Implementing a Clinical Research Department to Support Pediatric Studies: A SWOT Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Company Analysis
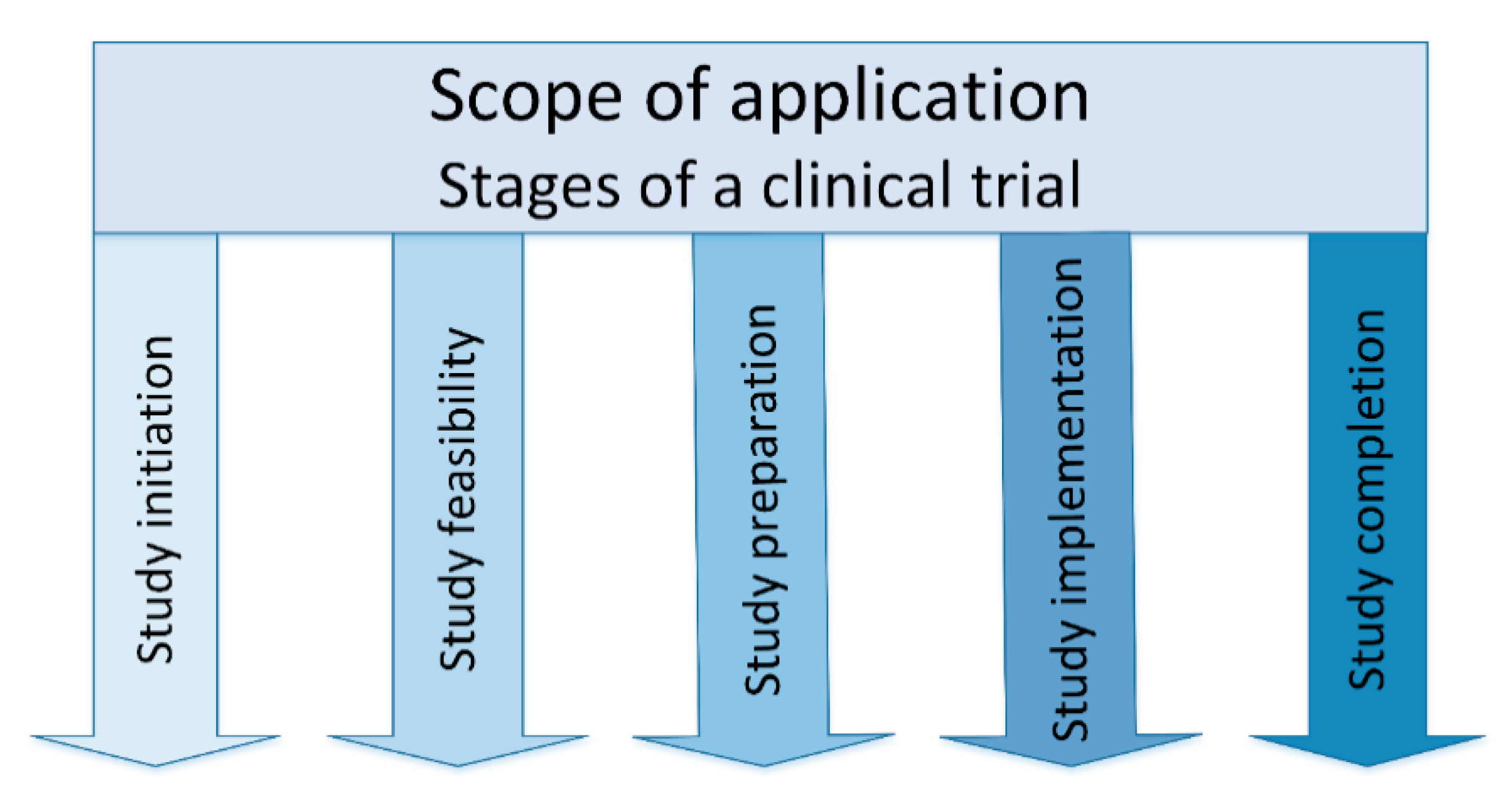
- Study initiation: In the stage of study initiation, a sponsor approaches potential investigators with the question of whether there is interest in conducting a study.
- Study feasibility: The feasibility of the planned clinical study is checked and evaluated in the next stage. The sponsor provides a synopsis of the study and the key facts, such as study title, objectives, study design, inclusion and exclusion criteria for the study patients, treatment, investigational product, study procedures, sample size and duration of study. The feasibility of the study, including the site feasibility, is evaluated using a questionnaire.
- Study preparation: The study preparation stage is very time-consuming and resource-intensive. The entire study team is determined on site and the study is reported to the local ethics committee (EC), the national competent authority (NCA) and the hospital management. The clinical trial agreement (CTA) and the patient insurance are concluded. The study team must attend Good Clinical Practice (GCP) [27,28] and randomization training, as well as training in how to handle the investigational medicinal product (IMP), including storage, that is usually provided online by the sponsor. In the study preparation stage, the investigational medicinal products including the necessary study materials, such as files, informed consent forms (ICFs), logs, laboratory materials, are sent to the study center.
- Study implementation: The study begins after all preparations are complete. The patient comes to the clinic for the study visits according to the clinical trial protocol and receives all study-related examinations and the investigational product (IP). The implementation stage starts with the initiation visit (IV), which is followed by interim (regular/routine monitoring) visits (RMV) [29].
- Study completion: The final stage is the completion of the study with the last monitoring visit, the so-called close out visit (COV) [29]. The end of the study must be reported to the ethics committee, the responsible national competent authority and the hospital management.
