Hajdu–Cheney Syndrome: A Systematic Review of the Literature
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search
2.4. Data Collection Process
2.5. Study Selection
2.6. Data Collection Process and Data Items
2.7. Synthesis of the Results
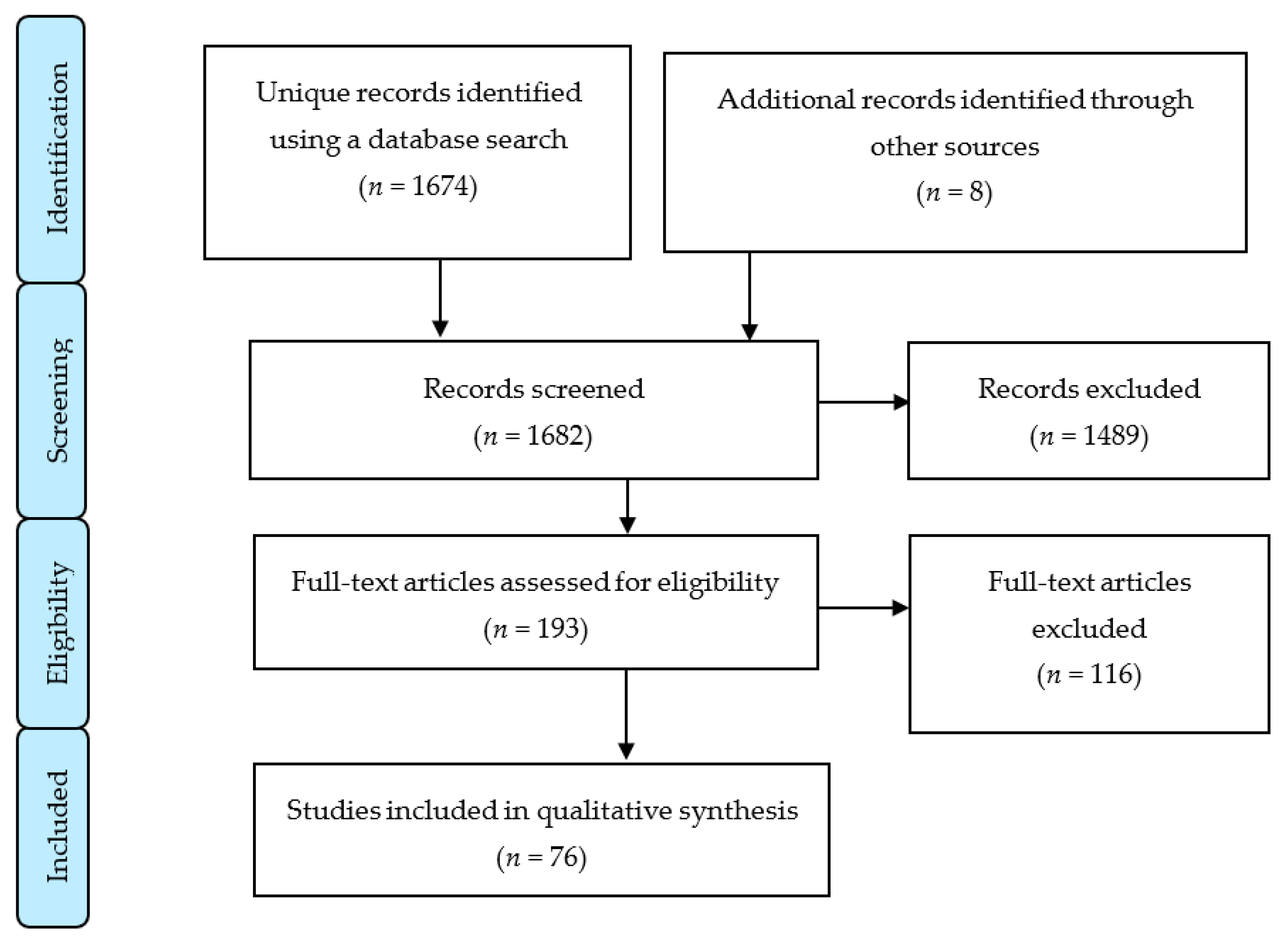
3. Results
3.1. Study Selection
3.2. Disease Genetics
3.3. Description of the Disease and Evolution of the Phenotype
3.4. Diagnosis and Differential Diagnosis
- -
- Acroosteolysis plus three clinical manifestations, except for a documented positive family history.
