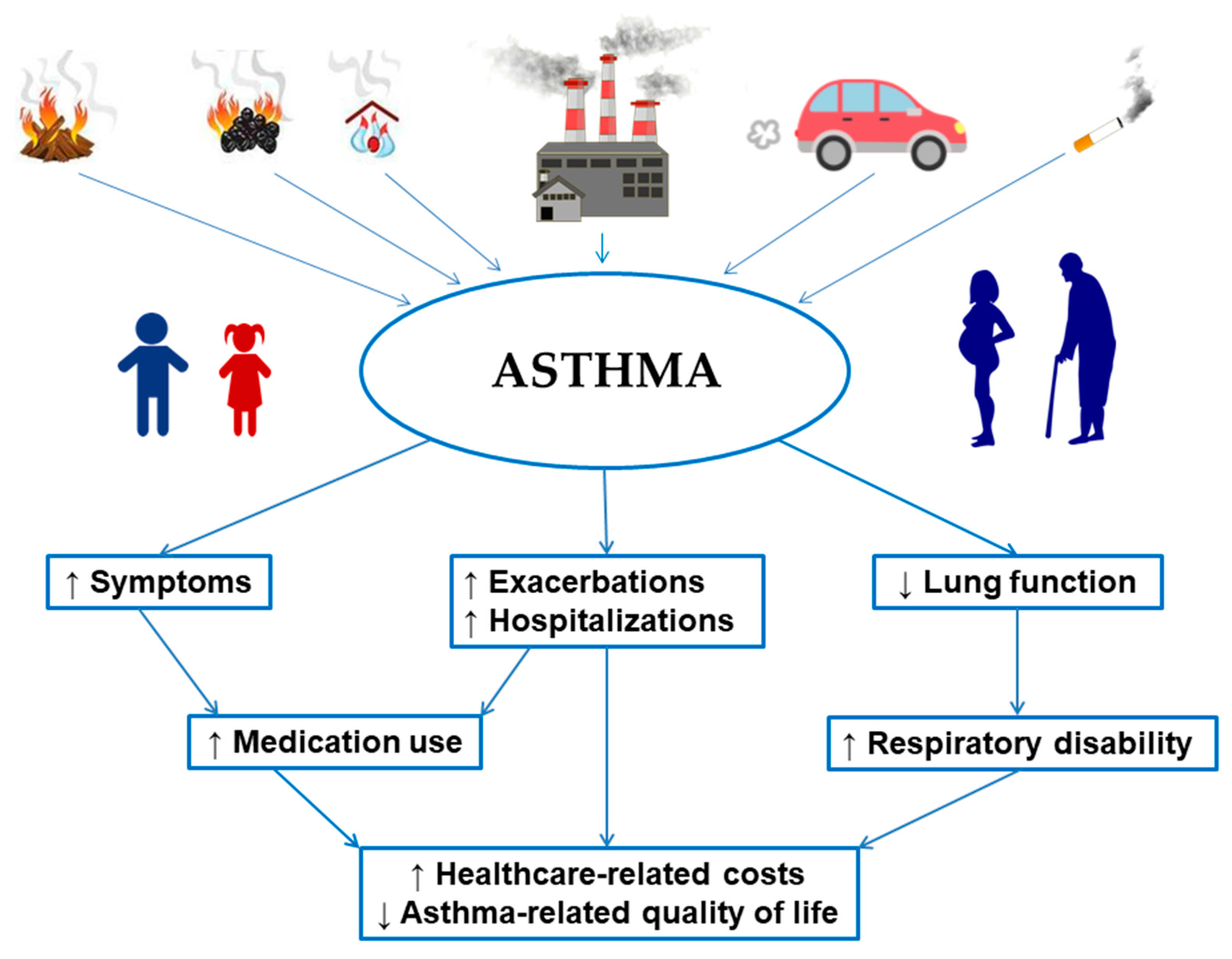
1. Introduction
2. Search Strategy, Data Sources and Selection Criteria
3. Air Pollution and Risk of Asthma
4. Outdoor Air Pollution
4.1. Ozone
4.2. Nitrogen Dioxide
4.3. Carbon Monoxide and Carbon Dioxide
4.4. Sulfur Dioxide
4.5. Particulate Matter
4.6. Outdoor Air Pollution and Asthma Outcomes
4.7. Outdoor Air Pollution and Asthma Management
5. Indoor Air Pollution
5.1. Tobacco Smoke
5.2. Wood-Burning and Unflued Gas Heaters, Cooking Behaviors (Using Wood or Coal), Molds
5.3. Indoor Air Pollution and Asthma Outcomes
5.4. Indoor Air Pollution and Asthma Management
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 14 August 2020).
- Liu, Y.; Pan, J.; Zhang, H.; Shi, C.; Li, G.; Peng, Z.; Ma, J.; Zhou, Y.; Zhang, L. Short-Term Exposure to Ambient Air Pollution and Asthma Mortality. Am. J. Respir. Crit. Care Med. 2019, 200, 24–32. [Google Scholar] [CrossRef]
- World Health Organization. Ambient Air Pollution. Available online: https://apps.who.int/gho/data/node.main.151?lang=en (accessed on 13 June 2020).
- United States Environmental Protection Agency. Air Quality—National Summary. Available online: https://www.epa.gov/air-trends/air-quality-national-summary (accessed on 13 June 2020).
- World Health Organization. WHO Global Ambient Air Quality Database (Update 2018). Available online: http://www.who.int/airpollution/data/cities/en/ (accessed on 13 June 2020).
- Sompornrattanaphan, M.; Thongngarm, T.; Ratanawatkul, P.; Wongsa, C.; Swigris, J.J. The contribution of particulate matter to respiratory allergy. Asian Pac. J. Allergy Immunol. 2020, 38, 19–28. [Google Scholar] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- GINA Reports. Global Initiative for Asthma-GINA. Available online: https://ginasthma.org/gina-reports/ (accessed on 13 June 2020).
- Delfino, R.J.; Wu, J.; Tjoa, T.; Gullesserian, S.K.; Nickerson, B.; Gillen, D.L. Asthma morbidity and ambient air pollution: Effect modification by residential traffic-related air pollution. Epidemiology 2014, 25, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Veremchuk, L.V.; Tsarouhas, K.; Vitkina, T.I.; Mineeva, E.E.; Gvozdenko, T.A.; Antonyuk, M.V.; Rakitskii, V.N.; Sidletskaya, K.A.; Tsatsakis, A.M.; Golokhvast, K.S. Impact evaluation of environmental factors on respiratory function of asthma patients living in urban territory. Environ. Pollut. 2018, 235, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Khreis, H.; Cirach, M.; Mueller, N.; de Hoogh, K.; Hoek, G.; Nieuwenhuijsen, M.J.; Rojas-Rueda, D. Outdoor air pollution and the burden of childhood asthma across Europe. Eur. Respir. J. 2019, 54, 1802194. [Google Scholar] [CrossRef]
- Pierangeli, I.; Nieuwenhuijsen, M.J.; Cirach, M.; Rojas-Rueda, D. Health equity and burden of childhood asthma - related to air pollution in Barcelona. Environ. Res. 2020, 22, 109067. [Google Scholar] [CrossRef]
- Bowatte, G.; Lodge, C.; Lowe, A.J.; Erbas, B.; Perret, J.; Abramson, M.J.; Matheson, M.; Dharmage, S.C. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: A systematic review and a meta-analysis of birth cohort studies. Allergy 2015, 70, 245–256. [Google Scholar] [CrossRef]
- Khreis, H.; Kelly, C.; Tate, J.; Parslow, R.; Lucas, K.; Nieuwenhuijsen, M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 2017, 100, 1–31. [Google Scholar] [CrossRef]
- Gehring, U.; Wijga, A.H.; Koppelman, G.H.; Vonk, J.M.; Smit, H.A.; Brunekreef, B. Air pollution and the development of asthma from birth until young adulthood. Eur. Respir. J. 2020, 56, 2000147. [Google Scholar] [CrossRef]
- Sbihi, H.; Koehoorn, M.; Tamburic, L.; Brauer, M. Asthma Trajectories in a Population-based Birth Cohort. Impacts of Air Pollution and Greenness. Am. J. Respir. Crit. Care Med. 2017, 195, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-R.; Chen, W.-T.; Tang, Y.-H.; Hwang, B.-F. Fine particulate matter exposure during pregnancy and infancy and incident asthma. J. Allergy Clin. Immunol. 2019, 143, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Hehua, Z.; Qing, C.; Shanyan, G.; Qijun, W.; Yuhong, Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environ. Res. 2017, 159, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wang, X.; Dong, T.; Sun, M.; Zhang, M.; Fang, K.; Chen, Y.; Chen, R.; Sun, Z.; Xia, Y. The impact of prenatal exposure to PM2.5 on childhood asthma and wheezing: A meta-analysis of observational studies. Environ. Sci. Pollut. Res. Int. 2020. [Google Scholar] [CrossRef]
- Thurston, G.D.; Balmes, J.R.; Garcia, E.; Gilliland, F.D.; Rice, M.B.; Schikowski, T.; Van Winkle, L.S.; Annesi-Maesano, I.; Burchard, E.G.; Carlsten, C.; et al. Outdoor Air Pollution and New-Onset Airway Disease. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2020, 17, 387–398. [Google Scholar] [CrossRef]
- Lin, W.; Brunekreef, B.; Gehring, U. Meta-analysis of the effects of indoor nitrogen dioxide and gas cooking on asthma and wheeze in children. Int. J. Epidemiol. 2013, 42, 1724–1737. [Google Scholar] [CrossRef]
- Hüls, A.; Vanker, A.; Gray, D.; Koen, N.; MacIsaac, J.L.; Lin, D.T.S.; Ramadori, K.E.; Sly, P.D.; Stein, D.J.; Kobor, M.S.; et al. Genetic susceptibility to asthma increases the vulnerability to indoor air pollution. Eur. Respir. J. 2020, 55, 1901831. [Google Scholar] [CrossRef]
- Brooks, C.C.; Martin, L.J.; Pilipenko, V.; He, H.; LeMasters, G.K.; Lockey, J.E.; Bernstein, D.I.; Ryan, P.H.; Khurana Hershey, G.K.; Biagini Myers, J.M. NAT1 genetic variation increases asthma risk in children with secondhand smoke exposure. J. Asthma 2019, 1–9. [Google Scholar] [CrossRef]
- Silvestri, M.; Franchi, S.; Pistorio, A.; Petecchia, L.; Rusconi, F. Smoke exposure, wheezing, and asthma development: A systematic review and meta-analysis in unselected birth cohorts. Pediatr. Pulmonol. 2015, 50, 353–362. [Google Scholar] [CrossRef]
- Burke, H.; Leonardi-Bee, J.; Hashim, A.; Pine-Abata, H.; Chen, Y.; Cook, D.G.; Britton, J.R.; McKeever, T.M. Prenatal and passive smoke exposure and incidence of asthma and wheeze: Systematic review and meta-analysis. Pediatrics 2012, 129, 735–744. [Google Scholar] [CrossRef]