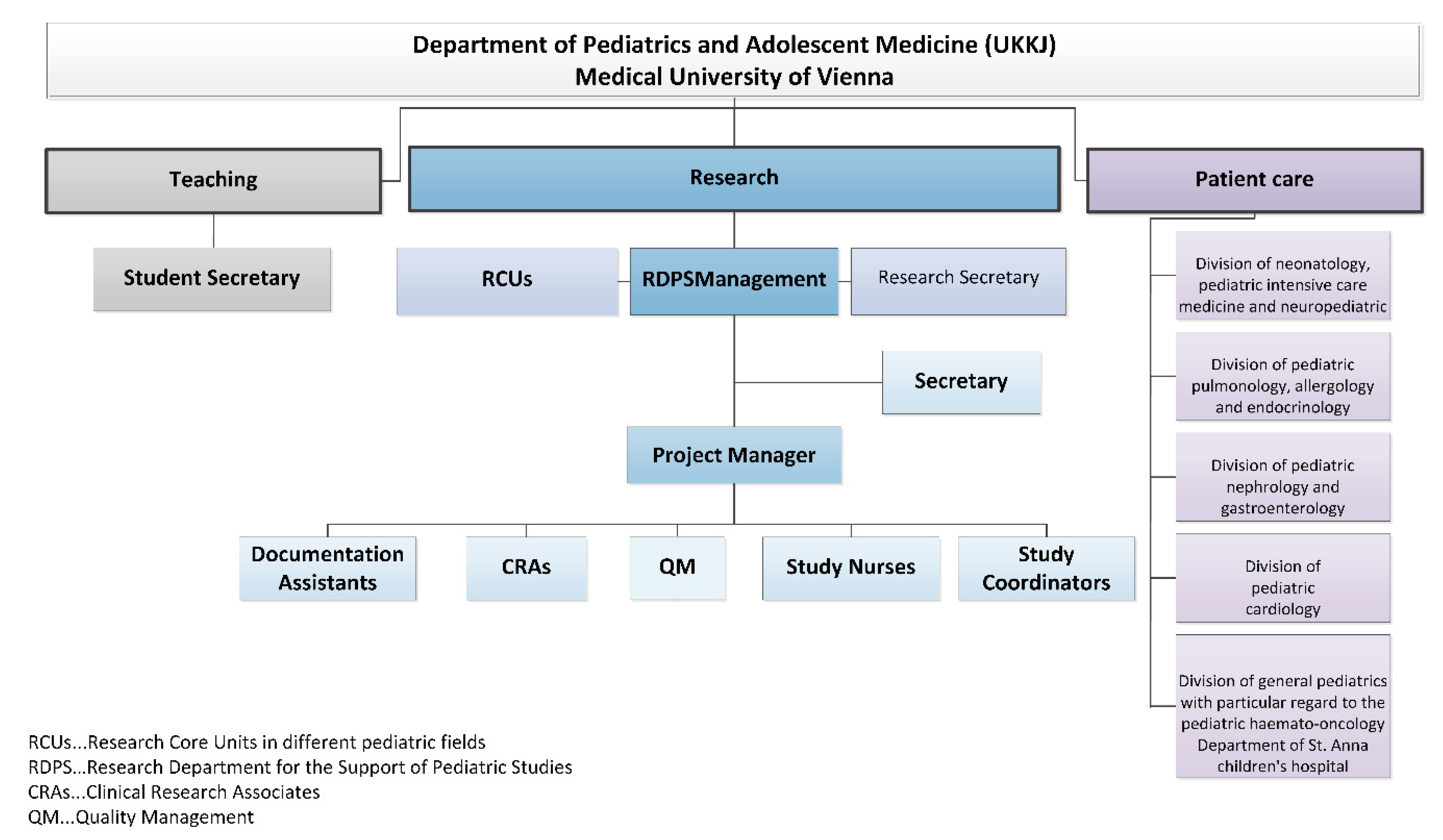
- RDPS management: In addition to staff and cost responsibility, RDPS management is also responsible for the promotion of employees and the study development and management.
- Secretary: The secretary provides administrative support to management.
- Project manager: The project manager (PM) represents the interface between RDPS management and other employees. The main tasks of the project manager comprise communication, advice (initiation and feasibility of studies), support (conduct of studies), review (study documents) and management. Management tasks comprise support of pharmacovigilance and evaluation of resources, budget, study contract, standardized operation procedures and monitoring plans.
- Documentation assistants: The documentation assistants are responsible for the entire documentation process of patient records in paper form, the collection of patient data from the archive or from the electronic system of the Vienna General Hospital. The Vienna General Hospital is the public hospital with which the Medical University of Vienna cooperates in performing their clinical research, patient care and teaching tasks. The documentation assistants are in charge of data entry into the study-related (electronic) case report forms (CRFs).
- Study coordinators: The study coordinators are responsible for the overall communication and coordination in all stages of a study. They represent the interface between the sponsor and the entire study team. The study coordinators support the completion of feasibility questionnaires, the process of drafting contracts and compliance with regulatory requirements and are responsible for shipping inspection, documentation, archiving, communication with suppliers, to name just a few.
- Study nurses: A study nurse plays a key role in conducting clinical trials and represents an important interface between the monitor and the study team. The focus is on the activities with, on and for the patient and everything related to the investigational medicinal product.
- Clinical research associates: The clinical research associate (CRA) is responsible for quality assurance and developing a monitoring plan for each clinical trial. The preparation, implementation and follow-up of pre-study visits, initiation visits, routine monitoring visits and close-out visits is the responsibility of a clinical research associate. Other tasks include monitoring of the investigator site file (ISF) and the trial master file (TMF), regulatory affairs, source data verification, data quality verification, patient insurance, laboratory, pharmacy and documenting adverse events. The monitor represents the interface between the sponsor and the study team.
- Quality management: With quality management (QM), all activities within the RDPS should be coordinated in such a way that quality can be ensured, checked and if necessary improved and that quality objectives can be met.