- -
- Acroosteolysis plus a documented positive family history.
- -
- Documented positive family history plus two other manifestations, except for acroosteolysis.
- -
- Four clinical manifestations, except for a documented positive family history.
- -
- Documented positive family history plus two manifestations.
3.5. Treatment
4. Discussion
4.1. Summary of Evidence
4.1.1. Epidemiology
4.1.2. Etiology
- CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy): mutations of EGF-like repeats of NOTCH3.
- Bicuspid aortic valve: protein-truncating mutations of NOTCH1.
- LAL-T: protein-truncating mutations of the PEST domain of NOTCH1.
- Alagille syndrome: mutation of the splice acceptor of exon 33 of NOTCH2.
4.1.3. Pathophysiology
4.1.4. Clinical Manifestations
- Cranial alterations: bathrocephaly, presence of multiple wormian bones, delayed suture closure, thickened dome of the skull, absent frontal sinuses, elongated sella turcica, small jaw, basilar invagination, dolichocephaly, and occipital prominence.
- Facial alterations: coarse and dysmorphic facies, elongated philtrum, micrognathia, low-set ears, telecanthus, sinofridia, bushy eyebrows, long eyelashes, wide nose, high arched palate, premature denture loss, jaw malocclusion, hirsutism, and hypertelorism.
- Musculoskeletal alterations: short stature, short neck, fractures of long bones, joint laxity, biconcave vertebrae, kyphoscoliosis, cervical instability, vertebral collapse, genu valgum, serpentine fibula, acroosteolysis, pseudoclubbing, short fingers, Hippocratic fingers, progressive distal bone resorption, bone demineralization, osteopenia, and osteoporosis.
- Cardiovascular alterations: congenital heart disease, patent arterial duct, and sept defects.
- Digestive alterations: intestinal malrotation.
- Neurological alterations: hydrocephalus and lateral meningocele.
- Renal alterations: hypospadias, cryptorchidism, renal cysts, and kidney failure.
- Respiratory alterations: thoracic deformities, ventilatory restriction, and recurrent infections.
- Other alterations: delayed motor development, hearing loss, changes of the voice, deep voice, short nails, plantar ulcers, and hernias.
- birth (<1 year old)
- early childhood (ages 1–5)
- childhood (ages 6–12)
- adolescence (ages 13–19)
- early adulthood (ages 20–33)
- middle adulthood (ages 36–65)
- late adulthood (65+).
4.1.5. Diagnosis
4.1.6. Treatment
4.1.7. Prognosis
4.2. Limitations
4.3. Possible Future Lines of Research
- Revise all the cases described in the literature and describe new cases to obtain a large and reliable sample of patients with HCS with whom a descriptive study could be carried out. Such a study would serve not only to identify the cases of this disorder and study its prevalence but also to analyze the complete phenotype of HCS in depth, allowing for a greater understanding of this syndrome and contribute to an earlier diagnosis in new cases.
- Standardize protocols for the evaluation of signs and symptoms, diagnostic orientation, and disease management. Action protocols and specific intervention plans are basic and necessary tools for the universalization of care for patients with HCS. The use of a nursing methodology and its taxonomy NANDA (North American Nursing Diagnosis Association) - NIC (Nursing Interventions Classification) - NOC (Nursing Outcomes Classification) would provide a universal, individualized, and multidisciplinary approach to this disorder.
- Perform a qualitative study on HCS to understand the impact on the quality of life and daily activities. Such a study would aim to report on the level of dependency and adaptation of these patients and evaluate possible future healthcare interventions.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, J.A.; Williams, C.B.; McAlister, W.H. Talo-patello-scaphoid osteolysis, synovitis, and short fourth metacarpals in sisters: A new syndrome? Am. J. Med. Genet. 2003, 121A, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Orphanet: Acroosteolisis Tipo Dominante. Available online: https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=ES&data_id=1276&Disease_Disease_Search_diseaseGroup=Hajducheney&Disease_Disease_Search_diseaseType=Pat&Enfermedad(es)/grupodeenfermedades=Acroosteolisis-tipo-dominante&title=Acroosteolisistipodominante&search=Disease_Search_Simple (accessed on 25 July 2020).
