Be Healthe for Your Heart: A Pilot Randomized Controlled Trial Evaluating a Web-Based Behavioral Intervention to Improve the Cardiovascular Health of Women with a History of Preeclampsia
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Intervention
- How Healthy is your Heart? A brief survey was available on the website from enrolment to evaluate each participant’s current eating habits, physical activity, stress levels and body weight. Automated individualized feedback was provided based on participant’s responses comparing current behaviors to the program recommendations.
- My goals: Allowed participants to select up to four behavior change goals consistent with the program recommendations, and to record strategies for achieving those goals. This component was available on the website throughout the three months.
- Track my progress: Allowed participants to self-monitor their progress by answering a series of questions related to their goals. Feedback was provided on their progress towards achieving their goals and the program recommendations. This component could only be completed once the My goals component was completed.
- Resources: Comprehensive written information related to the program recommendations was provided. All resources were available throughout the three months.
- Email newsletters: Participants were sent a weekly newsletter, which focused on a different program recommendation each week, and prompted participants to use the website components.
2.4. Control Group
2.5. Outcome Measures
2.5.1. Acceptability (Primary Outcome)
2.5.2. Preliminary Efficacy (Secondary Outcome)
2.5.3. Other Measures
2.6. Sample Size
2.7. Randomisation and Blinding
2.8. Statistical Methods
3. Results
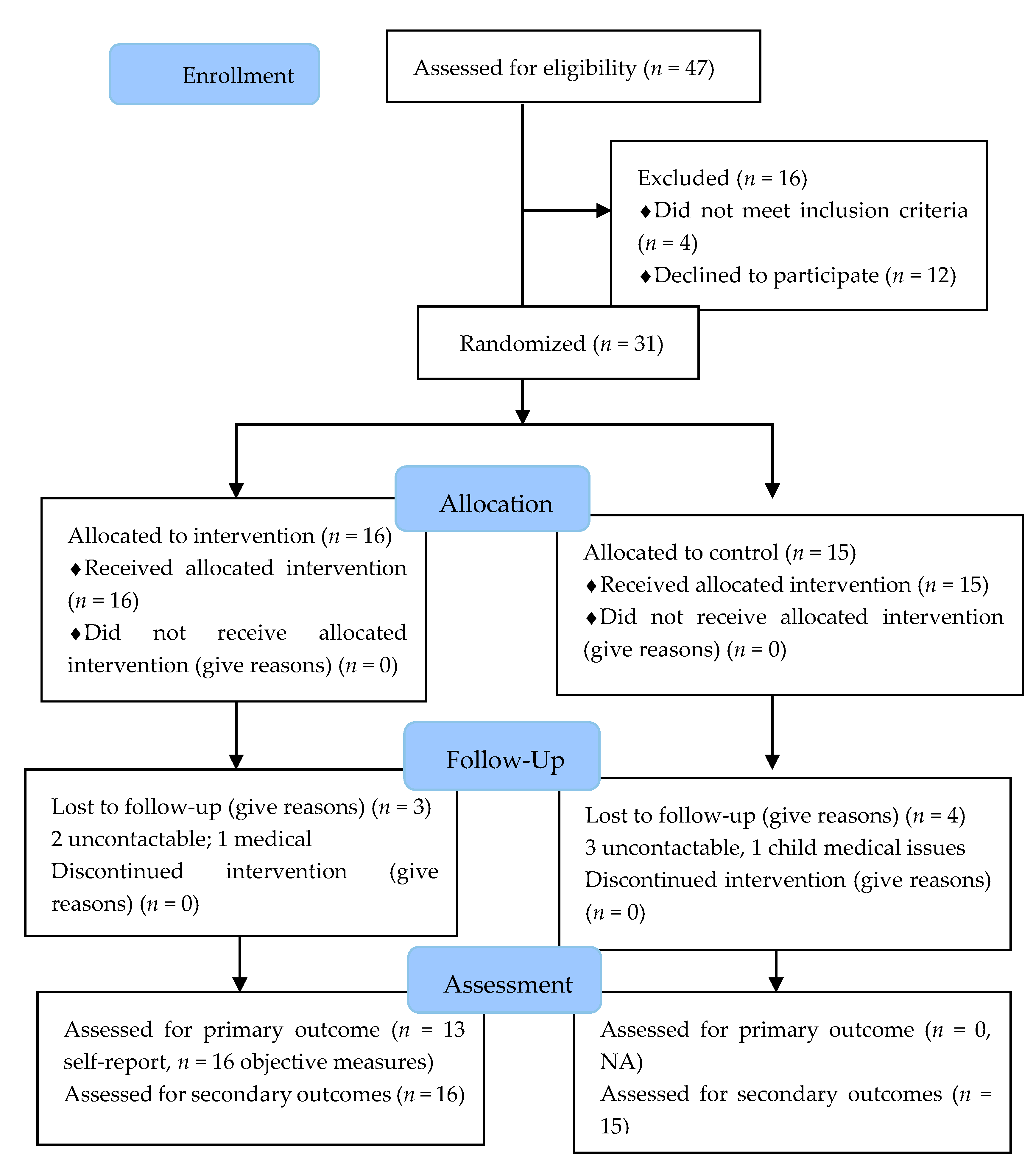
3.1. Recruitment
3.2. Participant Characteristics
3.3. Participant Retention
3.4. Acceptability
3.5. Preliminary Efficacy
4. Discussion
4.1. Participant Recruitment and Retention
4.2. Intervention Acceptability
4.3. Preliminary Efficacy
4.4. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease (GBD). Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy: The Task Force for the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef] [PubMed]
- Benschop, L.; Duvekot, J.J.; Roeters van Lennep, J.E. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart 2019, 105, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Vitalek, S.G.; Sapia, F.; Valenti, G.; Corrado, F.; Padula, F.; Rapisarda, A.; D’Anna, R. miRNA expression for early diagnosis of preeclampsia onset: Hope or hype? J. Matern. Fetal. Neonatal. Med. 2018, 31, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Favilli, A.; Triolo, O.; Granese, R.; Gerli, S. Early serum markers of pre-eclampsia: Are we stepping forward? J. Matern. Fetal. Neonatal. Med. 2016, 29, 3019–3023. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef]
- Grandi, S.M.; Filion, K.B.; Yoon, S.; Ayele, H.T.; Doyle, C.M.; Hutcheon, J.A.; Smith, G.N.; Gore, G.C.; Ray, J.G.; Nerenberg, K.; et al. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation 2019, 139, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 10, 974. [Google Scholar] [CrossRef]
- McDonald, S.D.; Malinowski, A.; Zhou, Q.; Yusuf, S.; Devereaux, P.J. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am. Heart J. 2008, 156, 918–930. [Google Scholar] [CrossRef]