- Joubert, B.R.; Felix, J.F.; Yousefi, P.; Bakulski, K.M.; Just, A.C.; Breton, C.; Reese, S.E.; Markunas, C.A.; Richmond, R.C.; Xu, C.-J.; et al. DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. Am. J. Hum. Genet. 2016, 98, 680–696. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, A.M.; Oh, S.S.; Hu, D.; Huntsman, S.; Eng, C.; Rodríguez-Santana, J.R.; Kumar, R.; Balmes, J.R.; Eisen, E.A.; Burchard, E.G. In utero tobacco smoke exposure, DNA methylation, and asthma in Latino children. Environ. Epidemiol. 2019, 3, e048. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Håberg, S.E.; Karlstad, Ø.; Nafstad, P.; London, S.J.; Nystad, W. Grandmother’s smoking when pregnant with the mother and asthma in the grandchild: The Norwegian Mother and Child Cohort Study. Thorax 2015, 70, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdóttir, B.; Bertelsen, R.J.; Bråbäck, L.; Carsin, A.-E.; Dharmage, S.C.; Dratva, J.; et al. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018, 47, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Hsu, T.-Y.; Chang, J.-C.; Ou, C.-Y.; Kuo, H.-C.; Liu, C.-A.; Wang, C.-L.; Chuang, H.; Chen, C.-P.; Yang, K.D. Paternal Tobacco Smoke Correlated to Offspring Asthma and Prenatal Epigenetic Programming. Front. Genet. 2019, 10, 471. [Google Scholar] [CrossRef]
- Toppila-Salmi, S.; Luukkainen, A.T.; Xu, B.; Lampi, J.; Auvinen, J.; Dhaygude, K.; Järvelin, M.-R.; Pekkanen, J. Maternal smoking during pregnancy affects adult onset of asthma in offspring: A follow up from birth to age 46 years. Eur. Respir. J. 2020, 55, 1901857. [Google Scholar] [CrossRef]
- Coogan, P.F.; Castro-Webb, N.; Yu, J.; O’Connor, G.T.; Palmer, J.R.; Rosenberg, L. Active and passive smoking and the incidence of asthma in the Black Women’s Health Study. Am. J. Respir. Crit. Care Med. 2015, 191, 168–176. [Google Scholar] [CrossRef]
- Flodin, U.; Jönsson, P.; Ziegler, J.; Axelson, O. An epidemiologic study of bronchial asthma and smoking. Epidemiology 1995, 6, 503–505. [Google Scholar] [CrossRef]
- Chen, Y.; Dales, R.; Krewski, D.; Breithaupt, K. Increased effects of smoking and obesity on asthma among female Canadians: The National Population Health Survey, 1994–1995. Am. J. Epidemiol. 1999, 150, 255–262. [Google Scholar] [CrossRef]
- Torén, K.; Olin, A.C.; Hellgren, J.; Hermansson, B.A. Rhinitis increase the risk for adult-onset asthma--a Swedish population-based case-control study (MAP-study). Respir. Med. 2002, 96, 635–641. [Google Scholar] [CrossRef]
- Piipari, R.; Jaakkola, J.J.K.; Jaakkola, N.; Jaakkola, M.S. Smoking and asthma in adults. Eur. Respir. J. 2004, 24, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Plaschke, P.P.; Janson, C.; Norrman, E.; Björnsson, E.; Ellbjär, S.; Järvholm, B. Onset and remission of allergic rhinitis and asthma and the relationship with atopic sensitization and smoking. Am. J. Respir. Crit. Care Med. 2000, 162, 920–924. [Google Scholar] [CrossRef]
- Polosa, R.; Knoke, J.D.; Russo, C.; Piccillo, G.; Caponnetto, P.; Sarvà, M.; Proietti, L.; Al-Delaimy, W.K. Cigarette smoking is associated with a greater risk of incident asthma in allergic rhinitis. J. Allergy Clin. Immunol. 2008, 121, 1428–1434. [Google Scholar] [CrossRef]
- Vignoud, L.; Pin, I.; Boudier, A.; Pison, C.; Nadif, R.; Le Moual, N.; Slama, R.; Makao, M.N.; Kauffmann, F.; Siroux, V. Smoking and asthma: Disentangling their mutual influences using a longitudinal approach. Respir. Med. 2011, 105, 1805–1814. [Google Scholar] [CrossRef]
- Torén, K.; Hermansson, B.A. Incidence rate of adult-onset asthma in relation to age, sex, atopy and smoking: A Swedish population-based study of 15813 adults. Int. J. Tuberc. Lung Dis. 1999, 3, 192–197. [Google Scholar] [PubMed]
- Verlato, G.; Nguyen, G.; Marchetti, P.; Accordini, S.; Marcon, A.; Marconcini, R.; Bono, R.; Fois, A.; Pirina, P.; de Marco, R. Smoking and New-Onset Asthma in a Prospective Study on Italian Adults. Int. Arch. Allergy Immunol. 2016, 170, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, E.; Kaprio, J.; Koskenvuo, M. Factors associated to lifestyle and risk of adult onset asthma. Respir. Med. 2003, 97, 273–280. [Google Scholar] [CrossRef]
- Vesterinen, E.; Kaprio, J.; Koskenvuo, M. Prospective study of asthma in relation to smoking habits among 14,729 adults. Thorax 1988, 43, 534–539. [Google Scholar] [CrossRef]
- Becklake, M.R.; Lalloo, U. The “healthy smoker”: A phenomenon of health selection? Respiration 1990, 57, 137–144. [Google Scholar] [CrossRef]
- Huff, R.D.; Carlsten, C.; Hirota, J.A. An update on immunologic mechanisms in the respiratory mucosa in response to air pollutants. J. Allergy Clin. Immunol. 2019, 143, 1989–2001. [Google Scholar] [CrossRef]
- Bontinck, A.; Maes, T.; Joos, G. Asthma and air pollution: Recent insights in pathogenesis and clinical implications. Curr. Opin. Pulm. Med. 2020, 26, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-G.; Lee, P.-H.; Lee, S.-H.; Park, C.-S.; Jang, A.-S. Impact of ozone on claudins and tight junctions in the lungs. Environ. Toxicol. 2018, 33, 798–806. [Google Scholar] [CrossRef]
- Heijink, I.; van Oosterhout, A.; Kliphuis, N.; Jonker, M.; Hoffmann, R.; Telenga, E.; Klooster, K.; Slebos, D.-J.; ten Hacken, N.; Postma, D.; et al. Oxidant-induced corticosteroid unresponsiveness in human bronchial epithelial cells. Thorax 2014, 69, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Esposito, S.; Tenconi, R.; Lelii, M.; Preti, V.; Nazzari, E.; Consolo, S.; Patria, M.F. Possible molecular mechanisms linking air pollution and asthma in children. BMC Pulm. Med. 2014, 14, 31. [Google Scholar] [CrossRef]
- Gref, A.; Merid, S.K.; Gruzieva, O.; Ballereau, S.; Becker, A.; Bellander, T.; Bergström, A.; Bossé, Y.; Bottai, M.; Chan-Yeung, M.; et al. Genome-Wide Interaction Analysis of Air Pollution Exposure and Childhood Asthma with Functional Follow-up. Am. J. Respir. Crit. Care Med. 2017, 195, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Piyadasa, H.; Ryu, M.H.; Rider, C.F.; Ezzati, P.; Spicer, V.; Carlsten, C. Inhaled diesel exhaust alters the allergen-induced bronchial secretome in humans. Eur. Respir. J. 2018, 51, 1701385. [Google Scholar] [CrossRef]
- Stevens, E.L.; Rosser, F.; Forno, E.; Peden, D.; Celedón, J.C. Can the effects of outdoor air pollution on asthma be mitigated? J. Allergy Clin. Immunol. 2019, 143, 2016–2018. [Google Scholar] [CrossRef]
- Nuvolone, D.; Petri, D.; Voller, F. The effects of ozone on human health. Environ. Sci. Pollut. Res. Int. 2018, 25, 8074–8088. [Google Scholar] [CrossRef]
- Anenberg, S.C.; Henze, D.K.; Tinney, V.; Kinney, P.L.; Raich, W.; Fann, N.; Malley, C.S.; Roman, H.; Lamsal, L.; Duncan, B.; et al. Estimates of the Global Burden of Ambient Ozone on Asthma Incidence and Emergency Room Visits. Environ. Health Perspect. 2018, 126, 107004. [Google Scholar] [CrossRef]
- Obeidat, M.; Li, X.; Burgess, S.; Zhou, G.; Fishbane, N.; Hansel, N.N.; Bossé, Y.; Joubert, P.; Hao, K.; Nickle, D.C.; et al. Surfactant protein D is a causal risk factor for COPD: Results of Mendelian randomisation. Eur. Respir. J. 2017, 50, 1700657. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, R.; Xia, Y.; Cai, J.; Lin, Z.; Liu, C.; Chen, C.; Peng, L.; Zhao, Z.; Zhou, W.; et al. Personal Ozone Exposure and Respiratory Inflammatory Response: The Role of DNA Methylation in the Arginase-Nitric Oxide Synthase Pathway. Environ. Sci. Technol. 2018, 52, 8785–8791. [Google Scholar] [CrossRef]