3.2. Environmental Analysis
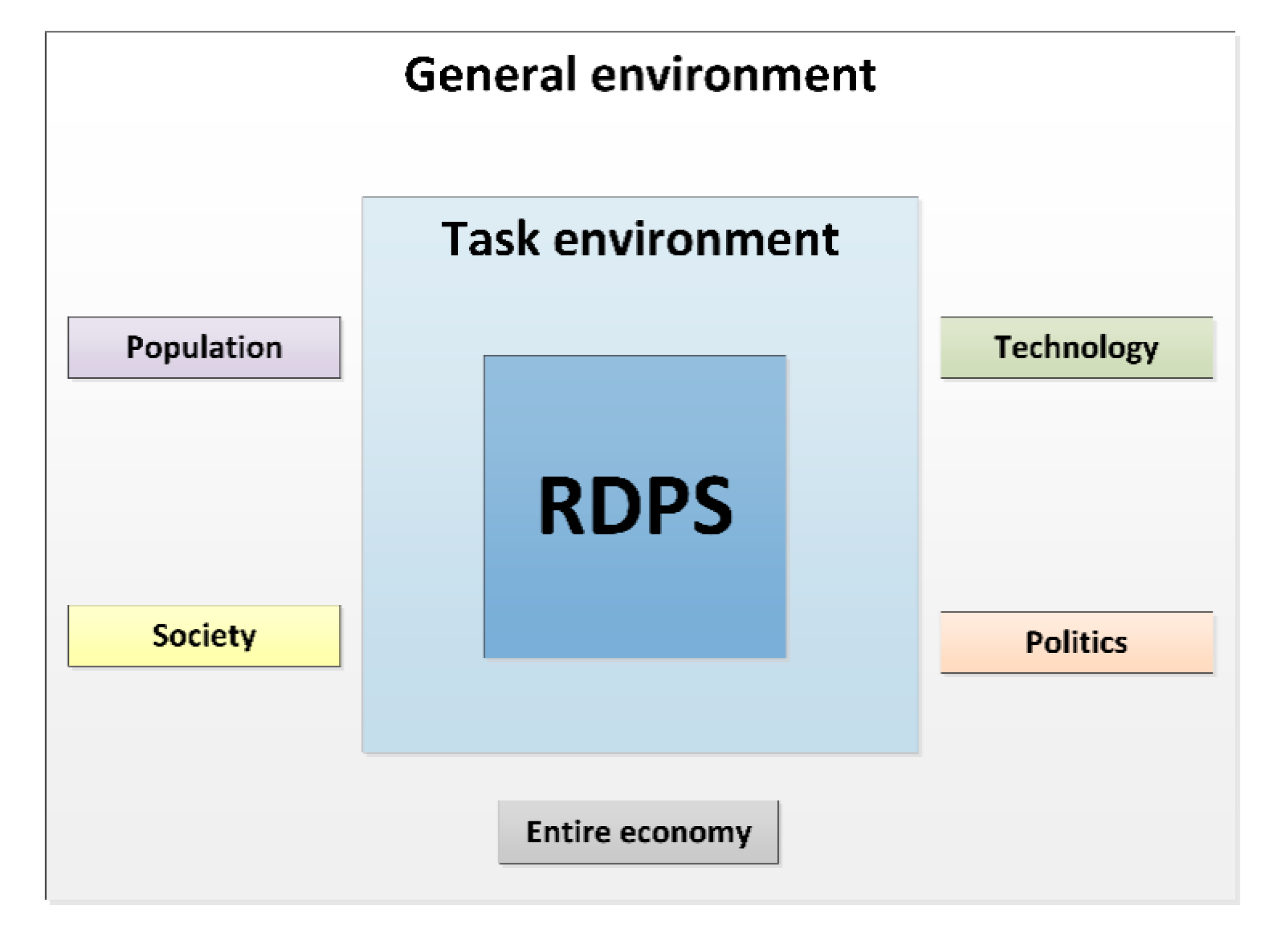
3.2.1. General Environment
- Population: Demographic factors, such as the availability of the pediatric study sample, are relevant for the selection as a study center. The population growth, which can also be attributed to migration, affects the spectrum of diseases that otherwise only occur in certain geographic regions. The Vienna General Hospital specializes in high-risk pregnancies. The sophisticated medical methods, such as those in neonatology, are the reason why extremely low birthweight infants and children with rare congenital diseases can survive.
- Society: Society’s understanding of the importance, necessity and significance of pediatric studies is constantly increasing. Nevertheless, the general population should be made even more sensitive to the importance of pediatric studies, e.g., in the form of access to research results in an easily understandable form. Medical experts also benefit by gaining experience and insight into how to use a new drug, which is then used in the particular indication and the corresponding patient sample. The transparent evidence-based approach not only promotes trust in pediatric clinical trials but also in subsequent treatment paths.
- Technology: Studies not only require working according to the “state of the art”, but rather working “beyond the state of the art”, which leads to scientific added value. Studies accelerate innovations that, through novel drugs, medical devices and therapeutic strategies, represent unique opportunities for improving medical care for future patients. A balance between sponsored clinical trials and competitive third-party funding for academic studies, however, is important. In Austria, the national research ratio, i.e., the research and development expenditures as a percentage of gross domestic product (GDP), amounted to 3.16 percent in the last decade [32]. This figure is above the European target (3 percent), thereby-comparable to some other European countries, such as Germany and Sweden - illustrating that Austria is an attractive study location.
- Politics: Medical universities have a research mission. The successful completion of studies reflects the high performance of clinical research, which in turn promotes Austria as a successful location for medicine and research. The UKKJ at the Medical University of Vienna is internationally recognized as a study center and is selected based on experience, expertise and patient population. When multi-center studies are carried out, cooperation between medical universities and hospitals is promoted at national and international level.
- Entire economy: The number of pediatric clinical trials is increasing. A higher number of studies usually goes hand in hand with an increased approval of pharmaceuticals and medical devices. This has immediate impact on the economy: Out of 5000–10,000 tested initial substances, only a single drug gets approved [32]. Pharmaceutical development takes about 10–12 years, with development costs of up to 2.4 billion Euros. In 2018, 84 new pharmaceuticals were approved in Europe. Between 2014 and 2018, an average of 41 new pharmaceuticals received marketing authorization in Austria [32]. Since 2007, 238 new drugs for the use on children and 39 child-friendly dosage forms have been approved [33].The increase in studies also requires human resources, i.e., research is an important employer for scientists, physicians, study nurses, study coordinators, clinical research associates, clinical research organizations, ethics committees, authorities, foundations, and pharmaceutical companies. Research also supports patient care with new scientific findings by uncovering direct and indirect efficiency potential.
