Physical Activity Promotes Health and Reduces Cardiovascular Mortality in Depressed Populations: A Literature Overview
Abstract
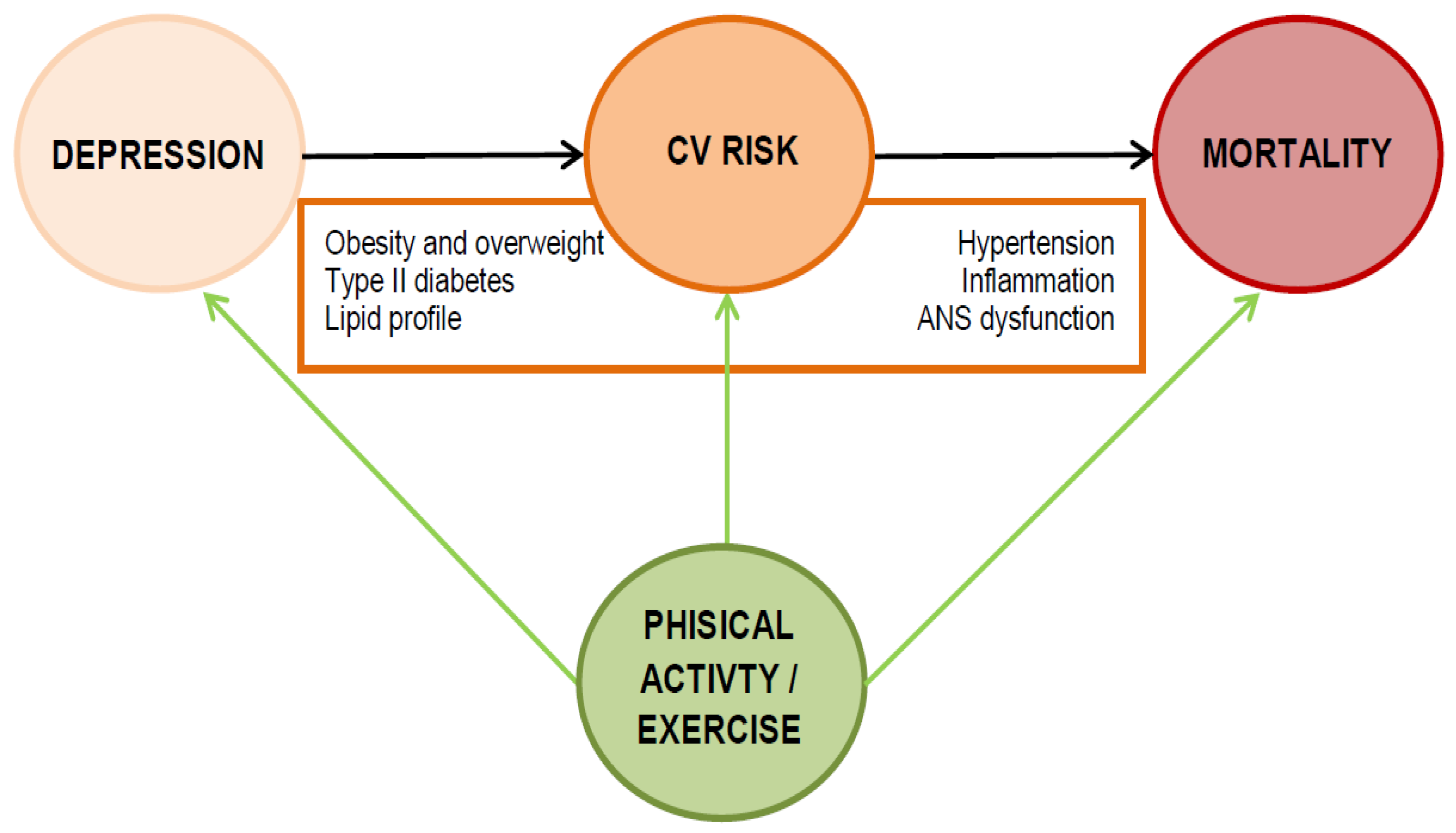
1. Introduction
2. Depression Increases Mortality
3. Which Mechanisms Are Involved in the Higher Cardiovascular Risk of Depression?
3.1. Biological Factors
3.2. Psychosocial Factors
4. Physical Activity May Narrow the Mortality Gap of Depression
5. How to Prescribe Physical Activity to Depressed Individuals
Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laursen, T.M.; Musliner, K.L.; Benros, M.E.; Vestergaard, M.; Munk-Olsen, T. Mortality and life expectancy in persons with severe unipolar depression. J. Affect. Disord. 2016, 193, 203–207. [Google Scholar] [CrossRef]
- Walker, E.R.; McGee, R.E.; Druss, B.G.; Reisinger, E.; McGee, R.E.; Druss, B.G. Mortality in Mental Disorders and Global Disease Burden Implications: A Systematic Review and Meta-analysis. JAMA Psychiatr. 2015, 72, 334–341. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Carnethon, M.R.; Matthews, K.A.; McIntyre, R.S.; Miller, G.E.; Raghuveer, G.; Stoney, C.M.; Wasiak, H.; McCrindle, B.W. Major Depressive Disorder and Bipolar Disorder Predispose Youth to Accelerated Atherosclerosis and Early Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2015, 132, 965–986. [Google Scholar] [CrossRef]
- Dhar, A.K.; Barton, D.A. Depression and the link with cardiovascular disease. Front. Psychiatr. 2016, 7, 33. [Google Scholar] [CrossRef]
- Wu, Q.; Kling, J.M. Depression and the Risk of Myocardial Infarction and Coronary Death. Medicine 2016, 95, e2815. [Google Scholar] [CrossRef]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A scientific statement from the american heart association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- Alvares, G.A.; Quintana, D.S.; Hickie, I.B.; Guastella, A.J. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. J. Psychiatr. Neurosci. 2016, 41, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Leighton, S.P.; Nerurkar, L.; Krishnadas, R.; Johnman, C.; Graham, G.J.; Cavanagh, J. Chemokines in depression in health and in inflammatory illness: A systematic review and meta-Analysis. Mol. Psychiatr. 2018, 23, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Naci, H.; Ioannidis, J.P.A. Comparative effectiveness of exercise and drug interventions on mortality outcomes: Metaepidemiological study. Br. J. Sports Med. 2015, 49, 1414–1422. [Google Scholar] [CrossRef]
- Schuch, F.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.; Reichert, T.; Bagatini, N.C.; Bgeginski, R.; Stubbs, B. Physical activity and sedentary behavior in people with major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2017, 210, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Ekkekakis, P.; Belvederi Murri, M. Exercise as antidepressant treatment: Time for the transition from trials to clinic? Gen. Hosp. Psychiatr. 2017, 49, A1–A5. [Google Scholar] [CrossRef] [PubMed]