- Havet, A.; Zerimech, F.; Sanchez, M.; Siroux, V.; Le Moual, N.; Brunekreef, B.; Stempfelet, M.; Künzli, N.; Jacquemin, B.; Matran, R.; et al. Outdoor air pollution, exhaled 8-isoprostane and current asthma in adults: The EGEA study. Eur. Respir. J. 2018, 51, 1702036. [Google Scholar] [CrossRef]
- Dai, Y.; Qiu, H.; Sun, S.; Yang, Y.; Lin, H.; Tian, L. Age-dependent effect of ambient ozone on emergency asthma hospitalizations in Hong Kong. J. Allergy Clin. Immunol. 2018, 141, 1532–1534. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Zheng, X.; Li, Y.; Han, M.; Liu, T.; Xiao, J.; Guo, L.; Zeng, W.; Zhang, J.; et al. Effects of ambient ozone concentrations with different averaging times on asthma exacerbations: A meta-analysis. Sci. Total Environ. 2019, 691, 549–561. [Google Scholar] [CrossRef]
- Zu, K.; Liu, X.; Shi, L.; Tao, G.; Loftus, C.T.; Lange, S.; Goodman, J.E. Concentration-response of short-term ozone exposure and hospital admissions for asthma in Texas. Environ. Int. 2017, 104, 139–145. [Google Scholar] [CrossRef]
- To, T.; Zhu, J.; Larsen, K.; Simatovic, J.; Feldman, L.; Ryckman, K.; Gershon, A.; Lougheed, M.D.; Licskai, C.; Chen, H.; et al. Progression from Asthma to Chronic Obstructive Pulmonary Disease. Is Air Pollution a Risk Factor? Am. J. Respir. Crit. Care Med. 2016, 194, 429–438. [Google Scholar] [CrossRef]
- Gruzieva, O.; Bellander, T.; Eneroth, K.; Kull, I.; Melén, E.; Nordling, E.; van Hage, M.; Wickman, M.; Moskalenko, V.; Hulchiy, O.; et al. Traffic-related air pollution and development of allergic sensitization in children during the first 8 years of life. J. Allergy Clin. Immunol. 2012, 129, 240–246. [Google Scholar] [CrossRef]
- Gruzieva, O.; Xu, C.-J.; Breton, C.V.; Annesi-Maesano, I.; Antó, J.M.; Auffray, C.; Ballereau, S.; Bellander, T.; Bousquet, J.; Bustamante, M.; et al. Epigenome-Wide Meta-Analysis of Methylation in Children Related to Prenatal NO2 Air Pollution Exposure. Environ. Health Perspect. 2017, 125, 104–110. [Google Scholar] [CrossRef]
- Kravitz-Wirtz, N.; Teixeira, S.; Hajat, A.; Woo, B.; Crowder, K.; Takeuchi, D. Early-Life Air Pollution Exposure, Neighborhood Poverty, and Childhood Asthma in the United States, 1990–2014. Int. J. Environ. Res. Public Health 2018, 15, 1114. [Google Scholar] [CrossRef]
- Achakulwisut, P.; Brauer, M.; Hystad, P.; Anenberg, S.C. Global, national, and urban burdens of paediatric asthma incidence attributable to ambient NO2 pollution: Estimates from global datasets. Lancet Planet Health 2019, 3, e166–e178. [Google Scholar] [CrossRef]
- To, T.; Zhu, J.; Stieb, D.; Gray, N.; Fong, I.; Pinault, L.; Jerrett, M.; Robichaud, A.; Ménard, R.; van Donkelaar, A.; et al. Early life exposure to air pollution and incidence of childhood asthma, allergic rhinitis and eczema. Eur. Respir. J. 2020, 55, 1900913. [Google Scholar] [CrossRef]
- Fuertes, E.; Sunyer, J.; Gehring, U.; Porta, D.; Forastiere, F.; Cesaroni, G.; Vrijheid, M.; Guxens, M.; Annesi-Maesano, I.; Slama, R.; et al. Associations between air pollution and pediatric eczema, rhinoconjunctivitis and asthma: A meta-analysis of European birth cohorts. Environ. Int. 2020, 136, 105474. [Google Scholar] [CrossRef]
- Barone-Adesi, F.; Dent, J.E.; Dajnak, D.; Beevers, S.; Anderson, H.R.; Kelly, F.J.; Cook, D.G.; Whincup, P.H. Long-Term Exposure to Primary Traffic Pollutants and Lung Function in Children: Cross-Sectional Study and Meta-Analysis. PLoS ONE 2015, 10, e0142565. [Google Scholar] [CrossRef]
- Knibbs, L.D.; Cortés de Waterman, A.M.; Toelle, B.G.; Guo, Y.; Denison, L.; Jalaludin, B.; Marks, G.B.; Williams, G.M. The Australian Child Health and Air Pollution Study (ACHAPS): A national population-based cross-sectional study of long-term exposure to outdoor air pollution, asthma, and lung function. Environ. Int. 2018, 120, 394–403. [Google Scholar] [CrossRef]
- Bougas, N.; Rancière, F.; Beydon, N.; Viola, M.; Perrot, X.; Gabet, S.; Lezmi, G.; Amat, F.; De Blic, J.; Just, J.; et al. Traffic-related Air Pollution, Lung Function, and Host Vulnerability. New Insights from the PARIS Birth Cohort. Ann. Am. Thorac. Soc. 2018, 15, 599–607. [Google Scholar] [CrossRef]
- Orellano, P.; Quaranta, N.; Reynoso, J.; Balbi, B.; Vasquez, J. Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta-analysis. PLoS ONE 2017, 12, e0174050. [Google Scholar] [CrossRef]
- Poole, J.A.; Barnes, C.S.; Demain, J.G.; Bernstein, J.A.; Padukudru, M.A.; Sheehan, W.J.; Fogelbach, G.G.; Wedner, J.; Codina, R.; Levetin, E.; et al. Impact of weather and climate change with indoor and outdoor air quality in asthma: A Work Group Report of the AAAAI Environmental Exposure and Respiratory Health Committee. J. Allergy Clin. Immunol. 2019, 143, 1702–1710. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Kabir, E. A review on human health perspective of air pollution with respect to allergies and asthma. Environ. Int. 2013, 59, 41–52. [Google Scholar] [CrossRef]
- Burbank, A.J.; Peden, D.B. Assessing the impact of air pollution on childhood asthma morbidity: How, when, and what to do. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 124–131. [Google Scholar] [CrossRef]
- Hong, E.; Lee, S.; Kim, G.-B.; Kim, T.-J.; Kim, H.-W.; Lee, K.; Son, B.-S. Effects of Environmental Air Pollution on Pulmonary Function Level of Residents in Korean Industrial Complexes. Int. J. Environ. Res. Public Health 2018, 15, 834. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Ross Anderson, H.; Ostro, B.; Pandey, K.D.; Krzyzanowski, M.; Künzli, N.; Gutschmidt, K.; Pope, A.; Romieu, I.; Samet, J.M.; et al. The global burden of disease due to outdoor air pollution. J. Toxicol. Environ. Health Part A 2005, 68, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Wu, J.-Z.; Ge, D.-D.; Zhou, L.-F.; Hou, L.-Y.; Zhou, Y.; Li, Q.-Y. Effects of particulate matter on allergic respiratory diseases. Chronic Dis. Transl. Med. 2018, 4, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.L.; Amin, N.; Robinson, S.D.; Anand, A.; Davies, J.; Patel, D.; de la Fuente, J.M.; Cassee, F.R.; Boon, N.A.; Macnee, W.; et al. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am. J. Respir. Crit. Care Med. 2006, 173, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, S.; Fan, C.; Bai, Z.; Yang, K. The impact of PM2.5 on asthma emergency department visits: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2016, 23, 843–850. [Google Scholar] [CrossRef]