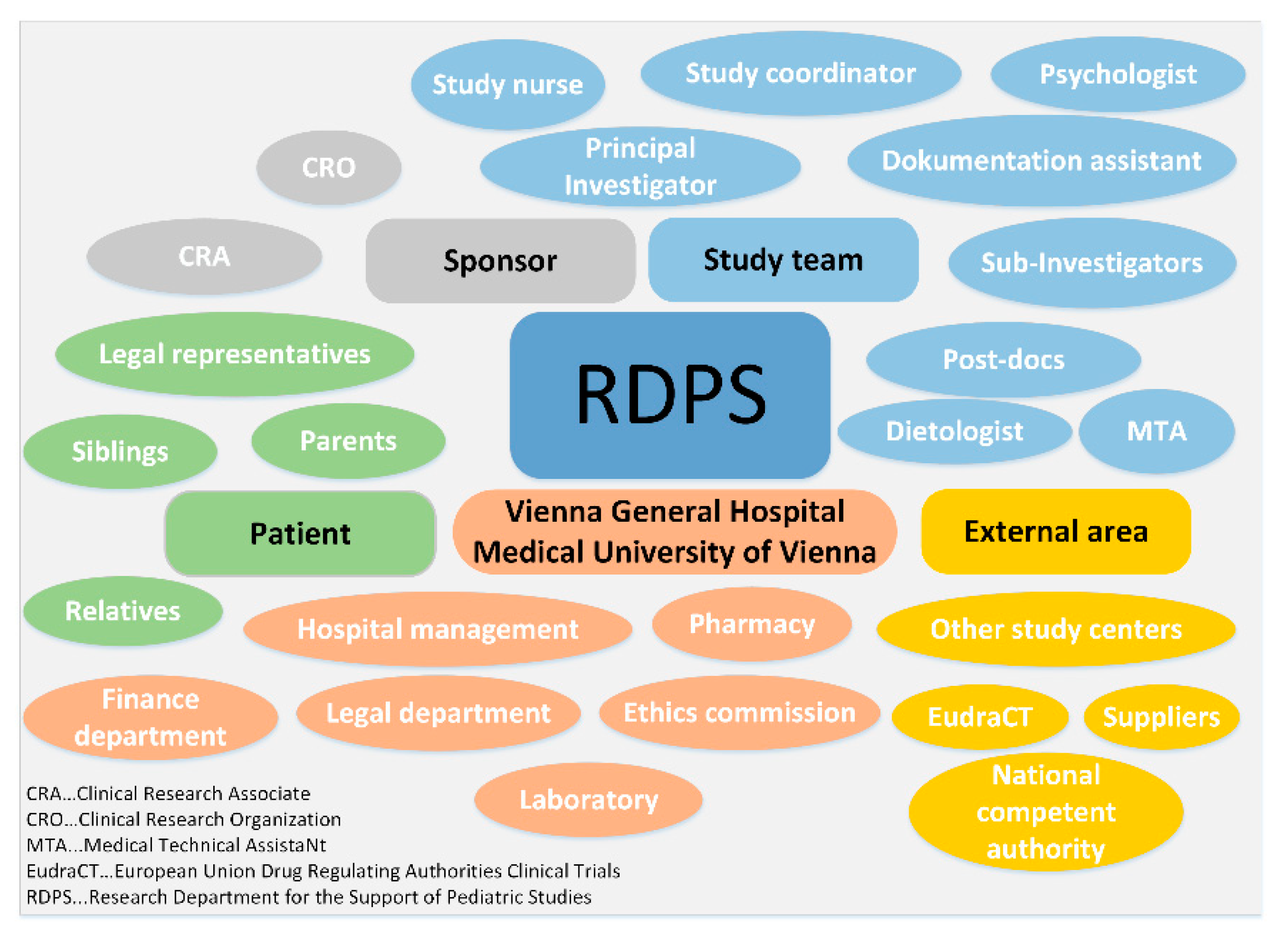
3.2.2. Task Environment
- Sponsor: The RDPS works with the sponsor at every stage of a clinical trial. Since the sponsor has many employees with different tasks, which in turn places high demands on communication, the sponsor often commissions clinical research organizations to plan, prepare and conduct a study. These clinical research organizations, also called contract research organizations (CROs), specialize in studies according to the Medicinal Products Act and the Medical Devices Act. The sponsor or clinical research organization has either its own or an external clinical research associate. The clinical research organization and the clinical research associate are therefore part of the close environment of the sponsor and thus of the RDPS. Staff fluctuations at the sponsor or the clinical research organization or a change of the clinical monitor lead to considerable information gaps. This adds to the workload and can affect the smooth running of the study. A RDPS, however, provides support in the event of sponsor-related staff fluctuations.
- Study team: There is a very close cooperation between the RDPS and the study team. At the stage of study initiation, there is mainly contact with the principal investigator (PI). After that, contact with the entire study team (sub-investigators, dietologist, psychologist, post-docs, medical technical assistant) is necessary, especially to plan, prepare and coordinate the visits with the study team so that all examinations can be carried out for each study visit. At the stage of study implementation, individual RDPS employees (study nurse, study coordinator, documentation assistant) are optionally members of the study team.
- Patient: Pediatric studies affect not only the patients, but also their families. Parents or legal representatives are involved in the entire study process. In addition, siblings and other relatives are part of the child’s environment and thus the RDPS.
- Vienna General Hospital/Medical University of Vienna: The Vienna General Hospital and the Medical University of Vienna are also part of the task environment of the RDPS. An important point of contact is the legal department to review the clinical trial agreement (CTA) between sponsor and the Medical University of Vienna. Although sponsors often use sample agreements that are already used for other studies at the Medical University of Vienna, adjustments to the sample agreements are often necessary before both contracting parties agree. The ethics committee, the hospital management, the finance department, the laboratory and the pharmacy are also part of the task environment.
- External area: The external area comprises the national competent authority, the different European drug regulating authorities, the European Medicines Agency (EMA), which administers the clinical trials database (EudraCT), other study centers, and external suppliers.