- Simpson, M.A.; Irving, M.D.; Asilmaz, E.; Gray, M.J.; Dafou, D.; Elmslie, F.V.; Mansour, S.; Holder, S.E.; Brain, C.E.; Burton, B.K.; et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat. Genet. 2011, 43, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Descartes, M.; Rojnueangnit, K.; Cole, L.; Sutton, A.; Morgan, S.L.; Patry, L.; Samuels, M.E. Hajdu-Cheney syndrome: Phenotypical progression with de-novo NOTCH2 mutation. Clin. Dysmorphol. 2014, 23, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, N.; Kauntze, R. Cranio-skeletal dysplasia. Br. J. Radiol. 1948, 21, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Cheney, W. Acro-Osteolysis. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1965, 94, 595–607. [Google Scholar] [PubMed]
- Brown, D.M.; Bradford, D.S.; Gorlin, R.J.; Desnick, R.J.; Langer, L.O.; Jowsey, J.; Sauk, J.J. The acro-osteolysis syndrome: Morphologic and biochemical studies. J. Pediatr. 1976, 88, 573–580. [Google Scholar] [CrossRef]
- Brennan, A.M.; Pauli, R.M. Hajdu-Cheney syndrome: Evolution of phenotype and clinical problems. Am. J. Med. Genet. 2001, 100, 292–310. [Google Scholar] [CrossRef]
- PROSPERO [Internet]. Available online: https://www.crd.york.ac.uk/PROSPERO/ (accessed on 24 August 2020).
- Gibofsky, A. Genetics of the hajdu-cheney syndrome. Arthritis Rheum. 1987, 30, 718. [Google Scholar] [CrossRef] [PubMed]
- Le Caignec, C. Pathologies humaines et récepteurs NOTCH. Med./Sci. 2011, 27, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Canalis, E. NOTCH regulation of bone development and remodeling and related skeletal disorders. Calcif. Tissue Int. 2012, 90, 69–75. [Google Scholar] [CrossRef]
- Canalis, E. The fate of circulating osteoblasts. N. Engl. J. Med. 2005, 352, 2014–2016. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Schilling, L.; Yee, S.P.; Lee, S.K.; Zanotti, S. Hajdu cheney mouse mutants exhibit osteopenia, increased osteoclastogenesis, and bone resorption. J. Biol. Chem. 2016, 291, 1538–1551. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.J.; Kaplan, B.S.; Bellah, R.D.; Zackai, E.H.; Kaplan, P. Further evidence that the Hajdu-Cheney syndrome and the “serpentine fibula-polycystic kidney syndrome” are a single entity. Am. J. Med. Genet. 1998, 78, 474–481. [Google Scholar] [CrossRef]
- Nunziata, V.; di Giovanni, G.; Ballanti, P.; Bonucci, E. High turnover osteoporosis in acro-osteolysis (Hajdu-Cheney syndrome). J. Endocrinol. Investig. Off. J. Ital. Soc. Endocrinol. 1990, 13, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Siklar, Z.; Tanyer, G.; Dallar, Y.; Aksoy, F.G. Hajdu-Cheney syndrome with growth hormone deficiency and neuropathy. J. Pediatr. Endocrinol. Metab. 2000, 13, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.J.; Saba, C.; Rennert, O.M. Kidney abnormalities in Hajdu-Cheney syndrome. Pediatr. Nephrol. 1996, 10, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Ades, L.C.; Morris, L.L.; Haan, E.A. Hydrocephalus in Hajdu-Cheney syndrome. J. Med. Genet. 1993, 30, 175. [Google Scholar] [CrossRef] [PubMed]
- Currarino, G. Hajdu-Cheney syndrome associated with serpentine fibulae and polycystic kidney disease. Pediatr. Radiol. 2009, 39, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Isidor, B.; Lindenbaum, P.; Pichon, O.; Bézieau, S.; Dina, C.; Jacquemont, S.; Martin-Coignard, D.; Thauvin-Robinet, C.; Le Merrer, M.; Mandel, J.L.; et al. Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nat. Genet. 2011, 43, 306–308. [Google Scholar] [CrossRef]