- Mazenq, J.; Dubus, J.-C.; Gaudart, J.; Charpin, D.; Nougairede, A.; Viudes, G.; Noel, G. Air pollution and children’s asthma-related emergency hospital visits in southeastern France. Eur. J. Pediatr. 2017, 176, 705–711. [Google Scholar] [CrossRef]
- Li, Q.; Yi, Q.; Tang, L.; Luo, S.; Tang, Y.; Zhang, G.; Luo, Z. Influence of Ultrafine Particles Exposure on Asthma Exacerbation in Children: A Meta-Analysis. Curr. Drug Targets 2019, 20, 412–420. [Google Scholar] [CrossRef]
- Borchers Arriagada, N.; Horsley, J.A.; Palmer, A.J.; Morgan, G.G.; Tham, R.; Johnston, F.H. Association between fire smoke fine particulate matter and asthma-related outcomes: Systematic review and meta-analysis. Environ. Res. 2019, 179, 108777. [Google Scholar] [CrossRef]
- Walter, C.M.; Schneider-Futschik, E.K.; Knibbs, L.D.; Irving, L.B. Health impacts of bushfire smoke exposure in Australia. Respirology 2020, 25, 495–501. [Google Scholar] [CrossRef]
- Haikerwal, A.; Akram, M.; Sim, M.R.; Meyer, M.; Abramson, M.J.; Dennekamp, M. Fine particulate matter (PM2.5) exposure during a prolonged wildfire period and emergency department visits for asthma. Respirology 2016, 21, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Thompson, L.A.; Gross, H.E.; Shenkman, E.A.; DeWalt, D.A.; Huang, I.-C. Longitudinal Effect of Ambient Air Pollution and Pollen Exposure on Asthma Control: The Patient-Reported Outcomes Measurement Information System (PROMIS) Pediatric Asthma Study. Acad. Pediatr. 2019, 19, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, P.; Canova, C.; Scapellato, M.L.; Visentin, A.; Tessari, R.; Bartolucci, G.B.; Simonato, L.; Lotti, M. Personal exposure to particulate matter is associated with worse health perception in adult asthma. J. Investig. Allergol. Clin. Immunol. 2011, 21, 120–128. [Google Scholar] [PubMed]
- Yu, S.; Park, S.; Park, C.-S.; Kim, S. Association between the Ratio of FEV₁ to FVC and the Exposure Level to Air Pollution in Neversmoking Adult Refractory Asthmatics Using Data Clustered by Patient in the Soonchunhyang Asthma Cohort Database. Int. J. Environ. Res. Public Health 2018, 15, 2349. [Google Scholar] [CrossRef]
- Park, J.W.; Lim, Y.H.; Kyung, S.Y.; An, C.H.; Lee, S.P.; Jeong, S.H.; Ju, Y.-S. Effects of ambient particulate matter on peak expiratory flow rates and respiratory symptoms of asthmatics during Asian dust periods in Korea. Respirology 2005, 10, 470–476. [Google Scholar] [CrossRef]
- Yamazaki, S.; Shima, M.; Ando, M.; Nitta, H.; Watanabe, H.; Nishimuta, T. Effect of hourly concentration of particulate matter on peak expiratory flow in hospitalized children: A panel study. Environ. Health 2011, 10, 15. [Google Scholar] [CrossRef]
- Mentz, G.; Robins, T.G.; Batterman, S.; Naidoo, R.N. Effect modifiers of lung function and daily air pollutant variability in a panel of schoolchildren. Thorax 2019, 74, 1055–1062. [Google Scholar] [CrossRef]
- Williams, A.M.; Phaneuf, D.J.; Barrett, M.A.; Su, J.G. Short-term impact of PM2.5 on contemporaneous asthma medication use: Behavior and the value of pollution reductions. Proc. Natl. Acad. Sci. USA 2019, 116, 5246–5253. [Google Scholar] [CrossRef]
- Pepper, J.R.; Barrett, M.A.; Su, J.G.; Merchant, R.; Henderson, K.; Van Sickle, D.; Balmes, J.R. Geospatial-temporal analysis of the impact of ozone on asthma rescue inhaler use. Environ. Int. 2020, 136, 105331. [Google Scholar] [CrossRef]
- Kowalska, M.; Skrzypek, M.; Kowalski, M.; Cyrys, J. Effect of NOx and NO2 Concentration Increase in Ambient Air to Daily Bronchitis and Asthma Exacerbation, Silesian Voivodeship in Poland. Int. J. Environ. Res. Public Health 2020, 17, 754. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhang, Y.; Lin, J.; Xia, G.; Zhang, W.; Knibbs, L.D.; Morgan, G.G.; Jalaludin, B.; Marks, G.; Abramson, M.; et al. Multi-city study on air pollution and hospital outpatient visits for asthma in China. Environ. Pollut. 2020, 257, 113638. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Liu, C.; Chen, R.; Kan, H.; Sun, J.; Zhao, J.; Wang, C.; Sun, Q.; Bai, H. Associations between short-term exposure to fine particulate matter and acute exacerbation of asthma in Yancheng, China. Chemosphere 2019, 237, 124497. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Berhane, K.T.; Islam, T.; McConnell, R.; Urman, R.; Chen, Z.; Gilliland, F.D. Association of Changes in Air Quality with Incident Asthma in Children in California, 1993–2014. JAMA 2019, 321, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Chi, R.; Li, H.; Wang, Q.; Zhai, Q.; Wang, D.; Wu, M.; Liu, Q.; Wu, S.; Ma, Q.; Deng, F.; et al. Association of emergency room visits for respiratory diseases with sources of ambient PM2.5. J. Environ. Sci. 2019, 86, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Alcala, E.; Brown, P.; Capitman, J.A.; Gonzalez, M.; Cisneros, R. Cumulative Impact of Environmental Pollution and Population Vulnerability on Pediatric Asthma Hospitalizations: A Multilevel Analysis of CalEnviroScreen. Int. J. Environ. Res. Public Health 2019, 16, 2683. [Google Scholar] [CrossRef]
- Marques Mejías, M.A.; Tomás Pérez, M.; Hernández, I.; López, I.; Quirce, S. Asthma Exacerbations in the Pediatric Emergency Department at a Tertiary Hospital: Association with Environmental Factors. J. Investig. Allergol. Clin. Immunol. 2019, 29, 365–370. [Google Scholar] [CrossRef]
- Baek, J.; Kash, B.A.; Xu, X.; Benden, M.; Roberts, J.; Carrillo, G. Association between Ambient Air Pollution and Hospital Length of Stay among Children with Asthma in South Texas. Int. J. Environ. Res. Public Health 2020, 17, 3812. [Google Scholar] [CrossRef]
- Lim, H.; Kwon, H.-J.; Lim, J.-A.; Choi, J.H.; Ha, M.; Hwang, S.-S.; Choi, W.-J. Short-term Effect of Fine Particulate Matter on Children’s Hospital Admissions and Emergency Department Visits for Asthma: A Systematic Review and Meta-analysis. J. Prev. Med. Public Health 2016, 49, 205–219. [Google Scholar] [CrossRef]
- Zheng, X.; Ding, H.; Jiang, L.; Chen, S.; Zheng, J.; Qiu, M.; Zhou, Y.; Chen, Q.; Guan, W. Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0138146. [Google Scholar] [CrossRef]
- Hopke, P.K.; Croft, D.; Zhang, W.; Lin, S.; Masiol, M.; Squizzato, S.; Thurston, S.W.; van Wijngaarden, E.; Utell, M.J.; Rich, D.Q. Changes in the acute response of respiratory diseases to PM2.5 in New York State from 2005 to 2016. Sci. Total Environ. 2019, 677, 328–339. [Google Scholar] [CrossRef]
- Mendy, A.; Wilkerson, J.; Salo, P.M.; Weir, C.H.; Feinstein, L.; Zeldin, D.C.; Thorne, P.S. Synergistic Association of House Endotoxin Exposure and Ambient Air Pollution with Asthma Outcomes. Am. J. Respir. Crit. Care Med. 2019, 200, 712–720. [Google Scholar] [CrossRef]
- World Health Organization. The Data Repository. Available online: http://www.who.int/gho/database/en/ (accessed on 14 June 2020).