3.3. SWOT Analysis
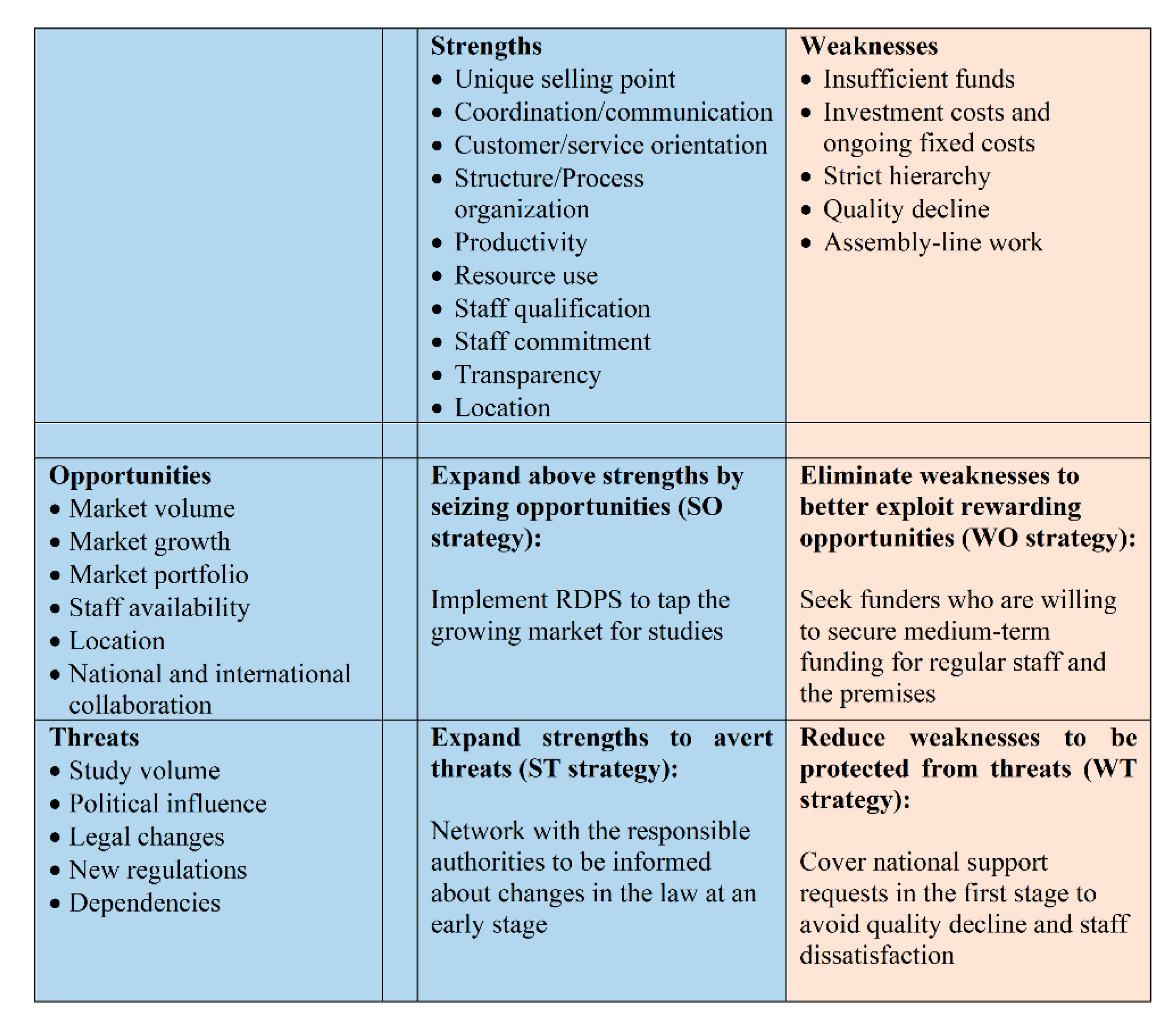
- Strengths: There are several strengths when implementing a RDPS. However, due to the wide range of tasks and the variety of internal and external people involved, we refrain from discussing the strengths (and later on the weaknesses) along the management and service delivery sub-systems, as suggested in the strategic management approach.Since a RDPS team consists of employees, who have many years of experience in pediatric clinical studies and have contacts to experts and potential national and international partners, extensive and well-founded advice and support for studies is ensured. A high level of professionalism among team members can be assured for a team with clearly assigned tasks through relevant and continuous further education and training in connection with clinical studies. Clearly defined processes as well as clear responsibilities and work tasks of the employees not only lead to high efficiency, flexibility and service orientation, but also have a positive effect on the collaboration with cooperation partners such as pharmaceutical companies, study teams, patients and legal representatives. The own area of responsibility and the possibility of working independently promote employee satisfaction and reduce staff fluctuations, so that optimal conditions are created to keep competent employees and their know-how in the company. The many stakeholders involved in the different stages of a study also benefit from a trusted contact person at the study center.Professional study support is not limited to pharmaceutical studies. Hence, other studies with a high workload also benefit from the support of a RDPS. Another strength is that many different indications and studies with large and low sample sizes (e.g., rare diseases) can be carried out. A RDPS ensures that laws, regulations, and national and international guidelines are observed. The easy accessibility of the UKKJ is another advantage, not least because many patients have to undergo routine and control examinations at the UKKJ anyway.
- Weaknesses: The weaknesses include the high personnel costs and the hierarchical structure that is required for the organizational and internal processes for the planning and implementing of studies. If too many studies are supported at the same time, quality loss can occur. If resources are insufficient, study requests run the risk of being rejected. The standardization of processes can give employees the impression of assembly line work. Although, a high degree of standardization increases efficiency in the different stages of a study, there is a risk that the individual needs of stakeholders are not adequately addressed.