- Gray, M.J.; Kim, C.A.; Bertola, D.R.; Arantes, P.R.; Stewart, H.; Simpson, M.A.; Irving, M.D.; Robertson, S.P. Serpentine fibula polycystic kidney syndrome is part of the phenotypic spectrum of Hajdu-Cheney syndrome. Eur. J. Hum. Genet. 2012, 20, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, J.; Miki, Y.; Yamazaki, S.; Ogawa, M. Hajdu-Cheney Syndrome: MR imaging. Neuroradiology 1991, 33, 441–442. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.A.R.; Shaw, D.G. Hajdu-Cheney syndrome. Ann. Rheum. Dis. 1994, 53, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Gripp, K.W. Lateral meningocele syndrome and Hajdu-Cheney syndrome: Different disorders with overlapping phenotypes. Am. J. Med. Genet. Part A 2011, 155, 1773–1774. [Google Scholar] [CrossRef] [PubMed]
- Sawin, P.D.; Menezes, A.H. Basilar invagination in osteogenesis imperfecta and related osteochondrodysplasias: Medical and surgical management. J. Neurosurg. 1997, 86, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Ornetti, P.; Tavernier, C. Osteoporotic compression fracture revealing Hajdu-Cheney syndrome. Jt. Bone Spine 2012, 79, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Murtagh-Schaffer, C.; Moquin, R.R. Spinal reconstruction in Hajdu-Cheney syndrome. JAAPA 2008, 21, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Schawo, S.; Weber, M.A.; Libicher, M. Junge frau mit rückenschmerzen und akroosteolysen. Radiologe 2006, 46, 901–904. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, F.E. Integrated anti-remodeling and anabolic therapy for the osteoporosis of Hajdu-Cheney syndrome. Osteoporos. Int. 2007, 18, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.; Gafni, R.I.; Davies, J.H.; Clarke, B.; Tebben, P.; Samuels, M.; Saraff, V.; Klaushofer, K.; Fratzl-Zelman, N.; Roschger, P.; et al. Bone Structural Characteristics and Response to Bisphosphonate Treatment in Children With Hajdu-Cheney Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 4163–4172. [Google Scholar] [CrossRef] [PubMed]
- Engin, F.; Yao, Z.; Yang, T.; Zhou, G.; Bertin, T.; Jiang, M.M.; Chen, Y.; Wang, L.; Zheng, H.; Sutton, R.E.; et al. Dimorphic effects of NOTCH signaling in bone homeostasis. Nat. Med. 2008, 14, 299–305. [Google Scholar] [CrossRef]
- Sahlgren, C.; Lendahl, U. NOTCH signaling and its integration with other signaling mechanisms. Regen. Med. 2006, 1, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Canalis, E. NOTCH1 and NOTCH2 expression in osteoblast precursors regulates femoral microarchitecture. Bone 2014, 62, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Yu, J.; Bridgewater, D.; Wolf, J.M.; Canalis, E. Mice harboring a Hajdu Cheney Syndrome mutation are sensitized to osteoarthritis. Bone 2018, 114, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Vollersen, N.; Hermans-Borgmeyer, I.; Cornils, K.; Fehse, B.; Rolvien, T.; Triviai, I.; Jeschke, A.; Oheim, R.; Amling, M.; Schinke, T.; et al. High Bone Turnover in Mice Carrying a Pathogenic NOTCH2 Mutation Causing Hajdu-Cheney Syndrome. J. Bone Miner. Res. 2018, 33, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Regev, M.; Pode-Shakked, B.; Jacobson, J.M.; Raas-Rothschild, A.; Goldstein, D.B.; Anikster, Y. Phenotype variability in Hajdu-Cheney syndrome. Eur. J. Med. Genet. 2019, 62, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Jirečková, J.; Magner, M.; Lambert, L.; Baxová, A.; Leiská, A.; Kopečková, L.; Fajkusová, L.; Zeman, J. The Age Dependent Progression of Hajdu-Cheney Syndrome in Two Families. Prague Med. Rep. 2018, 119, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Majewski, J.; Schwartzentruber, J.A.; Caqueret, A.; Patry, L.; Marcadier, J.; Fryns, J.P.; Boycott, K.M.; Ste-Marie, L.G.; Mckiernan, F.E.; Marik, I.; et al. Mutations in NOTCH2 in families with Hajdu-Cheney syndrome. Hum. Mutat. 2011, 32, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Rosenmann, E.; Penchas, S.; Cohen, T.; Aviad, I. Sporadic idiopathic acro-osteolysis with cranio-skeletal dysplasia, polycystic kidneys and glomerulonephritis A case of the hajdu-cheney syndrome. Pediatr. Radiol. 1977, 6, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.N.; Pinals, R.S.; Clarke Anderson, H.; Gould, L.V.; Streeten, D.H.P. Hereditary osteodysplasia with acro-osteolysis (the Hajdu-Cheney syndrome). Am. J. Med. 1978, 65, 627–636. [Google Scholar] [CrossRef]