- Tooher, J.; Chiu, C.L.; Yeung, K.; Lupton, S.J.; Thornton, C.; Makris, A.; Loughlin, A.; Hennessy, A.; Lind, J.M. High blood pressure during pregnancy is associated with future cardiovascular disease: An observational cohort study. BMJ Open 2013, 3, e002964. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, L.; van der Meiden-van Roest, A.J.; Savelkoul, C.; Vogelvang, T.E.; Lely, A.T.; Franx, A.; van Rijn, B.B. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: A systematic review and meta-analysis. BJOG 2018, 125, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.A.; Bowyer, L.; Lust, K.; McMahon, L.P.; Morton, M.; North, R.A.; Paech, M.; Said, J.M. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust. N.Z. J. Obstet. Gynaecol. 2015, 55, e1–e29. [Google Scholar] [CrossRef] [PubMed]
- Roth, H.; LeMarquand, G.; Henry, A.; Homer, C. Assessing Knowledge Gaps of Women and Healthcare Providers Concerning Cardiovascular Risk After Hypertensive Disorders of Pregnancy—A Scoping Review. Front. Cardiovasc. Med. 2019, 29, 178. [Google Scholar] [CrossRef]
- Lui, N.A.; Jeyaram, G.; Henry, A. Postpartum Interventions to Reduce Long-Term Cardiovascular Disease Risk in Women after Hypertensive Disorders of Pregnancy: A Systematic Review. Front. Cardiovasc. Med. 2019, 15, 160. [Google Scholar] [CrossRef]
- Carter-Edwards, L.; Østbye, T.; Bastian, L.A.; Yarnall, K.S.; Krause, K.M.; Simmons, T.J. Barriers to adopting a healthy lifestyle: Insight from postpartum women. BMC Res. Notes 2009, 2, 161. [Google Scholar] [CrossRef]
- Nicklas, J.M.; Zera, C.A.; Seely, E.W.; Abdul-Rahim, Z.S.; Rudloff, N.D.; Levkoff, S.E. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy Childb. 2011, 11, 23. [Google Scholar] [CrossRef]
- Lim, S.; Dunbar, J.A.; Versace, V.L.; Janus, E.; Wildey, C.; Skinner, T.; O’Reilly, S. Comparing a telephone- and a group-delivered diabetes prevention program: Characteristics of engaged and non-engaged postpartum mothers with a history of gestational diabetes. Diabetes Res. Clin. Pract. 2017, 126, 254–262. [Google Scholar] [CrossRef]
- McKinley, M.C.; Allen-Walker, V.; McGirr, C.; Rooney, C.; Woodside, J.V. Weight loss after pregnancy: Challenges and opportunities. Nutr. Res. Rev. 2018, 31, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Shrewsbury, V.A.; Vincze, L.; Campbell, L.; Callister, R.; Park, F.; Schumacher, T.; Collins, C.; Hutchesson, M. Be Healthe for Your Heart: Protocol for a Pilot Randomized Controlled Trial Evaluating a Web-Based Behavioral Intervention to Improve the Cardiovascular Health of Women With a History of Preeclampsia. Front. Cardiovasc. Med. 2019, 26, 144. [Google Scholar] [CrossRef] [PubMed]
- Hutchesson, M.; Shrewsbury, V.; Park, F.; Callister, R.; Collins, C. Are women with a recent diagnosis of pre-eclampsia aware of their cardiovascular disease risk? A cross-sectional survey. Aust. N.Z. J. Obstet. Gynaecol. 2018, 58, E27–E28. [Google Scholar] [CrossRef] [PubMed]
- General Assembly of the World Medical Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- National Heart Foundation. Eating for Heart Health: Position Statement 2017; National Heart Foundation: Dhaka, Bangladesh, 2017. [Google Scholar]
- Australian Government Department of Health. Australia’s Physical Activity and Sedentary Behaviour Guidelines for Adults (18–64 Years) 2014; Australian Government Department of Health: Canberra, Australia, 2014.