- Opportunities: The RDPS would be the first research department to support pediatric studies at a medical university in Austria. This pioneering role ensures a very high market coverage, since basically all types of studies (drug studies, medical device studies, non-interventional drug studies, registry studies, epidemiological studies, academic or industry sponsored studies) can be supported. Although no funding is currently planned to support basic research, a RDPS can provide support if the need arises. While the focus is on successfully setting up a RDPS in Vienna, this RDPS serves as a role model for setting up additional RDPS at other medical universities in Austria.The integration of the RDPS into the Medical University of Vienna opens up a wider range of opportunities for employees to undergo further training on site with regard to clinical studies. At the Medical University of Vienna, employees are offered numerous seminars free of charge, such as training on medicinal products, medicinal devices, good clinical practice, study design, pharmacovigilance, and analysis and interpretation of clinical trials. Another option for further training is participation in congresses, symposia, external workshops and seminars. There are also comprehensive training and career opportunities for young people, such as medical students, who can be recruited at an early stage of their study as documentation assistants. The time-flexible tasks of a documentation assistant can easily be combined with the six-year medical studies in Austria. When students participate in pediatric trials during their medical studies, they gain insight and experience in the field. The close collaboration with the principal investigators also gives the students the opportunity to get to know potential supervisors of theses. This offers the opportunity to complete the diploma thesis in the field of pediatrics. As graduates, they will already have extensive experience in pediatric studies, which is a competitive advantage when looking for a job, especially at a medical university.With regard to communication and collaboration with the many different stakeholders, the RDPS enables efficient and effective working through clearly structured processes and work instructions. Smooth processes are guaranteed by ensuring that contact persons are always available. This facilitates the work of investigators and sub-investigators in that they can primarily deal with their clinical activities.Existing institutions should not be seen as competitors but as potential cooperation partners. In order to be able to use synergy effects, collaborations with the KKS and the Austrian OKIDS network should be sought.OKIDS offers one study nurse per location. However, OKIDS is third party funded, so the availability of the OKIDS study nurse and OKIDS in general is dependent on ongoing third-party funding. The limited funding also implies that the OKIDS study nurse can only be employed 30 h per week. Given the current workload and the continued increase in drug trials, this is not enough. Additionally, since OKIDS only supports drug studies, support is limited to this type of study. The RDPS can support the OKIDS study nurse. This creates synergy effects between OKIDS and the RDPS so that the aim of increasing pediatric drug trials at the UKKJ can be achieved.Since the Vienna KKS concentrates exclusively on studies with adults, the KKS is not a direct competitor. We expect that the cooperation with this institution will also result in synergies. The Vienna KKS can forward any pediatric study request to the RDPS, while the RDPS can work continuously with the KKS on patient insurance. If electronic care report forms (eCRFs) or queries to pharmacovigilance are required, the KKS Vienna is a very good service provider. Since KKS charges a fee per service, KKS also benefits from a collaboration with the RDPS. A RDPS therefore not only increases the number of studies, it also increases the attractiveness of the UKKJ, the Medical University and the Vienna General Hospital as a place of study.With the establishment of a RDPS, there is also the possibility of flexible structuring of employment contracts. This enables nurses, who are currently working in a strictly clinical routine and who want to change careers to work as study nurses with new and challenging tasks. However, this option should not be limited to permanent staff, but should also be offered to freshly graduated nurses in order to attract highly motivated and qualified staff.In the medium term, the expansion of the RDPS should also be considered. With more staff and adequate training, application preparation, third party funding, budget planning, medical writing and the publication process can also be supported.
- Threats: Personnel costs are a high-risk factor from an external perspective as well. The key personnel (RDPS management, project manager, study coordinator, and study nurse) should hold permanent positions that are publicly funded and thus covered by the university’s budget. However, cross financing of other employees such as documentation assistants must also be guaranteed. It should also be borne in mind that it can be difficult to find competent and qualified personnel. The high level of flexibility and commitment required can also be seen as a hurdle in this regard, since the respective area of responsibility is very complex.The availability and cost of the premises are a further risk factor as there must be a sufficient number of rooms for employees, meetings (face-to-face, video and telephone conferences), monitoring visits and for the storage of documents and investigational medicinal products. Study documents, investigator site files and trial master files must be kept locked. In addition, all essential documents must be retained for at least 15 years after the completion of the clinical study [34].Although it is quite unlikely, a RDPS could be a risk factor in the form of high overhead if the core tasks of the Medical University change in such a way that research is no longer one of the core competencies. However, there is a greater likelihood of a shortage of doctors and nurses in pediatrics, as the field of pediatrics is less attractive for doctors from a financial point of view than other medical subjects and nurses have to undergo additional training.
- SWOT Matrix: The strengths and weaknesses as well as the opportunities and risks of the SWOT analysis must be translated into a SWOT matrix, which then forms the basis for strategy development. However, the development of strategies is the responsibility of the decision-makers at the Medical University of Vienna and was therefore not the aim of the present study. For this reason, one exemplary strategy is provided per field (Figure 5).
4. Discussion
- Number of studies: There are several reasons for the increase in studies at the UKKJ in Vienna. In 2013, OKIDS, the Austrian research network for pediatric drug studies, was implemented at the Department of Pediatrics and Adolescent Medicine in Vienna. As a result, more drug trials were carried out and the study teams on site acquired specialist knowledge for the successful implementation of studies. This in turn led to highly satisfied investigators and sponsors, which increased the number study requests. Another reason is the research mandate of the medical universities in Austria. At the Medical University of Vienna, just like at other medical universities, research is a cornerstone in addition to teaching and patient care. This in turn creates the incentive to conduct studies and, as a result, to acquire third party funds in order to deliver a corresponding research output. Young scientists in particular need support in this regard.EU Regulation (EC) No. 1901/2006 supports pediatric drug studies so that children and adolescents receive specially tested and approved drugs [3], which in itself will lead to an increase in the number of studies. In addition, Vienna has a diverse and large clientele for studies compared to other cities, which is why the UKKJ is considered as an attractive site for the study center.
- Ethical and legal norms: Children and adolescents are a particularly vulnerable patient population. Therefore, comprehensive guidelines and regulations must be followed, and additional monitoring carried out, which increases the need for support. We assume that the increase in ethical and legal norms observed in the past will continue in the next few years in order to expand the protection of the vulnerable patient collective. Pediatric studies are complex and resource-intensive. Investigators and study teams do not always know what needs to be considered in clinical studies. A RDPS is therefore not only available as a service provider, but also as a control body to ensure that all ethical and legal standards are observed.For clinical studies, the sponsor is obliged to conduct monitoring visits at the study center. The Medical University of Vienna is the sponsor for academic studies. However, monitoring is costly. Clinical research organizations or the Vienna KKS can be commissioned for this, but they are cost-intensive. Since the budget for academic studies is generally limited and a RDPS also offers monitoring, the Medical University of Vienna incurs not only additional costs when implementing a RDPS, but also savings because the monitoring can be offered by the RDPS at significantly lower costs. However, monitoring services depend on the RDPS resources, the priority, the study type, the study protocol and the sample size. For example, monitoring is not required by law for non-clinical studies, such as registry or epidemiological studies, but is recommended. If sufficient resources are available, a RDPS can also support these study types as a monitoring body.