- Leidig-Bruckner, G.; Pfeilschifter, J.; Penning, N.; Limberg, B.; Priemel, M.; Delling, G.; Ziegler, R. Severe osteoporosis in familial Hajdu-Cheney syndrome: Progression of acro-osteolysis and osteoporosis during long-term follow-up. J. Bone Miner. Res. 1999, 14, 2036–2041. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, P.; Thumboo, J.; Leong, R.T.K. A patient with progressive shortening of the fingers. J. Rheumatol. 2009, 36, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, I.P.; Trovas, G.; Lampropoulou-Adamidou, K.; Koromila, T.; Kollia, P.; Papaioannou, N.A.; Lyritis, G. Severe osteoporosis and mutation in NOTCH2 gene in a woman with Hajdu-Cheney syndrome. Bone 2013, 52, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Hoey, H.; Hinde, F.; Grant, D.B. Hajdu-Cheney syndrome associated with intrauterine fractures and arachnoid cysts. J. R. Soc. Med. 1983, 76, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Battelino, N.; Writzl, K.; Bratanič, N.; Irving, M.D.; Novljan, G. End-Stage Renal Disease in an Infant With Hajdu-Cheney Syndrome. Ther. Apher. Dial. 2016, 20, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Herscovici, D.; Bowen, J.R.; Scott, C.I. Cervical instability as an unusual manifestation of Hajdu-Cheney syndrome of acroosteolysis. Clin. Orthop. Relat. Res. 1990, 111–116. [Google Scholar] [CrossRef]
- Bazopoulou-Kyrkanidou, E.; Vrahopoulos, T.P.; Eliades, G.; Vastardis, H.; Tosios, K.; Vrotsos, I.A. Periodontitis Associated With Hajdu-Cheney Syndrome. J. Periodontol. 2007, 78, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, K.; Kaklamanos, E.; Kavadia, S.; Hatzistilianou, M.; Antoniades, V. Hajdu-Cheney syndrome (acro-osteolysis): A case report of dental interest. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Fryns, J.-P.; Stinckens, C.; Feenstra, L. Vocal cord paralysis and cystic kidney disease in Hajdu-Cheney syndrome. Clin. Genet. 2008, 51, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Avela, K.; Valanne, L.; Helenius, I.; Mäkitie, O. Hajdu-Cheney syndrome with severe dural ectasia. Am. J. Med. Genet. Part A 2011, 155, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Mannstadt, M.; Lin, A.E.; Le, L.P. Case 24-2014: A 27-year-old man with severe osteoporosis and multiple bone fractures. N. Engl. J. Med. 2014, 371, 465–472. [Google Scholar] [CrossRef]
- Deepak Amalnath, S.; Babu, V. Hajdu-Cheney syndrome—A rare cause of micrognathia. Indian J. Med. Res. 2016, 143, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, T.; Ihara, K.; Makimura, M.; Kinjo, T.; Hara, T. A girl with Hajdu-Cheney syndrome and premature ovarian failure. J. Pediatr. Endocrinol. Metab. 2012, 25, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Sargin, G.; Cildag, S.; Senturk, T. Hajdu-Cheney syndrome with ventricular septal defect. Kaohsiung J. Med. Sci. 2013, 29, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Swan, L.; Gole, G.; Sabesan, V.; Cardinal, J.; Coman, D. Congenital Glaucoma: A Novel Ocular Manifestation of Hajdu-Cheney Syndrome. Case Rep. Genet. 2018, 2018, 2508345. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, G.; Aoaki, K.; Haga, N.; Hasegawa, T.; Aoki, K.; Haga, N.; Hasegawa, T. Syringohydromyelia in Hajdu-Cheney syndrome. Pediatr Radiol 1996, 26, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, A.; Tamaki, N.; Nagashima, T.; Nakamura, M. Syringomyelia associated with Hajdu-Cheney syndrome: Case report. Neurosurgery 1996, 39, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ito, Y.; Kawame, H.; Kikuchi, A.; Tanaka, H. Fatal case of Hajdu-Cheney syndrome with idiopathic pulmonary hemosiderosis. Pediatr. Int. 2019, 61, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Takatani, R.; Someya, T.; Kazukawa, I.; Nishimura, G.; Minagawa, M.; Kohno, Y. Hajdu-Cheney syndrome: Infantile onset of hydrocephalus and serpentine fibulae. Pediatr. Int. 2009, 51, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Niijima, K.H.; Kondo, A.; Ishikawa, J.I. Familial osteodysplasia associated with trigeminal neuralgia: Case report. Neurosurgery 1984, 15, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Fryns, J.P. Serpentine fibula syndrome: A variant clinical presentation of Hajdu-Cheney syndrome? Clin. Dysmorphol. 1997, 6, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Damian, L.O.; Simon, S.P.; Filipescu, I.; Bocsa, C.; Botar-Jid, C.; Rednic, S. Capillaroscopic findings in a case of Hajdu-Cheney syndrome. Osteoporos. Int. 2016, 27, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Gripp, K.W.; Robbins, K.M.; Sobreira, N.L.; Witmer, P.D.; Bird, L.M.; Avela, K.; Makitie, O.; Alves, D.; Hogue, J.S.; Zackai, E.H.; et al. Truncating mutations in the last exon of NOTCH3 cause lateral meningocele syndrome. Am. J. Med. Genet. Part A 2015, 167, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Albano, L.M.J.; Bertola, D.R.; Barba, M.F.; Valente, M.; Robertson, S.P.; Kim, C.A. Phenotypic overlap in Melnick-Needles, serpentine fibula-polycystic kidney and Hajdu-Cheney syndromes: A clinical and molecular study in three patients. Clin. Dysmorphol. 2007, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pittaway, J.F.H.; Harrison, C.; Rhee, Y.; Holder-Espinasse, M.; Fryer, A.E.; Cundy, T.; Drake, W.M.; Irving, M.D. Bisphosphonate therapy for spinal osteoporosis in Hajdu-Cheney syndrome—New data and literature review. Orphanet J. Rare Dis. 2018, 13, 47. [Google Scholar] [CrossRef]
- Adami, G.; Rossini, M.; Gatti, D.; Orsolini, G.; Idolazzi, L.; Viapiana, O.; Scarpa, A.; Canalis, E. Hajdu Cheney Syndrome; report of a novel NOTCH2 mutation and treatment with denosumab. Bone 2016, 92, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Galli-Tsinopoulou, A.; Kyrgios, I.; Giza, S.; Giannopoulou, E.Z.; Maggana, I.; Laliotis, N. Two-year cyclic infusion of pamidronate improves bone mass density and eliminates risk of fractures in a girl with osteoporosis due to Hajdu-Cheney syndrome. Minerva Endocrinol. 2012, 37, 283–289. [Google Scholar] [PubMed]
- Al-Mayouf, S.M.; Madi, S.M.; Bin-Abbas, B.S. Cyclic intravenous pamidronate treatment in children with nodulosis, arthropathy and osteolysis syndrome. Ann. Rheum. Dis. 2006, 65, 1672–1673. [Google Scholar] [CrossRef]
- Hwang, S.; Shin, D.Y.; Moon, S.H.; Lee, E.J.; Lim, S.K.; Kim, O.H.; Rhee, Y. Effect of Zoledronic Acid on Acro-Osteolysis and Osteoporosis in a Patient with Hajdu-Cheney Syndrome. Yonsei Med. J. 2011, 52, 543–546. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, F.E. Integrated anti-remodeling and anabolic therapy for the osteoporosis of Hajdu-Cheney syndrome: 2-Year follow-up. Osteoporos. Int. 2008, 19, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Mattei, T.A.; Rehman, A.A.; Issawi, A.; Fassett, D.R. Surgical challenges in the management of cervical kyphotic deformity in patients with severe osteoporosis: An illustrative case of a patient with Hajdu–Cheney syndrome. Eur. Spine J. 2015, 24, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoedt, E.; Bailleul-Forestier, I.; Fellus, P.; Schoenaers, J.; Frijns, J.P.; Carels, C. Syndrome of Hajdu-Cheney: Three case reports