- Carlsten, C.; Salvi, S.; Wong, G.W.K.; Chung, K.F. Personal strategies to minimise effects of air pollution on respiratory health: Advice for providers, patients and the public. Eur. Respir. J. 2020, 55, 1902056. [Google Scholar] [CrossRef]
- Houdouin, V.; Dubus, J.-C. What is the impact of outdoor pollution on children’s asthma? Arch. Pediatr. 2019, 26, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Whyand, T.; Hurst, J.R.; Beckles, M.; Caplin, M.E. Pollution and respiratory disease: Can diet or supplements help? A review. Respir. Res. 2018, 19, 79. [Google Scholar] [CrossRef]
- Gref, A.; Rautiainen, S.; Gruzieva, O.; Håkansson, N.; Kull, I.; Pershagen, G.; Wickman, M.; Wolk, A.; Melén, E.; Bergström, A. Dietary total antioxidant capacity in early school age and subsequent allergic disease. Clin. Exp. Allergy 2017, 47, 751–759. [Google Scholar] [CrossRef]
- Cruz, Á.A.; Stelmach, R.; Ponte, E.V. Asthma prevalence and severity in low-resource communities. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Glazener, A.; Khreis, H. Transforming Our Cities: Best Practices Towards Clean Air and Active Transportation. Curr. Environ. Health Rep. 2019, 6, 22–37. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tobacco. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 6 July 2020).
- National Research Council (US) Committee on Passive Smoking. Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects; National Academies Press (US): Washington, DC, USA, 1986; ISBN 978-0-309-03730-3. Available online: http://www.ncbi.nlm.nih.gov/books/NBK219205/ (accessed on 6 July 2020).
- Monograph 9: Cigars: Health Effects and Trends. Available online: https://cancercontrol.cancer.gov/brp/tcrb/monographs/9/index.html (accessed on 6 July 2020).
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014. Available online: http://www.ncbi.nlm.nih.gov/books/NBK179276/ (accessed on 6 July 2020).
- Jiménez-Ruiz, C.A.; Andreas, S.; Lewis, K.E.; Tonnesen, P.; van Schayck, C.P.; Hajek, P.; Tonstad, S.; Dautzenberg, B.; Fletcher, M.; Masefield, S.; et al. Statement on smoking cessation in COPD and other pulmonary diseases and in smokers with comorbidities who find it difficult to quit. Eur. Respir. J. 2015, 46, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.-P.; Lemière, C.; Archambault, F.; Carrier, G.; Descary, M.C.; Deschesnes, F. Smoking and asthma: Clinical and radiologic features, lung function, and airway inflammation. Chest 2006, 129, 661–668. [Google Scholar] [CrossRef]
- Chalmers, G.W.; MacLeod, K.J.; Thomson, L.; Little, S.A.; McSharry, C.; Thomson, N.C. Smoking and airway inflammation in patients with mild asthma. Chest 2001, 120, 1917–1922. [Google Scholar] [CrossRef]
- Chaudhuri, R.; McSharry, C.; Brady, J.; Donnelly, I.; Grierson, C.; McGuinness, S.; Jolly, L.; Weir, C.J.; Messow, C.M.; Spears, M.; et al. Sputum matrix metalloproteinase-12 in patients with chronic obstructive pulmonary disease and asthma: Relationship to disease severity. J. Allergy Clin. Immunol. 2012, 129, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Polosa, R.; Thomson, N.C. Smoking and asthma: Dangerous liaisons. Eur. Respir. J. 2013, 41, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Tsoumakidou, M.; Elston, W.; Zhu, J.; Wang, Z.; Gamble, E.; Siafakas, N.M.; Barnes, N.C.; Jeffery, P.K. Cigarette smoking alters bronchial mucosal immunity in asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Venarske, D.L.; Busse, W.W.; Griffin, M.R.; Gebretsadik, T.; Shintani, A.K.; Minton, P.A.; Peebles, R.S.; Hamilton, R.; Weisshaar, E.; Vrtis, R.; et al. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J. Infect. Dis. 2006, 193, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, G.W.; Macleod, K.J.; Little, S.A.; Thomson, L.J.; McSharry, C.P.; Thomson, N.C. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax 2002, 57, 226–230. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Livingston, E.; McMahon, A.D.; Thomson, L.; Borland, W.; Thomson, N.C. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am. J. Respir. Crit. Care Med. 2003, 168, 1308–1311. [Google Scholar] [CrossRef]
- Lazarus, S.C.; Chinchilli, V.M.; Rollings, N.J.; Boushey, H.A.; Cherniack, R.; Craig, T.J.; Deykin, A.; DiMango, E.; Fish, J.E.; Ford, J.G.; et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 783–790. [Google Scholar] [CrossRef]
- Tomlinson, J.E.M.; McMahon, A.D.; Chaudhuri, R.; Thompson, J.M.; Wood, S.F.; Thomson, N.C. Efficacy of low and high dose inhaled corticosteroid in smokers versus non-smokers with mild asthma. Thorax 2005, 60, 282–287. [Google Scholar] [CrossRef]
- Pedersen, B.; Dahl, R.; Karlström, R.; Peterson, C.G.; Venge, P. Eosinophil and neutrophil activity in asthma in a one-year trial with inhaled budesonide. The impact of smoking. Am. J. Respir. Crit. Care Med. 1996, 153, 1519–1529. [Google Scholar] [CrossRef]
- Pedersen, S.E.; Bateman, E.D.; Bousquet, J.; Busse, W.W.; Yoxall, S.; Clark, T.J. Gaining Optimal Asthma controL Steering Committee and Investigators Determinants of response to fluticasone propionate and salmeterol/fluticasone propionate combination in the Gaining Optimal Asthma controL study. J. Allergy Clin. Immunol. 2007, 120, 1036–1042. [Google Scholar] [CrossRef]
- Barnes, P.J. Corticosteroid resistance in airway disease. Proc. Am. Thorac. Soc. 2004, 1, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Lim, S.; Caramori, G.; Chung, K.F.; Barnes, P.J.; Adcock, I.M. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 2001, 15, 1110–1112. [Google Scholar] [CrossRef] [PubMed]
- Cosio, B.G.; Tsaprouni, L.; Ito, K.; Jazrawi, E.; Adcock, I.M.; Barnes, P.J. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J. Exp. Med. 2004, 200, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Cosío, B.G.; Mann, B.; Ito, K.; Jazrawi, E.; Barnes, P.J.; Chung, K.F.; Adcock, I.M. Histone acetylase and deacetylase activity in alveolar macrophages and blood mononocytes in asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Reduced histone deacetylase in COPD: Clinical implications. Chest 2006, 129, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Q.A.; Wenzel, S.E.; Hauk, P.J.; Tsicopoulos, A.; Wallaert, B.; Lafitte, J.J.; Chrousos, G.P.; Szefler, S.J.; Leung, D.Y. Increased glucocorticoid receptor beta in airway cells of glucocorticoid-insensitive asthma. Am. J. Respir. Crit. Care Med. 1999, 159, 1600–1604. [Google Scholar] [CrossRef]
- Sousa, A.R.; Lane, S.J.; Cidlowski, J.A.; Staynov, D.Z.; Lee, T.H. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J. Allergy Clin. Immunol. 2000, 105, 943–950. [Google Scholar] [CrossRef]
- Leung, D.Y.; Hamid, Q.; Vottero, A.; Szefler, S.J.; Surs, W.; Minshall, E.; Chrousos, G.P.; Klemm, D.J. Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor beta. J. Exp. Med. 1997, 186, 1567–1574. [Google Scholar] [CrossRef]
- Livingston, E.; Darroch, C.E.; Chaudhuri, R.; McPhee, I.; McMahon, A.D.; Mackenzie, S.J.; Thomson, N.C. Glucocorticoid receptor alpha:beta ratio in blood mononuclear cells is reduced in cigarette smokers. J. Allergy Clin. Immunol. 2004, 114, 1475–1478. [Google Scholar] [CrossRef]
- Smith, K.R.; Bruce, N.; Balakrishnan, K.; Adair-Rohani, H.; Balmes, J.; Chafe, Z.; Dherani, M.; Hosgood, H.D.; Mehta, S.; Pope, D.; et al. Millions dead: How do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu. Rev. Public Health 2014, 35, 185–206. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Phase I Recommendations by the Air Quality Management Work Group to the Clean Air Act Advisory Committee. Available online: https://www.epa.gov/caaac/phase-i-recommendations-air-quality-management-work-group-clean-air-act-advisory-committee (accessed on 8 July 2020).