- Financing: In times of limited resources, special attention is paid to the financing of a RDPS. The key staff consists of the RDPS management and at least one project manager, a study coordinator, a study nurse and a clinical research associate. Publicly funded positions should be created for key personnel, and the following options are available for financing other employees: A start-up fee can be negotiated for industry-sponsored studies. The start-up fee covers the services provided by the RDPS for the entire support right up to the initiation of the study. Charging a fee per patient is another financing option. Another source of funding is the monitoring. The sponsor can save the entire travel expenses for monitoring visits by commissioning a RDPS monitor. This is a great financial advantage for the sponsor, as well as for the RDPS, which means that funds are re-acquired and some of it can be passed on to the RDPS. However, services provided by a study nurse, a study coordinator or a documentation assistant are available exclusively for a single study and have to be budgeted accordingly.
- Limitations: As a typical strategic management tool, SWOT analysis was originally designed for private profit-oriented organizations. However, in view of the growing challenges in healthcare, including the aging of the population, the rapid technological change, and the increase in healthcare expenditure while financial resources are increasingly limited, strategic planning has also been used by healthcare organizations. For a discussion of the pros and cons of strategic planning in healthcare organizations see Rodríguez Perera and Peiró [35]. As indicated by Chermack and Kasshanna, if adequately used, SWOT analysis provides support in deriving appropriate strategies to meet overall goals by exploiting the organization’s strengths and the environmental opportunities and mitigating the organization’s weaknesses and environmental threats [36]. However, as with any other tool, pitfalls have also been identified in the use of SWOT analysis, not least because the user has a considerable leeway in performing a SWOT analysis. The authors argued that a “critical flaw in the development of SWOT analysis as a solid and reliable strategic tool is a lack of research” [36], thereby criticizing that theory building for SWOT analysis is based on empirical applications only. They also offered protocols to address various pitfalls in the uses of SWOT analysis. Pitfalls include, among others, the use of SWOT analysis as justification for decisions already made; the negligence of the close relationship between the results of the SWOT analysis and the subsequently derived strategy; the failure to link the results of the company analysis with those of the environmental analysis; and deriving strategies before all strategic options have been identified. For the comprehensive list of pitfalls see, e.g., Koch [37] and Kearns [38].
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.A.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.; Greenland, S.; Hlatky, M.A.; Khoury, M.J.; Macleod, M.R.; Moher, D.; Schulz, K.F.; Tibshirani, R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014, 383, 166–175. [Google Scholar] [CrossRef]
- Regulation (EC). No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on Medicinal Products for Paediatric Use and Amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004; The European Parliament and the Council of the European Union: Strasbourg, France, 2006; pp. 1–31. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006R1901 (accessed on 10 June 2020).
- Moulis, F.; Durrieu, G.; Lapeyre-Mestre, M. Off-label and unlicensed drug use in children population. Therapie 2018, 73, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Klassen, T.P.; Hartling, L.; Hamm, M.; van der Lee, J.H.; Ursum, J.; Offringa, M. StaR child health: An initiative for RCTs in children. Lancet 2009, 374, 1310–1312. [Google Scholar] [CrossRef]
- Kimland, E.; Odlind, V. Off-label drug use in pediatric patients. Clin. Pharmacol. Ther. 2012, 91, 796–801. [Google Scholar] [CrossRef]
- Kimland, E. Off-label and unlicensed drug use in children. Paediatr. Int. Child. Health 2014, 34, 1–2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magalhaes, J.; Rodrigues, A.T.; Roque, F.; Figueiras, A.; Falcao, A.; Herdeiro, M.T. Use of off-label and unlicenced drugs in hospitalised paediatric patients: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Neubert, A.; Rascher, W. Medication safety in children: What role do dosing and formulations play? Bundesgesundheitsblatt Gesundh. Gesundh. 2018, 61, 1139–1145. [Google Scholar] [CrossRef]
- Martinez-Castaldi, C.; Silverstein, M.; Bauchner, H. Child versus adult research: The gap in high-quality study design. Pediatrics 2008, 122, 52–57. [Google Scholar] [CrossRef]
- Tretter, F.; Löffler-Stastka, H. Medical knowledge integration and “systems medicine”: Needs, ambitions, limitations and options. Med. Hypotheses 2019, 133, 109386. [Google Scholar] [CrossRef]
- Tretter, F.; Löffler-Stastka, H. The human ecological perspective and biopsychosocial medicine. Int. J. Environ. Res. Public Health 2019, 16, 4230. [Google Scholar] [CrossRef] [PubMed]
- Croghan, I.T.; Viker, S.D.; Limper, A.H.; Evans, T.K.; Cornell, A.R.; Ebbert, J.O.; Gertz, M.A. Developing a clinical trial unit to advance research in an academic institution. Contemp Clin. Trials 2015, 45 (Pt B), 270–276. [Google Scholar] [CrossRef]
- Helms, M.M.; Nixon, J.V. Exploring SWOT analysis—Where are we now? A review of academic research from the last decade. J. Strategy Manag. 2010, 3, 215–251. [Google Scholar] [CrossRef]
- Pritchard, M.A.; Colditz, P.B.; Beller, E.M.; Queensland Optimising Preterm Infant Outcomes Group. Parental experiences and preferences which influence subsequent use of post-discharge health services for children born very preterm. J. Paediatr. Child Health 2008, 44, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Camden, C.; Swaine, B.; Tetreault, S.; Carriere, M. Going beyond the identification of change facilitators to effectively implement a new model of services: Lessons learned from a case example in paediatric rehabilitation. Dev. Neurorehabil. 2011, 14, 247–260. [Google Scholar] [CrossRef]
- Ferre-Eguiluz, I.; Buccini, G.; Hromi-Fiedler, A.; Rovelo, N.; Gonzalez de Cosio, T.; Perez-Escamilla-Costas, J.R.; Perez-Escamilla-Gonzalez, J.R.; Perez-Escamilla, R. Content analysis of media coverage of breastfeeding in Mexico. Matern. Child Nutr. 2020, 16, e12905. [Google Scholar] [CrossRef]
- Orzan, E.; Ruta, F.; Bolzonello, P.; Marchi, R.; Ceschin, F.; Ciciriello, E. Childhood hearing surveillance activity in Italy: Preliminary recommendations. Acta Otorhinolaryngol. Ital. 2016, 36, 15–20. [Google Scholar]
- Lawrence, G.F. The SWOT Analysis: Using Your Strength to Overcome Weaknesses, Using Opportunities to Overcome Threats; CreateSpace Independent Publishing Platform: North Charleston, SC, USA, 2010; p. 78. Available online: https://ogur.org/uploads-the-swot-analysis-using.pdf (accessed on 26 June 2020).