- National Health Medical Research Council (NHMRC). Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults, Adolescents and Children in Australia; National Health Medical Research Council: Canberra, Australia, 2013. [Google Scholar]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Pencina, M.J.; D’Agostino Sr, R.B.; Larson, M.G.; Massaro, J.M.; Vasan, R.S. Predicting the thirty-year risk of cardiovascular disease: The Framingham Heart Study. Circulation 2009, 119, 3078. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Marshall, A.L.; Miller, Y.D.; Burton, N.W.; Brown, W.J. Measuring total and domain-specific sitting: A study of reliability and validity. Med. Sci. Sports Exerc. 2010, 42, 1094. [Google Scholar] [CrossRef]
- Miller, R.; Brown, W. Steps and sitting in a working population. Int. J. Behav. Med. 2004, 11, 219–224. [Google Scholar] [CrossRef]
- Schumacher, T.; Burrows, T.; Rollo, M.; Wood, L.; Callister, R.; Collins, C. Comparison of fatty acid intakes assessed by a cardiovascular-specific food frequency questionnaire with red blood cell membrane fatty acids in hyperlipidaemic australian adults: A validation study. Eur. J. Clin. Nutr. 2016, 70, 1433–1438. [Google Scholar] [CrossRef]
- Henry, J.D.; Crawford, J.R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005, 44, 227–239. [Google Scholar] [CrossRef]
- Endicott, J.; Nee, J.; Harrison, W.; Blumenthal, R. Quality of Life Enjoyment and Satisfaction Questionnaire: A new measure. Psychopharmacol. Bull. 1993, 29, 321–326. [Google Scholar] [PubMed]
- Diener, E.; Emmons, R.A.; Larsen, R.J.; Griffin, S. The satisfaction with life scale. J. Pers. Assess. 1985, 49, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.C.; Davis, L.L.; Kraemer, H.C. The role and interpretation of pilot studies in clinical research. J. Psychiatr. Res. 2011, 45, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Centre for Epidemiology and Evidence. New South Wales Mothers and Babies 2018; Ministry of Health: Sydney, Australia, 2019. [Google Scholar]
- Rich-Edwards, J.W.; Stuart, J.J.; Skurnik, G.; Roche, A.T.; Tsigas, E.; Fitzmaurice, G.M.; Wilkins-Haug, L.E.; Levkoff, S.E.; Seely, E.W. Randomized Trial to Reduce Cardiovascular Risk in Women with Recent Preeclampsia. Int. J. Womens Health 2019, 28, 1493–1504. [Google Scholar] [CrossRef]
- Vincze, L.; Rollo, M.; Hutchesson, M.; Hauck, Y.; MacDonald-Wicks, L.; Wood, L.; Callister, R.; Collins, C. Interventions including a nutrition component aimed at managing gestational weight gain or postpartum weight retention: A systematic review and meta-analysis. JBI Database Syst. Rev. Implement. Rep. 2019, 17, 297–364. [Google Scholar] [CrossRef]
- Berger, A.A.; Peragallo-Urrutia, R.; Nicholson, W.K. Systematic review of the effect of individual and combined nutrition and exercise interventions on weight, adiposity and metabolic outcomes after delivery: Evidence for developing behavioral guidelines for post-partum weight control. BMC Pregnancy Childb. 2014, 14, 319. [Google Scholar] [CrossRef]