- Noonan, C.W.; Ward, T.J.; Semmens, E.O. Estimating the number of vulnerable people in the United States exposed to residential wood smoke. Environ. Health Perspect. 2015, 123, A30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.; Lange, T. The impact of wood smoke on ambient PM2.5 in northern Rocky Mountain valley communities. Environ. Pollut. 2010, 158, 723–729. [Google Scholar] [CrossRef]
- Sigsgaard, T.; Forsberg, B.; Annesi-Maesano, I.; Blomberg, A.; Bølling, A.; Boman, C.; Bønløkke, J.; Brauer, M.; Bruce, N.; Héroux, M.-E.; et al. Health impacts of anthropogenic biomass burning in the developed world. Eur. Respir. J. 2015, 46, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, J.C.; Lumley, T.; Sheppard, L.; Koenig, J.Q.; Shapiro, G.G. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann. Allergy Asthma Immunol. 2003, 91, 346–353. [Google Scholar] [CrossRef]
- McConnell, R.; Berhane, K.; Gilliland, F.; Molitor, J.; Thomas, D.; Lurmann, F.; Avol, E.; Gauderman, W.J.; Peters, J.M. Prospective study of air pollution and bronchitic symptoms in children with asthma. Am. J. Respir. Crit. Care Med. 2003, 168, 790–797. [Google Scholar] [CrossRef]
- Gauderman, W.J.; McConnell, R.; Gilliland, F.; London, S.; Thomas, D.; Avol, E.; Vora, H.; Berhane, K.; Rappaport, E.B.; Lurmann, F.; et al. Association between air pollution and lung function growth in southern California children. Am. J. Respir. Crit. Care Med. 2000, 162, 1383–1390. [Google Scholar] [CrossRef]
- Lee, K.; Xue, J.; Geyh, A.S.; Ozkaynak, H.; Leaderer, B.P.; Weschler, C.J.; Spengler, J.D. Nitrous acid, nitrogen dioxide, and ozone concentrations in residential environments. Environ. Health Perspect. 2002, 110, 145–150. [Google Scholar] [CrossRef]
- Brown, S.K.; Mahoney, K.J.; Cheng, M. Room chamber assessment of the pollutant emission properties of (nominally) low-emission unflued gas heaters. Indoor Air 2004, 14 (Suppl. 8), 84–91. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on the Assessment of Asthma and Indoor Air. Clearing the Air: Asthma and Indoor Air Exposures; National Academies Press (US): Washington, DC, USA, 2000; ISBN 978-0-309-06496-5. Available online: http://www.ncbi.nlm.nih.gov/books/NBK224477/ (accessed on 8 July 2020).
- Franklin, P.J.; Loveday, J.; Cook, A. Unflued gas heaters and respiratory symptoms in older people with asthma. Thorax 2012, 67, 315–320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alqahtani, J.M.; Asaad, A.M.; Awadalla, N.J.; Mahfouz, A.A. Environmental Determinants of Bronchial Asthma among Saudi School Children in Southwestern Saudi Arabia. Int. J. Environ. Res. Public Health 2016, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.-L.; Du, Y.; Xu, G.; Zhang, H.; Cheng, L.; Wang, Y.-J.; Zhu, D.-D.; Lv, W.; Liu, S.-X.; Li, P.-Z.; et al. Prevalence and Occupational and Environmental Risk Factors of Self-Reported Asthma: Evidence from a Cross-Sectional Survey in Seven Chinese Cities. Int. J. Environ. Res. Public Health 2016, 13, 1084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Reponen, T.; Hershey, G.K.K. Fungal Exposure and Asthma: IgE and Non-IgE-Mediated Mechanisms. Curr. Allergy Asthma Rep. 2016, 16, 86. [Google Scholar] [CrossRef]
- Caillaud, D.; Leynaert, B.; Keirsbulck, M.; Nadif, R.; Mould ANSES Working Group. Indoor mould exposure, asthma and rhinitis: Findings from systematic reviews and recent longitudinal studies. Eur. Respir. Rev. 2018, 27, 170137. [Google Scholar] [CrossRef]
- Vincent, M.; Corazza, F.; Chasseur, C.; Bladt, S.; Romano, M.; Huygen, K.; Denis, O.; Michel, O. Relationship between mold exposure, specific IgE sensitization, and clinical asthma: A case-control study. Ann. Allergy Asthma Immunol. 2018, 121, 333–339. [Google Scholar] [CrossRef]
- Harley, K.G.; Macher, J.M.; Lipsett, M.; Duramad, P.; Holland, N.T.; Prager, S.S.; Ferber, J.; Bradman, A.; Eskenazi, B.; Tager, I.B. Fungi and pollen exposure in the first months of life and risk of early childhood wheezing. Thorax 2009, 64, 353–358. [Google Scholar] [CrossRef]
- Howard, E.; Orhurhu, V.; Huang, L.; Guthrie, B.; Phipatanakul, W. The Impact of Ambient Environmental Exposures to Microbial Products on Asthma Outcomes from Birth to Childhood. Curr. Allergy Asthma Rep. 2019, 19, 59. [Google Scholar] [CrossRef]
- Pulimood, T.B.; Corden, J.M.; Bryden, C.; Sharples, L.; Nasser, S.M. Epidemic asthma and the role of the fungal mold Alternaria alternata. J. Allergy Clin. Immunol. 2007, 120, 610–617. [Google Scholar] [CrossRef]
- Siroux, V.; Pin, I.; Oryszczyn, M.P.; Le Moual, N.; Kauffmann, F. Relationships of active smoking to asthma and asthma severity in the EGEA study. Epidemiological study on the Genetics and Environment of Asthma. Eur. Respir. J. 2000, 15, 470–477. [Google Scholar] [CrossRef]
- Austin, J.B.; Selvaraj, S.; Godden, D.; Russell, G. Deprivation, smoking, and quality of life in asthma. Arch. Dis. Child. 2005, 90, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Eisner, M.D.; Iribarren, C. The influence of cigarette smoking on adult asthma outcomes. Nicotine Tob. Res. 2007, 9, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Shavit, O.; Swern, A.; Dong, Q.; Newcomb, K.; Sazonov Kocevar, V.; Taylor, S.D. Impact of smoking on asthma symptoms, healthcare resource use, and quality of life outcomes in adults with persistent asthma. Qual. Life Res. 2007, 16, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Ulrik, C.S.; Frederiksen, J. Mortality and markers of risk of asthma death among 1075 outpatients with asthma. Chest 1995, 108, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Marquette, C.H.; Saulnier, F.; Leroy, O.; Wallaert, B.; Chopin, C.; Demarcq, J.M.; Durocher, A.; Tonnel, A.B. Long-term prognosis of near-fatal asthma. A 6-year follow-up study of 145 asthmatic patients who underwent mechanical ventilation for a near-fatal attack of asthma. Am. Rev. Respir. Dis. 1992, 146, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Polosa, R.; Russo, C.; Caponnetto, P.; Bertino, G.; Sarvà, M.; Antic, T.; Mancuso, S.; Al-Delaimy, W.K. Greater severity of new onset asthma in allergic subjects who smoke: A 10-year longitudinal study. Respir. Res. 2011, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; May, S.M.; Charoenlap, S.; Pyle, R.; Ott, N.L.; Mohammed, K.; Joshi, A.Y. Effects of secondhand smoke exposure on asthma morbidity and health care utilization in children: A systematic review and meta-analysis. Ann. Allergy Asthma Immunol. 2015, 115, 396–401. [Google Scholar] [CrossRef]
- Merianos, A.L.; Jandarov, R.A.; Mahabee-Gittens, E.M. Association of secondhand smoke exposure with asthma symptoms, medication use, and healthcare utilization among asthmatic adolescents. J. Asthma 2019, 56, 369–379. [Google Scholar] [CrossRef]
- Jing, W.; Wang, W.; Liu, Q. Passive smoking induces pediatric asthma by affecting the balance of Treg/Th17 cells. Pediatr. Res. 2019, 85, 469–476. [Google Scholar] [CrossRef]
- Office on Smoking and Health (US). The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2006. Available online: http://www.ncbi.nlm.nih.gov/books/NBK44324/ (accessed on 6 July 2020).