of orofacial interest. Cleft Palate-Craniofac. J. 2010, 47, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Liljeström, M.R.; Närhi, T.O. Occlusal rehabilitation of a patient with hereditary multicentric osteolysis. J. Prosthet. Dent. 2003, 89, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, H.; Tanaka, J.; Yoshiya, S.; Kokubu, T.; Fujita, K.; Makino, T.; Mizuno, K. Proximal translation of the radius following arthroplasty of the distal radioulnar joint in Hajdu-Cheney syndrome. J. Shoulder Elb. Surg. 2003, 12, 97–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamaguchi, S.; Nakamura, K.; Takahashi, Y. A case report of anesthesia for a child with Hajdu-Cheney syndrome. J. Anesth. 2013, 27, 949–950. [Google Scholar] [CrossRef] [PubMed]
- August, D.A.; Ramos, D.C. Anesthesia for a child with Hajdu-Cheney syndrome. Paediatr. Anaesth. 2009, 19, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Zietz, D.F.; Aubel, E.B.; Tao, W. Continuous spinal labor analgesia in a patient with Hajdu-Cheney syndrome. Reg. Anesth. Pain Med. 2013, 38, 466–467. [Google Scholar] [CrossRef] [PubMed]




| Information Source | Search Chain |
|---|---|
| ORPHANET | Código ORPHA: 955 |
| PUBMED | ((((Hajdu-Cheney Syndrome) OR (Acro-Osteolysis)) OR (Receptor, NOTCH2)) OR (Connective tissue)) OR (Rare diseases) (((((((“hajdu-cheney syndrome”[MeSH Terms] OR (“hajdu cheney”[All Fields] AND “syndrome”[All Fields])) OR “hajdu cheney syndrome”[All Fields]) OR ((“hajdu”[All Fields] AND “cheney”[All Fields]) AND “syndrome”[All Fields])) OR “hajdu cheney syndrome”[All Fields]) OR (((“acro-osteolysis”[MeSH Terms] OR “acro osteolysis”[All Fields]) OR (“acro”[All Fields] AND “osteolysis”[All Fields])) OR “acro osteolysis”[All Fields])) OR ((((“receptor, NOTCH2”[MeSH Terms] OR (“receptor”[All Fields] AND “NOTCH2”[All Fields])) OR “NOTCH2 receptor”[All Fields]) OR (“receptor”[All Fields] AND “NOTCH2”[All Fields])) OR “receptor NOTCH2”[All Fields])) OR ((“connective tissue”[MeSH Terms] OR (“connective”[All Fields] AND “tissue”[All Fields])) OR “connective tissue”[All Fields])) OR ((“rare diseases”[MeSH Terms] OR (“rare”[All Fields] AND “diseases”[All Fields])) OR “rare diseases”[All Fields]) |
| SCIELO | (Hajdu-Cheney Syndrome) OR (Acro-Osteolysis) OR (Receptor, NOTCH2) |
| Authors | Article | Thematic Block | Results |
|---|---|---|---|
| Gibofsky [10] (1987) | Genetics of the Hajdu–Cheney Syndrome | Disease genetics | The existing association between NOTCH and Hajdu–Cheney syndrome (HCS), focusing on the signaling pathway and other disorders caused by NOTCH mutations. |
| Le Caignec [11] (2011) | Pathologies humaines et récepteurs NOTCH | ||
| Zanotti et al. [12] (2012) | NOTCH regulation of bone development and remodeling and related skeletal disorders. | ||
| Canalis et al. [13] (2005) | The fate of circulating osteoblasts | ||
| Canalis et al. [14] (2016) | Hajdu Cheney mouse mutants exhibit osteopenia, increased osteoclastogenesis, and bone resorption | ||
| Ramos et al. [15] (1998) | Further evidence that the Hajdu-Cheney Syndrome and the ‘‘Serpentine Fibula-Polycystic Kidney Syndrome’’ are a single entity | Description of the disease and evolution of the phenotype | Descriptions of the clinical manifestations of the disease, highlighting its variable phenotype, the wide spectrum of clinical presentation, and the age-dependent progression and possible complications. Resolution of the controversy between serpentine fibula-polycystic kidney syndrome and HCS, proving it is only another manifestation of HCS, not an independent disorder as was previously believed. |
| Nunziata et al. [16] (1990) | High turnover osteoporosis in acro-osteolysis (Hajdu-Cheney Syndrome) | ||