- Kvam, S.; Kleppe, C.L.; Nordhus, I.H.; Hovland, A. Exercise as a treatment for depression: A meta-analysis. J. Affect. Disord. 2016, 202, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Vogelzangs, N.; Twisk, J.; Kleiboer, A.; Li, J.; Penninx, B.W. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am. J. Psychiatr. 2014, 171, 453–462. [Google Scholar] [CrossRef]
- Cuijpers, P.; Vogelzangs, N.; Twisk, J.; Kleiboer, A.; Li, J.; Penninx, B.W. Is excess mortality higher in depressed men than in depressed women? A meta-analytic comparison. J. Affect. Disord. 2014, 161, 47–54. [Google Scholar] [CrossRef]
- Lynch, J.; Smith, G.D.; Harper, S.; Hillemeier, M.; Ross, N.; Kaplan, G.A.; Wolfson, M. Is income inequality a determinant of population health? Part 1: A systematic review. Milbank Q. 2004, 82, 5–99. [Google Scholar] [CrossRef]
- Brandão, D.J.; Fontenelle, L.F.; da Silva, S.A.; Menezes, P.R.; Pastor-Valero, M. Depression and excess mortality in the elderly living in low- and middle-income countries: Systematic review and meta-analysis. Int. J. Geriatr. Psychiatr. 2019, 34, 22–30. [Google Scholar] [CrossRef]
- Machado, M.O.; Veronese, N.; Sanches, M.; Stubbs, B.; Koyanagi, A.; Thompson, T.; Tzoulaki, I.; Solmi, M.; Vancampfort, D.; Schuch, F.B.; et al. The association of depression and all-cause and cause-specific mortality: An umbrella review of systematic reviews and meta-analyses. BMC Med. 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Leung, Y.W.; Flora, D.B.; Gravely, S.; Irvine, J.; Carney, R.M.; Grace, S.L. The impact of premorbid and postmorbid depression onset on mortality and cardiac morbidity among patients with coronary heart disease: Meta-analysis. Psychosom. Med. 2012, 74, 786. [Google Scholar] [CrossRef]
- Gathright, E.C.; Goldstein, C.M.; Josephson, R.A.; Hughes, J.W. Depression increases the risk of mortality in patients with heart failure: A meta-analysis. J. Psychosom. Res. 2017, 94, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Vogelzangs, N.; Twisk, J.; Kleiboer, A.; Li, J.; Penninx, B.W. Differential mortality rates in major and subthreshold depression: Meta-analysis of studies that measured both. Br. J. Psychiatr. 2013, 202, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa-Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E.; et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatr. 2017, 16, 163–180. [Google Scholar] [CrossRef]