- Vincze, L.; Rollo, M.E.; Hutchesson, M.J.; Burrows, T.L.; MacDonald-Wicks, L.; Blumfield, M.; Collins, C.E. A cross sectional study investigating weight management motivations, methods and perceived healthy eating and physical activity influences in women up to five years following childbirth. Midwifery 2017, 49, 124–133. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| History of preeclampsia (within four years of diagnosis) | Currently or recently pregnant (<3 months postpartum) |
| Aged 18 to 45 years | Planning to become pregnant within the next three months |
| Internet access and email address | Non-English speaking |
| Able to attend assessments at The University of Newcastle Callaghan campus | Type I or II Diabetes Mellitus due to potential impact on secondary outcomes |
| Interested in all or some of the topics below: (a) Improving eating habits. (b) Improving physical activity levels (c) Managing their body weight (d) Managing their stress | Currently participating in another lifestyle behavior intervention |
| Completed postpartum check-up at six weeks with no further follow-up required | Unable to provide the contact details of their General Practitioner to allow for follow-up pf any identified concerns from measurement of cardiovascular risk markers |
| Outcome | Description |
|---|---|
| Absolute CVD Risk | |
| Absolute full CVD risk | Determined using the Framingham CVD 30-year risk score [28]. The score considers age, sex, total and high-density lipoprotein cholesterol (HDL-C), current smoking status, systolic blood pressure, use of antihypertensive treatment, and diagnosis of diabetes |
| CVD Risk Markers | |
| Weight | Measured to the nearest 0.01 kg on a digital scale |
| Body mass index (BMI) | Calculated from measured height and weight using the standard equation: weight (kg)/height (m2) |
| Waist circumference | Measured to the nearest 0.1 cm using a non-extensible steel tape measure |
| Blood pressure | Systolic and diastolic blood pressure were measured using an automatic sphygmomanometer |
| Cardiovascular biomarkers | Fasted blood samples were collected to measure total cholesterol, high-density lipoprotein (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, glucose and insulin |
| Health Behaviors | |
| Physical activity | The International Physical Activity Questionnaire (IPAQ) (short-form) was used to calculate MET-minutes per week and categorize physical activity level (low, medium or high) [29]. Participation in resistance-based physical activity was also assessed (duration and frequency). |
| Sitting time | The Domain-Specific Sitting Questionnaire (adapted version) was used to assess weekday and weekend-day sitting time across five domains [30,31] |
| Dietary intake | The Australian Eating Survey Food Frequency Questionnaire (AES FFQ)—CVD version was used to assess dietary intake, including 66 supplementary questions specific to foods and nutrients related to CVD health [32] |
| Depression, anxiety and stress | The Depression, Anxiety and Stress Scale (DASS) (long version) was used to assess depression, anxiety and stress on individual scales [33] 2 |
| General Health and Wellbeing | |