| Brown et al. [7] (1976) | The acro-osteolysis syndrome: morphologic and biochemical studies | ||
| Brennan et al. [8] (2001) | Hajdu-Cheney Syndrome: evolution of phenotype and clinical problems | ||
| Siklar et al. [17] (2000) | Hajdu-Cheney Syndrome with growth hormone deficiency and neuropathy | ||
| Barakat et al. [18] (1996) | Kidney abnormalities in Hajdu-Cheney Syndrome | ||
| Ades et al. [19] (1993) | Hydrocephalus in Hajdu-Cheney Syndrome | ||
| Currarino [20] (2009) | Hajdu-Cheney Syndrome associated with serpentine fibulae and polycystic kidney disease | ||
| Isidor et al. [21] (2011) | Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis | ||
| Gray et al. [22] (2012) | Serpentine fibula polycystic kidney syndrome is part of the phenotypic spectrum of Hajdu–Cheney Syndrome | ||
| Kawamura et al. [23] (1991) | Hajdu-Cheney Syndrome: MR imaging | Diagnosis and differential diagnosis | We establish the necessary conditions for a diagnostic orientation toward HCS and propose several related disorders that are useful for a differential diagnosis. |
| O’Reilly et al. [24] (1994) | Hajdu-Cheney Syndrome | ||
| Singh et al. [1] (2003) | Talo-patello-scaphoid osteolysis, synovitis, and short fourth metacarpals in sisters: A new syndrome? | ||
| Gripp et al. [25] (2011) | Lateral meningocele syndrome and Hajdu–Cheney Syndrome: different disorders with overlapping phenotypes | ||
| Sawin et al. [26] (1997) | Basilar invagination in osteogenesis imperfecta and related osteochondrodysplasias: medical and surgical management | ||
| Ornetti et al. [27] (2012) | Osteoporotic compression fracture revealing Hajdu-Cheney Syndrome | Treatment | There is no curative treatment. There are several studies on bisphosphonates, although there is no clear evidence of their effectiveness. Surgical intervention to prevent complications is effective in certain cases. The current treatment of HCS is focused on the management of complications and underlying problems to improve the quality of life and life expectancy. |
| Murtagh-Schaffer et al. [28] (2008) | Spinal reconstruction in Hajdu-Cheney Syndrome | ||
| Schawo et al. [29] (2006) | Junge frau mit rückenschmerzen und akroosteolysen | ||
| McKiernan et al. [30] (2007) | Integrated anti-remodeling and anabolic therapy for the osteoporosis of Hajdu–Cheney Syndrome | ||
| Sakka et al. [31] (2017) | Bone structural characteristics and response to bisphosphonate treatment in children with Hajdu-Cheney Syndrome |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Martín, J.; Díaz-Rodríguez, L.; Piqueras-Sola, B.; Rodríguez-Blanque, R.; Bermejo-Fernández, A.; Sánchez-García, J.C. Hajdu–Cheney Syndrome: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 6174. https://doi.org/10.3390/ijerph17176174
Cortés-Martín J, Díaz-Rodríguez L, Piqueras-Sola B, Rodríguez-Blanque R, Bermejo-Fernández A, Sánchez-García JC. Hajdu–Cheney Syndrome: A Systematic Review of the Literature. International Journal of Environmental Research and Public Health. 2020; 17(17):6174. https://doi.org/10.3390/ijerph17176174
Chicago/Turabian StyleCortés-Martín, Jonathan, Lourdes Díaz-Rodríguez, Beatriz Piqueras-Sola, Raquel Rodríguez-Blanque, Antonio Bermejo-Fernández, and Juan Carlos Sánchez-García. 2020. "Hajdu–Cheney Syndrome: A Systematic Review of the Literature" International Journal of Environmental Research and Public Health 17, no. 17: 6174. https://doi.org/10.3390/ijerph17176174
APA StyleCortés-Martín, J., Díaz-Rodríguez, L., Piqueras-Sola, B., Rodríguez-Blanque, R., Bermejo-Fernández, A., & Sánchez-García, J. C. (2020). Hajdu–Cheney Syndrome: A Systematic Review of the Literature. International Journal of Environmental Research and Public Health, 17(17), 6174. https://doi.org/10.3390/ijerph17176174