- Gan, Y.; Gong, Y.; Tong, X.; Sun, H.; Cong, Y.; Dong, X.; Wang, Y.; Xu, X.; Yin, X.; Deng, J.; et al. Depression and the risk of coronary heart disease: A meta-analysis of prospective cohort studies. BMC Psychiatr. 2014, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Liu, T.; Liang, J.; Hu, D.; Yang, B. Depression and Risk of Sudden Cardiac Death and Arrhythmias: A Meta-Analysis. Psychosom. Med. 2017, 79, 153–161. [Google Scholar] [CrossRef]
- Mannan, M.; Mamun, A.; Doi, S.; Clavarino, A. Is there a bi-directional relationship between depression and obesity among adult men and women? Systematic review and bias-adjusted meta analysis. Asian J. Psychiatr. 2016, 21, 51–66. [Google Scholar] [CrossRef]
- Solmi, M.; Köhler, C.A.; Stubbs, B.; Koyanagi, A.; Bortolato, B.; Monaco, F.; Vancampfort, D.; Machado, M.O.; Maes, M.; Tzoulaki, I.; et al. Environmental risk factors and nonpharmacological and nonsurgical interventions for obesity: An umbrella review of meta-analyses of cohort studies and randomized controlled trials. Eur. J. Clin. Investig. 2018, 48. [Google Scholar] [CrossRef]
- Vancampfort, D.; Mitchell, A.J.; De Hert, M.; Sienaert, P.; Probst, M.; Buys, R.; Stubbs, B. Type 2 diabetes in patients with major depressive disorder: A meta-analysis of prevalence estimates and predictors. Depress. Anxiety 2015, 32, 763–773. [Google Scholar] [CrossRef]
- Meng, L.; Chen, D.; Yang, Y.; Zheng, Y.; Hui, R. Depression increases the risk of hypertension incidence: A meta-analysis of prospective cohort studies. J. Hypertens. 2012, 30, 842–851. [Google Scholar] [CrossRef]
- Vancampfort, D.; Correll, C.U.; Wampers, M.; Sienaert, P.; Mitchell, A.J.; De Herdt, A.; Probst, M.; Scheewe, T.W.; De Hert, M. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: A meta-analysis of prevalences and moderating variables. Psychol. Med. 2014, 44, 2017–2028. [Google Scholar] [CrossRef]
- Persons, J.E.; Fiedorowicz, J.G. Depression and serum low-density lipoprotein: A systematic review and meta-analysis. J. Affect. Disord. 2016, 206, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Belvederi Murri, M.; Pariante, C.; Mondelli, V.; Masotti, M.; Atti, A.R.; Mellacqua, Z.; Antonioli, M.; Ghio, L.; Menchetti, M.; Zanetidou, S.; et al. HPA axis and aging in depression: Systematic review and meta-analysis. Psychoneuroendocrinology 2014, 41, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatr. 2015, 20, 32–47. [Google Scholar] [CrossRef]
- Kemp, A.H.; Quintana, D.S.; Gray, M.A.; Felmingham, K.L.; Brown, K.; Gatt, J.M. Impact of Depression and Antidepressant Treatment on Heart Rate Variability: A Review and Meta-Analysis. Biol. Psychiatr. 2010, 67, 1067–1074. [Google Scholar] [CrossRef]
- Poole, L.; Dickens, C.; Steptoe, A. The puzzle of depression and acute coronary syndrome: Reviewing the role of acute inflammation. J. Psychosom. Res. 2011, 71, 61–68. [Google Scholar] [CrossRef]