| Quality of life | The Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (Q-LES-Q-SF) [34] and Satisfaction with Life Scale (SWLS) were used to assess quality of life and satisfaction with life [35] |
| Characteristic | Total (n = 31) | Intervention (n = 16) | Control (n = 15) |
|---|---|---|---|
| Socio-Demographic Characteristics | |||
| Age (years) | 33.4 ± 4.6 | 33.6 ± 4.6 | 33.1 ± 5.1 |
| Country of birth | |||
| Australia | 93.6 (29) | 93.8 (15) | 93.3 (14) |
| Other | 6.4 (2) | 6.3 (1) | 6.7 (1) |
| Marital status | |||
| Never married | 16.1 (5) | 18.8 (3) | 13.3 (2) |
| Married | 80.7 (25) | 75.0 (12) | 86.7 (13) |
| Separated/divorced | 3.2 (1) | 6.3 (1) | 0 (0) |
| Highest education level completed | |||
| University degree | 48.4 (15) | 43.8 (7) | 53.3 (8) |
| Diploma or Trade | 35.5 (11) | 15.1 (4) | 46.7 (7) |
| High school | 16.2 (5) | 14.3 (5) | 0 |
| Combined household income | |||
| ≥$2000/week | 32.2 (10) | 37.5 (6) | 26.7 (4) |
| ≥$1000/week and <$2000/week | 45.2 (14) | 56.3 (9) | 33.3 (5) |
| <$1000/week | 3.2 (1) | 0 | 6.7 (1) |
| Do not know or wish to answer | 5 | 0 | 33.3 (5) |
| Number of children/dependents | |||
| Total | 1.9 ± 0.9 | 1.9 ± 1.1 | 1.9 ± 0.7 |
| Under 2 years | 0.6 ± 0.6 | 0.6 ± 0.5 | 0.6 ± 0.6 |
| 2 to 5 years | 1.0 ± 0.8 | 0.9 ± 0.7 | 1.1 ± 0.8 |
| 6 to 10 years | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.1 ± 0.4 |
| 11 years and over | 0.1 ± 0.5 | 0.2 ± 0.8 | 0 ± 0 |
| Pregnancy and preeclampsia history | |||
| Number of pregnancies | 2.6 ± 2.0 | 3.1 ± 2.5 | 2.1 ± 1.4 |
| Number of births | 1.7 ± 0.7 | 1.8 ± 0.6 | 1.6 ± 0.9 |
| Number of pregnancies complicated by preeclampsia | |||
| One | 80.7 (25) | 81.3 (13) | 80.0 (12) |
| Two | 19.4 (6) | 18.8 (3) | 20.0 (3) |
| Time since most recent pregnancy complicated by preeclampsia | |||
| 3 months to <1 year | 22.6 (7) | 18.8 (3) | 26.7 (4) |
| ≥1 to <2 years | 9.7 (3) | 6.3 (1) | 13.3 (2) |
| ≥2 to 4 years | 67.7 (21) | 75.0 (12) | 60.0 (9) |
| Time of preeclampsia diagnosis a | |||
| < 34 weeks gestation | 60.0 (18) | 73.3 (11) | 46.7 (7) |
| 34–37 weeks gestation | 10.0 (3) | 6.7 (1) | 13.3 (2) |
| ≥ 37 weeks gestation | 20.0 (6) | 20.0 (3) | 20.0 (3) |
| Postpartum gestation | 10.0 (3) | 0 | 20.0 (3) |
| Pregnancy outcome | |||
| Live birth (> 37 weeks) | 51.6 (16) | 56.3 (9) | 46.7 (7) |
| Live preterm birth (< 37 weeks) | 45.2 (14) | 37.5 (6) | 53.3 (8) |
| Stillbirth | 3.2 (1) | 6.3 (1) | 0 |
| Absolute CVD risk | |||
| Framingham CVD 30-year Risk (Hard) (%) | 7.2 ± 4.2 | 5.8 ± 3.4 | 8.7 ± 4.6 |
| Low risk (<10%) | 80.0 (24) | 86.7 (13) | 73.3 (11) |
| Intermediate risk (10–20%) | 20.0 (6) | 13.3 (2) | 26.7 (4) |
| High risk (>20%) | 0 | 0 | 0 |
| CVD Risk Markers | |||
| Weight (kg) | 80.3 ± 20.6 | 72.6 ± 11.7 | 88.5 ± 25.0 |
| Body mass index (kg/m2) | 29.8 ± 7.3 | 27.1 ± 4.4 | 32.7 ± 8.7 |
| Healthy (18.5 to 24.9) | 19.4 (6) | 37.5 (6) | 0 |
| Overweight (25.0 to 29.9) | 45.2 (14) | 43.8 (7) | 46.7 (7) |
| Obese (≥ 30.0) | 35.5 (11) | 18.8 (3) | 53.3 (8) |
| Waist circumference (cm) | 91.9 ± 13.7 | 87.1 ± 9.1 | 96.9 ± 16.2 |
| Body fat (%) | 38.6 ± 8.2 | 35.9 ± 7.6 | 41.5 ± 7.9 |
| Blood pressure (mmHg) | |||