- Lange, P.; Parner, J.; Vestbo, J.; Schnohr, P.; Jensen, G. A 15-year follow-up study of ventilatory function in adults with asthma. N. Engl. J. Med. 1998, 339, 1194–1200. [Google Scholar] [CrossRef]
- Apostol, G.G.; Jacobs, D.R.; Tsai, A.W.; Crow, R.S.; Williams, O.D.; Townsend, M.C.; Beckett, W.S. Early life factors contribute to the decrease in lung function between ages 18 and 40: The Coronary Artery Risk Development in Young Adults study. Am. J. Respir. Crit. Care Med. 2002, 166, 166–172. [Google Scholar] [CrossRef] [PubMed]
- James, A.L.; Palmer, L.J.; Kicic, E.; Maxwell, P.S.; Lagan, S.E.; Ryan, G.F.; Musk, A.W. Decline in lung function in the Busselton Health Study: The effects of asthma and cigarette smoking. Am. J. Respir. Crit. Care Med. 2005, 171, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Tommola, M.; Ilmarinen, P.; Tuomisto, L.E.; Haanpää, J.; Kankaanranta, T.; Niemelä, O.; Kankaanranta, H. The effect of smoking on lung function: A clinical study of adult-onset asthma. Eur. Respir. J. 2016, 48, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, J.J.K.; Hernberg, S.; Lajunen, T.K.; Sripaijboonkij, P.; Malmberg, L.P.; Jaakkola, M.S. Smoking and lung function among adults with newly onset asthma. BMJ Open Respir. Res. 2019, 6, e000377. [Google Scholar] [CrossRef]
- Kiljander, T.; Helin, T.; Venho, K.; Jaakkola, A.; Lehtimäki, L. Prevalence of asthma-COPD overlap syndrome among primary care asthmatics with a smoking history: A cross-sectional study. NPJ Prim. Care Respir. Med. 2015, 25, 15047. [Google Scholar] [CrossRef]
- Gibson, P.G.; Simpson, J.L. The overlap syndrome of asthma and COPD: What are its features and how important is it? Thorax 2009, 64, 728–735. [Google Scholar] [CrossRef]
- Oluwole, O.; Arinola, G.O.; Huo, D.; Olopade, C.O. Biomass fuel exposure and asthma symptoms among rural school children in Nigeria. J. Asthma 2017, 54, 347–356. [Google Scholar] [CrossRef]
- Li, S.; Xu, J.; Jiang, Z.; Luo, Y.; Yang, Y.; Yu, J. Correlation between indoor air pollution and adult respiratory health in Zunyi City in Southwest China: Situation in two different seasons. BMC Public Health 2019, 19, 723. [Google Scholar] [CrossRef]
- Segura-Medina, P.; Vargas, M.H.; Aguilar-Romero, J.M.; Arreola-Ramírez, J.L.; Miguel-Reyes, J.L.; Salas-Hernández, J. Mold burden in house dust and its relationship with asthma control. Respir. Med. 2019, 150, 74–80. [Google Scholar] [CrossRef]
- Fernandes, A.G.O.; de Souza-Machado, C.; Pinheiro, G.P.; de Oliva, S.T.; Mota, R.C.L.; de Lima, V.B.; Cruz, C.S.; Chatkin, J.M.; Cruz, Á.A. Dual exposure to smoking and household air pollution is associated with an increased risk of severe asthma in adults in Brazil. Clin. Transl. Allergy 2018, 8, 48. [Google Scholar] [CrossRef]
- Tønnesen, P.; Pisinger, C.; Hvidberg, S.; Wennike, P.; Bremann, L.; Westin, A.; Thomsen, C.; Nilsson, F. Effects of smoking cessation and reduction in asthmatics. Nicotine Tob. Res. 2005, 7, 139–148. [Google Scholar] [CrossRef]
- Piccillo, G.; Caponnetto, P.; Barton, S.; Russo, C.; Origlio, A.; Bonaccorsi, A.; Di Maria, A.; Oliveri, C.; Polosa, R. Changes in airway hyperresponsiveness following smoking cessation: Comparisons between Mch and AMP. Respir. Med. 2008, 102, 256–265. [Google Scholar] [CrossRef]
- Oelsner, E.C.; Balte, P.P.; Bhatt, S.P.; Cassano, P.A.; Couper, D.; Folsom, A.R.; Freedman, N.D.; Jacobs, D.R.; Kalhan, R.; Mathew, A.R.; et al. Lung function decline in former smokers and low-intensity current smokers: A secondary data analysis of the NHLBI Pooled Cohorts Study. Lancet Respir. Med. 2020, 8, 34–44. [Google Scholar] [CrossRef]
- Chatkin, J.M.; Dullius, C.R. The management of asthmatic smokers. Asthma Res. Pract. 2016, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Polosa, R.; Morjaria, J.; Caponnetto, P.; Caruso, M.; Strano, S.; Battaglia, E.; Russo, C. Effect of smoking abstinence and reduction in asthmatic smokers switching to electronic cigarettes: Evidence for harm reversal. Int. J. Environ. Res. Public Health 2014, 11, 4965–4977. [Google Scholar] [CrossRef] [PubMed]
- Clapp, P.W.; Jaspers, I. Electronic Cigarettes: Their Constituents and Potential Links to Asthma. Curr. Allergy Asthma Rep. 2017, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Osei, A.D.; Mirbolouk, M.; Orimoloye, O.A.; Dzaye, O.; Uddin, S.M.I.; Dardari, Z.A.; DeFilippis, A.P.; Bhatnagar, A.; Blaha, M.J. The association between e-cigarette use and asthma among never combustible cigarette smokers: Behavioral risk factor surveillance system (BRFSS) 2016 & 2017. BMC Pulm. Med. 2019, 19, 180. [Google Scholar]
- Dijkstra, A.; Vonk, J.M.; Jongepier, H.; Koppelman, G.H.; Schouten, J.P.; ten Hacken, N.H.T.; Timens, W.; Postma, D.S. Lung function decline in asthma: Association with inhaled corticosteroids, smoking and sex. Thorax 2006, 61, 105–110. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Lamm, C.J.; Busse, W.W.; Tan, W.C.; Pedersen, S. START Investigators Group The effects of inhaled budesonide on lung function in smokers and nonsmokers with mild persistent asthma. Chest 2009, 136, 1514–1520. [Google Scholar]
- Van Schayck, O.C.P.; Haughney, J.; Aubier, M.; Selroos, O.; Ekström, T.; Ostinelli, J.; Buhl, R. Do asthmatic smokers benefit as much as non-smokers on budesonide/formoterol maintenance and reliever therapy? Results of an open label study. Respir. Med. 2012, 106, 189–196. [Google Scholar] [CrossRef]
- Clearie, K.L.; McKinlay, L.; Williamson, P.A.; Lipworth, B.J. Fluticasone/Salmeterol combination confers benefits in people with asthma who smoke. Chest 2012, 141, 330–338. [Google Scholar] [CrossRef]