- Pahl, N.; Richter, A. SWOT Analysis. Idea, Methodology And A Practical Approach; GRIN Verlag GmbH: Nordestedt, Germany, 2007. [Google Scholar]
- Harrison, J.P. Essentials of Strategic Planning in Healthcare; Health Administration Press: Chicago, IL, USA, 2010. Available online: https://www.ache.org/learning-center/publications/books/2420I (accessed on 27 June 2020).
- Bea, F.X.; Haas, J. Umweltanalyse. In Strategisches Management; 10 Auflage; Bea, F.X., Scheurer, S., Eds.; UVK Verlag: München, Germany, 2019; pp. 99–132. [Google Scholar]
- Bea, F.X.; Haas, J. Unternehmensanalyse. In Strategisches Management; 10 Auflage; UKV Verlag: München, Germany, 2019; pp. 133–182. [Google Scholar]
- Böhm, A. The SWOT Analysis, 1st ed.; GRIN Verlag GmbH: Norsestedt, Germany, 2008. [Google Scholar]
- Hill, T.; Westbrook, R. SWOT analysis: It’s time for a product recall. Long Range Plan. 1997, 30, 46–52. [Google Scholar] [CrossRef]
- Van Wijngaarden, J.D.; Scholten, G.R.; van Wijk, K.P. Strategic analysis for health care organizations: The suitability of the SWOT-analysis. Int. J. Health Plann. Manag. 2012, 27, 34–49. [Google Scholar] [CrossRef]
- GCP. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Guideline E6: Note for Guidance on Good Clinical Practice (GCP); European Medicines Agency: London, UK, 1996; pp. 1–59. Available online: https://ichgcp.net/ (accessed on 29 June 2020).
- GCP. Guideline for Good Clinical Practice E6(R2); European Medicines Agency, Committee for Human Medicinal Products, European Medicines Agency: London, UK, 2016; pp. 1–68. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf (accessed on 30 June 2020).
- Molloy, S.F.; Henley, P. Monitoring clinical trials: A practical guide. Trop. Med. Int. Health 2016, 21, 1602–1611. [Google Scholar] [CrossRef]
- Crossan, M.M.; Fry, J.N.; Killing, J.P. Strategic Analysis and Action, 6th ed.; Prentice Hall: Toronto, ON, Canada, 2005. [Google Scholar]
- International Organization for Standardization. ISO 9001:2015(en) Quality Management Systems—Requirements. Available online: https://www.iso.org/obp/ui/#iso:std:iso:9001:ed-5:v1:en (accessed on 4 August 2020).
- Pharmig. Daten und Fakten 2019—Arzneimittel und Gesundheitswesen in Österreich; Verband der Pharmazeutischen Industrie Österreichs: Wien, Austria, 2019; pp. 1–104. [Google Scholar]
- EMA. 10-year Report to the European Commission. General Report on the Experience Acquired as a Result of the Application of the Paediatric Regulation; European Medicines Agency and its Paediatric Committee, European Medicines Agency: London, UK, 2016; pp. 1–97. Available online: https://ec.europa.eu/health/sites/health/files/files/paediatrics/2016_pc_report_2017/ema_10_year_report_for_consultation.pdf (accessed on 30 June 2020).
- EMA. Guideline on the Content, Management and Archiving of the Clinical Trial Master File (Paper and/or Electronic); European Medicines Agency: London, UK, 2018; pp. 1–17. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-content-management-archiving-clinical-trial-master-file-paper/electronic_en.pdf (accessed on 30 June 2020).
- Rodriguez Perera, P.; Peiro, M. Strategic planning in healthcare organizations. Rev. Esp. Cardiol. (Engl. Ed.) 2012, 65, 749–754. [Google Scholar] [CrossRef]
- Chermack, T.J.; Kasshanna, B.K. The use and misuse of SWOT analysis and implications for HRD professionals. Hum. Resour. Dev. Int. 2007, 10, 383–399. [Google Scholar] [CrossRef]
- Koch, A. SWOT Does Not Need to Be Recalled: It Needs to Be Enhanced. Available online: https://www.westga.edu/~bquest/2000/swot1.html (accessed on 5 August 2020).
- Kearns, K.K. From comparative advantage to damage control: Clarifying strategic issues using swot analysis. Nonprofit Manag. Leadersh. 1992, 3, 3–22. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thajer, A.; Sommersguter-Reichmann, M.; Löffler-Stastka, H. Implementing a Clinical Research Department to Support Pediatric Studies: A SWOT Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6211. https://doi.org/10.3390/ijerph17176211
Thajer A, Sommersguter-Reichmann M, Löffler-Stastka H. Implementing a Clinical Research Department to Support Pediatric Studies: A SWOT Analysis. International Journal of Environmental Research and Public Health. 2020; 17(17):6211. https://doi.org/10.3390/ijerph17176211
Chicago/Turabian StyleThajer, Alexandra, Margit Sommersguter-Reichmann, and Henriette Löffler-Stastka. 2020. "Implementing a Clinical Research Department to Support Pediatric Studies: A SWOT Analysis" International Journal of Environmental Research and Public Health 17, no. 17: 6211. https://doi.org/10.3390/ijerph17176211
APA StyleThajer, A., Sommersguter-Reichmann, M., & Löffler-Stastka, H. (2020). Implementing a Clinical Research Department to Support Pediatric Studies: A SWOT Analysis. International Journal of Environmental Research and Public Health, 17(17), 6211. https://doi.org/10.3390/ijerph17176211