| Systolic | 111.6 ± 13.9 | 104.9 ± 10.5 | 118.6 ± 13.8 |
| Diastolic | 76.0 ± 8.5 | 73.4 ± 7.1 | 78.7 ± 9.2 |
| Cardiovascular blood biomarkers a: | |||
| Total cholesterol (mmol/L) | 4.7 ± 0.9 | 4.5 ± 1.1 | 4.8 ± 0.8 |
| HDL-C (mmol/L) | 1.4 ± 0.3 | 1.5 ± 0.3 | 1.2 ± 0.3 |
| LDL-C (mmol/L) | 2.8 ± 0.8 | 2.6 ± 1.0 | 3.0 ± 0.6 |
| Triglycerides (mmol/L) | 1.0 ± 0.6 | 0.8 ± 0.6 | 1.2 ± 0.6 |
| Glucose (mmol/L) | 4.7 ± 0.6 | 4.5 ± 0.6 | 4.8 ± 0.6 |
| Insulin (mIU/L) | 10.0 ± 8.5 | 6.5 ± 3.3 | 13.4 ± 10.7 |
| Health Behaviours | |||
| Physical activity (MET min/week) b: | 2304 ± 2497 | 2345 ± 2720 | 2256 ± 2337 |
| Resistance-based activities (minutes/week) | 31±63 | 28±58 | 33±70 |
| Sitting time | |||
| Weekdays (minutes/day) | 495 ± 214 | 423 ± 176 | 571 ± 230 |
| Weekend days (minutes/day) | 480 ± 161 | 473 ± 181 | 488 ± 142 |
| Dietary intake | |||
| Total energy (kJ/day) (kcal/day) | 9097 ± 2852 2174 ± 682 | 9712 ± 2414 2321 ± 577 | 8441 ± 3207 2018 ± 767 |
| Discretionary energy (kJ/day) (kcal/day) | 3132 ± 1487 749 ± 355 | 3318 ± 1443 793 ± 345 | 2934 ± 1558 701 ± 372 |
| Discretionary (% energy) | 34.8 ± 12.2 | 34.3 ± 11.9 | 35.3 ± 13.0 |
| Protein (% energy) | 18.9 ± 3.8 | 18.8 ± 3.5 | 19.1 ± 4.3 |
| Fats (% energy) | 37.7 ± 5.6 | 37.9 ± 6.3 | 37.6 ± 5.1 |
| Saturated fat (% energy) | 13.4 ± 2.7 | 13.2 ± 2.4 | 13.6 ± 3.0 |
| Monounsaturated fat (% energy) | 15.3 ± 2.9 | 15.6 ± 3.0 | 14.9 ± 2.9 |
| Polyunsaturated fat (% energy) | 5.9 ± 1.6 | 5.9 ± 1.6 | 6.0 ± 1.6 |
| Fibre (g) | 28.7 ± 11.8 | 30.8 ± 10.9 | 26.4 ± 12.6 |
| Sodium (mg) | 2034 ±586 | 2165 ± 546 | 1904 ± 615 |
| Fruit (serves/day) | 1.3 ± 1.0 | 1.4 ± 1.1 | 1.3 ± 1.0 |
| Vegetable (serves/day) | 4.2 ± 2.0 | 4.6 ± 2.1 | 3.9 ± 1.8 |
| Nuts (serves/day) | 0.4 ± 0.5 | 0.5 ± 0.5 | 0.4 ± 0.5 |
| Fish (serves/day) | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.2 |
| Legumes (serves/day) | 0.4 ± 0.7 | 0.4 ± 0.6 | 0.4 ± 0.8 |
| Depression, Anxiety and Stress Scale | |||
| Depression Score (0–42 points) | 4.7 ± 4.5 | 4.0 ± 3.5 | 5.4 ± 5.5 |
| Anxiety Score (0–42 points) | 6.3 ± 5.6 | 5.6 ± 4.8 | 7.1 ± 6.5 |
| Stress Score (0–42 points) | 8.5 ± 5.7 | 7.9 ± 4.4 | 9.1 ± 7.0 |
| General Health and Well-Being | |||
| Quality of Life | |||
| Q-LES-Q-SF Score (%) | 59.9 ± 16.0 | 58.5 ± 14.9 | 61.3 ± 17.5 |
| Satisfaction with Life Scale | |||
| Overall score (Max: 35 points) | 25.8 ± 5.2 | 25.9 ± 3.8 | 25.7 ± 6.6 |
| Extremely satisfied (31–35 points) | 12.9 (4) | 6.3 (1) | 20.0 (3) |
| Satisfied (26–30 points) | 51.6 (16) | 62.5 (10) | 40.0 (6) |
| Slightly satisfied (21–25 points) | 19.4 (6) | 18.8 (3) | 20.0 (3) |
| Neutral (20 points) | 6.5 (2) | 6.3 (1) | 6.7 (1) |
| Slightly dissatisfied (15–19 points) | 3.2 (1) | 6.3 (1) | 0 |
| Dissatisfied (10–14 points) | 6.5 (2) | 0 | 13.3 (2) |
| Program Components | How Healthy is Your Heart? (n = 11) | My Goals (n = 7) | Track My Progress (n = 4) | Website Resources (n = 12) | Email Newsletters (n = 10) |
|---|---|---|---|---|---|
| Useful information about healthy eating | 4.4 ± 0.5 | NA | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.2 ± 0.4 |
| Useful information about exercise | 4.3 ± 0.4 | NA | 4.3 ± 0.4 | 4.1 ± 0.3 | 4.2 ± 0.4 |