- Thomson, N.C.; Chaudhuri, R. GINA recommendations in adults with symptomatic mild asthma and a smoking history. Eur. Respir. J. 2020, 55, 1902043. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Popov, T.A.; Bjermer, L.; Lu, S.; Petrovic, R.; Vandormael, K.; Mehta, A.; Strus, J.D.; Polos, P.G.; Philip, G. Effect of montelukast for treatment of asthma in cigarette smokers. J. Allergy Clin. Immunol. 2013, 131, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kaneko, Y.; Ishimatsu, A.; Komori, M.; Iwanaga, T.; Inoue, H. Effects of tiotropium on lung function in current smokers and never smokers with bronchial asthma. Pulm. Pharmacol. Ther. 2017, 42, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.C. Challenges in the management of asthma associated with smoking-induced airway diseases. Expert Opin. Pharmacother. 2018, 19, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.; Park, J.-Y.; Seo, D.-C. Comprehensive US Statewide Smoke-Free Indoor Air Legislation and Secondhand Smoke Exposure, Asthma Prevalence, and Related Doctor Visits: 2007–2011. Am. J. Public Health 2015, 105, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.S.; Hristakeva, S.; Gottlieb, M.; Baum, C.F. Reduction in emergency department visits for children’s asthma, ear infections, and respiratory infections after the introduction of state smoke-free legislation. Prev. Med. 2016, 89, 278–285. [Google Scholar] [CrossRef]
- Landers, G.M.; Ketsche, P.; Diana, M.L.; Campbell, C. County Smoke-Free Laws and Asthma Discharges: Evidence from 17 US States. Can. Respir. J. 2017, 2017, 6321258. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Y.; Liu, Y.-Q.; Liu, Y.; Sun, J.; Huang, M.-M.; Liu, Y.; Dong, G.-H. Asthma and asthma related symptoms in 23,326 Chinese children in relation to indoor and outdoor environmental factors: The Seven Northeastern Cities (SNEC) Study. Sci. Total Environ. 2014, 497–498, 10–17. [Google Scholar] [CrossRef]
- Kile, M.L.; Coker, E.S.; Smit, E.; Sudakin, D.; Molitor, J.; Harding, A.K. A cross-sectional study of the association between ventilation of gas stoves and chronic respiratory illness in U.S. children enrolled in NHANESIII. Environ. Health 2014, 13, 71. [Google Scholar] [CrossRef]
- Butz, A.M.; Matsui, E.C.; Breysse, P.; Curtin-Brosnan, J.; Eggleston, P.; Diette, G.; Williams, D.; Yuan, J.; Bernert, J.T.; Rand, C. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch. Pediatr. Adolesc. Med. 2011, 165, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, P.A.; Butz, A.; Rand, C.; Curtin-Brosnan, J.; Kanchanaraksa, S.; Swartz, L.; Breysse, P.; Buckley, T.; Diette, G.; Merriman, B.; et al. Home environmental intervention in inner-city asthma: A randomized controlled clinical trial. Ann. Allergy Asthma Immunol. 2005, 95, 518–524. [Google Scholar] [CrossRef]
- Morgan, W.J.; Crain, E.F.; Gruchalla, R.S.; O’Connor, G.T.; Kattan, M.; Evans, R.; Stout, J.; Malindzak, G.; Smartt, E.; Plaut, M.; et al. Results of a home-based environmental intervention among urban children with asthma. N. Engl. J. Med. 2004, 351, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Howden-Chapman, P.; Pierse, N.; Nicholls, S.; Gillespie-Bennett, J.; Viggers, H.; Cunningham, M.; Phipps, R.; Boulic, M.; Fjällström, P.; Free, S.; et al. Effects of improved home heating on asthma in community dwelling children: Randomised controlled trial. BMJ 2008, 337, a1411. [Google Scholar] [CrossRef]
- Pilotto, L.S.; Nitschke, M.; Smith, B.J.; Pisaniello, D.; Ruffin, R.E.; McElroy, H.J.; Martin, J.; Hiller, J.E. Randomized controlled trial of unflued gas heater replacement on respiratory health of asthmatic schoolchildren. Int. J. Epidemiol. 2004, 33, 208–214. [Google Scholar] [CrossRef]
- Noonan, C.W.; Semmens, E.O.; Smith, P.; Harrar, S.W.; Montrose, L.; Weiler, E.; McNamara, M.; Ward, T.J. Randomized Trial of Interventions to Improve Childhood Asthma in Homes with Wood-burning Stoves. Environ. Health Perspect. 2017, 125, 097010. [Google Scholar] [CrossRef]
- Burr, M.L.; Matthews, I.P.; Arthur, R.A.; Watson, H.L.; Gregory, C.J.; Dunstan, F.D.J.; Palmer, S.R. Effects on patients with asthma of eradicating visible indoor mould: A randomised controlled trial. Thorax 2007, 62, 767–772. [Google Scholar] [CrossRef]
- Tiotiu, A.; Metz-Favre, C.; Reboux, G.; Kessler, R.; de Blay, F. Hypersensitivity pneumonitis related to Penicillium chrysogenum and mesophilic Streptomyces: The usefulness of the Medical Indoor Environment Councelor (MIEC). Rev. Pneumol. Clin. 2013, 69, 278–282. [Google Scholar] [CrossRef]
| Pollutant | Concentration μg/m3 | Asthma Symptoms | Exacerbations | Hospitalizations | Lung Function |
|---|---|---|---|---|---|
| O3 | 100 (8-h mean) | - | ↑ | ↑ | ↓ |
| NO2 | 200 (1-h mean) | ↑ | ↑ | ↑ | ↓ |
| CO | 30 (1-h mean) | - | ↑ | - | - |
| SO2 | 20 (24-h mean) | ↑ | ↑ | ↑ | ↓ |
| PM2.5 | 10 (annual mean) 25 (24-h mean) | ↑ | ↑ | ↑ | ↓ |
| PM10 | 20 (annual mean) 50 (24-h mean) | ↑ | ↑ | ↑ | ↓ |
| Source | Asthma Symptoms | Exacerbations | Hospitalizations | Asthma Medication Use | Lung Function |
|---|---|---|---|---|---|
| Cigarette Smoke | |||||
| Active Smoking | ↑ | ↑ | ↑ | ↑ | ↓ |
| SHS | ↑ | ↑ | ↑ | ↑ | ↓ |
| Heating Sources | |||||
| Wood | ↑ | ↑ | ↑ | ↑ | ↓ |
| Gas | ↑ | ↑ | - | - | ↓ |
| Cooking Smoke | |||||
| Wood | ↑ | - | - | - | - |
| Coal | ↑ | - | - | - | ↓ |
| Molds | ↑ | ↑ | - | - | - |
| Recommendations to Minimise Air Pollution from Wood-Burning Heater |
|
| Recommendations to Minimise Air Pollution fromUFGHs |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. https://doi.org/10.3390/ijerph17176212
Tiotiu AI, Novakova P, Nedeva D, Chong-Neto HJ, Novakova S, Steiropoulos P, Kowal K. Impact of Air Pollution on Asthma Outcomes. International Journal of Environmental Research and Public Health. 2020; 17(17):6212. https://doi.org/10.3390/ijerph17176212
Chicago/Turabian StyleTiotiu, Angelica I., Plamena Novakova, Denislava Nedeva, Herberto Jose Chong-Neto, Silviya Novakova, Paschalis Steiropoulos, and Krzysztof Kowal. 2020. "Impact of Air Pollution on Asthma Outcomes" International Journal of Environmental Research and Public Health 17, no. 17: 6212. https://doi.org/10.3390/ijerph17176212
APA StyleTiotiu, A. I., Novakova, P., Nedeva, D., Chong-Neto, H. J., Novakova, S., Steiropoulos, P., & Kowal, K. (2020). Impact of Air Pollution on Asthma Outcomes. International Journal of Environmental Research and Public Health, 17(17), 6212. https://doi.org/10.3390/ijerph17176212