| Useful information about weight management | 4.1 ± 0.5 | NA | 3.8 ± 0.8 | 4.1 ± 0.5 | 4.2 ± 0.4 |
| Useful information about stress management | 4.1 ± 0.5 | NA | 4.3 ± 0.4 | 4.1 ± 0.5 | 4.2 ± 0.4 |
| Helped me to attain my goals | 3.6 ± 0.9 | 3.4 ± 0.5 | 4.0 ± 0.7 | 3.6 ± 0.8 | 3.8 ± 0.7 |
| Motivated me | 4.0 ± 0.7 | 3.9 ± 0.6 | 4.0 ± 1.2 | 3.8 ± 0.6 | 4.1 ± 0.7 |
| Made me feel accountable | 4.1 ± 0.9 | 3.9 ± 0.6 | 4.3 ± 0.8 | 3.8 ± 0.6 | 4.2 ± 0.6 |
| Was easy to access/use | 4.2 ± 0.4 | 4 ± 0.0 | 4.3 ± 0.4 | 4.0 ± 0.4 | 4.2 ± 0.4 |
| Was visually appealing | 4.4 ± 0.5 | NA | NA | 4.1 ± 0.3 | 4.2 ± 0.4 |
| Overall Component satisfaction (n = 13) | 4.2 ± 0.4 | 3.7 ± 0.4 | 3.6 ± 0.8 | 4.0 ± 0.6 | 4.2 ± 0.7 |
| Outcome Measures | Mean (95% CI) Change from Baseline to 3 Months | Mean Difference between Groups | Effect Size (Cohens d) | |
|---|---|---|---|---|
| Intervention (n = 16) | Control (n = 15) | |||
| Absolute CVD Risk | ||||
| Framingham CVD-30 years Risk Score | 0.4 (−0.5, 1.3) | 0.9 (−0.1, 1.9) | −0.5 (−0.9, 1.9) | −0.12 |
| CVD Risk Markers | ||||
| Weight (kg) | −0.1 (−1.5, 1.3) | −0.1 (−1.6, 1.4) | 0.1 (−2.1, 2.0) | 0.00 |
| Body mass index (kg/m2) | −0.04 (−0.6, 0.5) | −0.1 (−0.7, 0.5) | 0.1 (−0.8, 0.7) | 0.00 |
| Waist circumference (cm) | −0.7 (−3.0, 1.7) | −0.6 (−3.2, 1.9) | −0.1 (−3.4, 3.5) | −0.00 |
| Body fat (%) | 0.9 (−1.3, 3.1) | 0.4 (−2.0, 2.8) | −0.5 (−3.7, 2.8) | −0.06 |
| Blood pressure (mmHg) | ||||
| Systolic | 3.2 (−1.6, 8.0) | −1.0 (−6.2, 4.1) | 4.2 (−11.3, 2.8) | 0.30 |
| Diastolic | 3.1 (−1.1, 7.3) | 1.2 (−3.3, 5.7) | 1.9 (−8.1, 4.3) | 0.23 |
| CVD biomarkers | ||||
| Total chol. (mmol/L) | 0.01 (−0.5, 0.5) | 0.5 (0.04, 1.04) * | −0.5 (−0.2, 1.2) | −0.58 |
| HDL-C (mmol/L) | −0.0001 (−0.2, 0.1) | 0.1 (−0.1, 0.2) | −0.1 (−0.1, 0.3) | −0.27 |
| LDL-C (mmol/L) | −0.1 (−0.5, 0.4) | 0.4 (−0.1, 0.8) | −0.5 (−0.2, 1.1) | −0.56 |
| Triglycerides (mmol/L) | 0.1 (−0.1, 0.4) | 0.1 (−0.2, 0.4) | 0.002 (−0.4, 0.4) | 0.00 |
| Glucose (mmol/L) | 0.03 (−0.3, 0.4) | −0.3 (−0.7, 0.03) | 0.4 (-0.9, 0.1) | 0.60 |
| Insulin (mIU/L) | 1.0 (−1.8, 3.6) | −2.5 (−5.4, 0.4) | 3.4 (−7.4, 0.5) | 0.40 |
| Health Behaviours | ||||
| Physical activity | ||||
| Physical activity (MET min/week) | −863 (−1965, 239) | 551 (−829, 1930) | −1413 (−354, 3181) | −0.57 |
| Resistance training (min/week) | 25 (−7,57) | −21 (−56,14) | −47 (−94, 1) | −0.75 |
| Sitting time | ||||
| Weekdays (min/day) | −32 (−128, 65) | −1 (−105, 103) | −30 (−112, 173) | −0.14 |
| Weekend days (min/day) | −53 (−133, 27) | −45 (−131, 41) | −8 (−109, 125) | −0.05 |
| Dietary intake | ||||
| Total energy (kJ/day) (Kcal/day) | −466 (−1555, 622) −111 (−372,149) | 97 (−1080, 1274) 23 (−258,305) | −563 (−1041, 2167) −135 (−249,518) | −0.20 |
| Discretionary (% energy) | −0.03 (−4.5, 4.4) | 0.8 (−4.1, 5.6) | −0. 8 (−5. 8, 7.3) | −0.06 |
| Protein (% energy) | 0.2 (−1.8, 2.2) | −0.1 (−2.2, 2.1) | 0.3 (−3.2, 2.7) | 0.08 |
| Fats (% energy) | −0.4 (−2.1, 1.2) | −2.5 (−4.3, −0.7) | 2.1 (−4.5, 0.3) | 0.37 |
| Saturated fat (% energy) | −0.01 (−0.9, 0.9) | −1.2 (−2.2, -0.2) | 1.2 (−2.5, 0.2) | 0.43 |
| MUFA (% energy) | −1.0 (−2.1, 0.1) | −0.6 (−1.8, 0.6) | 0.4 (−1.3, 2.0) | 0.13 |
| PUFA (% energy) | 0.3 (−0.6, 1.3) | −0.5 (−1.5, 0.5) | 0.8 (−2.2, 0.6) | 0.51 |
| Fibre (g) | 0.3 (−3.9, 4.4) | 1.2 (−3.2, 5.7) | −1.0 (−5.1, 7.0) | −0.08 |
| Sodium (mg) | −94 (−361, 173) | 267 (−22, 556) | −361 (−32, 754) | −0.62 |
| Fruit (serves/day) | 0.4 (−0.03, 0.7) | 0.3 (−0.1, 0.7) | 0.1 (−0.6, 0.5) | 0.07 |
| Vegetable (serves/day) | 0.4 (−0.5, 1.2) | −0.2 (−1.2, 0.7) | 0.6 (−1.9, 0.7) | 0.31 |
| Nuts (serves/day) | −0.3 (−0.5, 0.01) | −0.1 (−0.3, 0.2) | −0.2 (−0.2, 0.6) | −0.38 |
| Fish (serves/day) | 0.1 (−0.01, 0.1) | −0.01 (−0.1, 0.1) | 0.1 (−0.1, 0.03) | 0.30 |
| Legumes (serves/day) | 0.1 (−0.03, 0.1) | 0.1 (−0.03, 0.2) | −0.01 (−0.1, 0.1) | −0.01 |
| Depression, Anxiety and Stress Scale | ||||
| Depression | −0.3 (−2.4, 1.8) | −1.7 (−4.0, 0.5) | 1.5 (−4.5, 1.6) | 0.32 |
| Anxiety | −0.5 (−3.2, 2.3) | −2.4 (−5.3, 0.6) | 1.9 (−5.9, 2.1) | 0.34 |
| Stress | −0.9 (−3.6, 1.7) | −2.2 (−5.1, 0.7) | 1.2 (−5.2, 2.7) | 0.22 |
| General health and wellbeing | ||||
| Q-LES-Q-SF Score (%) | 5.7 (−1.9, 13.2) | 4.5 (−3.7, 12.6) | 1.2 (−12.3, 9.9) | 0.08 |
| Satisfaction with Life Scale overall score | 0.6 (−1.8, 2.9) | 1.1 (−1.5, 3.6) | −0.5 (−3.0, 4.0) | −0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutchesson, M.J.; Taylor, R.; Shrewsbury, V.A.; Vincze, L.; Campbell, L.E.; Callister, R.; Park, F.; Schumacher, T.L.; Collins, C.E. Be Healthe for Your Heart: A Pilot Randomized Controlled Trial Evaluating a Web-Based Behavioral Intervention to Improve the Cardiovascular Health of Women with a History of Preeclampsia. Int. J. Environ. Res. Public Health 2020, 17, 5779. https://doi.org/10.3390/ijerph17165779
Hutchesson MJ, Taylor R, Shrewsbury VA, Vincze L, Campbell LE, Callister R, Park F, Schumacher TL, Collins CE. Be Healthe for Your Heart: A Pilot Randomized Controlled Trial Evaluating a Web-Based Behavioral Intervention to Improve the Cardiovascular Health of Women with a History of Preeclampsia. International Journal of Environmental Research and Public Health. 2020; 17(16):5779. https://doi.org/10.3390/ijerph17165779
Chicago/Turabian StyleHutchesson, Melinda J., Rachael Taylor, Vanessa A. Shrewsbury, Lisa Vincze, Linda E. Campbell, Robin Callister, Felicity Park, Tracy L. Schumacher, and Clare E. Collins. 2020. "Be Healthe for Your Heart: A Pilot Randomized Controlled Trial Evaluating a Web-Based Behavioral Intervention to Improve the Cardiovascular Health of Women with a History of Preeclampsia" International Journal of Environmental Research and Public Health 17, no. 16: 5779. https://doi.org/10.3390/ijerph17165779
APA StyleHutchesson, M. J., Taylor, R., Shrewsbury, V. A., Vincze, L., Campbell, L. E., Callister, R., Park, F., Schumacher, T. L., & Collins, C. E. (2020). Be Healthe for Your Heart: A Pilot Randomized Controlled Trial Evaluating a Web-Based Behavioral Intervention to Improve the Cardiovascular Health of Women with a History of Preeclampsia. International Journal of Environmental Research and Public Health, 17(16), 5779. https://doi.org/10.3390/ijerph17165779