- Alexopoulos, G.S. Mechanisms and treatment of late-life depression. Transl. Psychiatr. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- McNutt, M.D.; Liu, S.; Manatunga, A.; Royster, E.B.; Raison, C.L.; Woolwine, B.J.; Demetrashvili, M.F.; Miller, A.H.; Musselman, D.L. Neurobehavioral effects of interferon-α in patients with hepatitis-C: Symptom dimensions and responsiveness to paroxetine. Neuropsychopharmacology 2012, 37, 1444–1454. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Hepgul, N.; Pariante, C.M. Inflammation and Depression. Curr. Top. Behav. Neurosci. 2013, 14, 135–151. [Google Scholar]
- Baumeister, D.; Akhtar, R.; Ciufolini, S.; Pariante, C.M.; Mondelli, V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatr. 2016, 21, 642–649. [Google Scholar] [CrossRef]
- Barnes, J.; Mondelli, V.; Pariante, C.M. Genetic Contributions of Inflammation to Depression. Neuropsychopharmacology 2017, 42, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur. Neuropsychopharmacol. 2017, 27, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain. Behav. Immun. 2019, 81, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Escelsior, A.; Sterlini, B.; Belvederi Murri, M.; Valente, P.; Amerio, A.; di Brozolo, M.R.; da Silva, B.P.; Amore, M. Transient receptor potential vanilloid 1 antagonism in neuroinflammation, neuroprotection and epigenetic regulation: Potential therapeutic implications for severe psychiatric disorders treatment. Psychiatr. Genet. 2020, 30, 39–48. [Google Scholar] [CrossRef]
- Moriarity, D.P.; Kautz, M.M.; Mac Giollabhui, N.; Klugman, J.; Coe, C.L.; Ellman, L.M.; Abramson, L.Y.; Alloy, L.B. Bidirectional associations between inflammatory biomarkers and depressive symptoms in adolescents: Potential causal relationships. Clin. Psychol. Sci. 2020. [Google Scholar] [CrossRef]
- Kahl, K.G.; Stapel, B.; Frieling, H. Link between depression and cardiovascular diseases due to epigenomics and proteomics: Focus on energy metabolism. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2019, 89, 146–157. [Google Scholar] [CrossRef]
- Carnevali, L.; Montano, N.; Statello, R.; Sgoifo, A. Rodent models of depression-cardiovascular comorbidity: Bridging the known to the new. Neurosci. Biobehav. Rev. 2017, 76, 144–153. [Google Scholar] [CrossRef]
- Carnevali, L.; Montano, N.; Tobaldini, E.; Thayer, J.F.; Sgoifo, A. The contagion of social defeat stress: Insights from rodent studies. Neurosci. Biobehav. Rev. 2020, 111, 12–18. [Google Scholar] [CrossRef]
- Quirk, S.E.; Williams, L.J.; O’Neil, A.; Pasco, J.A.; Jacka, F.N.; Housden, S.; Berk, M.; Brennan, S.L. The association between diet quality, dietary patterns and depression in adults: A systematic review. BMC Psychiatr. 2013, 13, 1. [Google Scholar] [CrossRef]
- Boden, J.M.; Fergusson, D.M. Alcohol and depression. Addiction 2011, 106, 906–914. [Google Scholar] [CrossRef]
- Chaiton, M.O.; Cohen, J.E.; O’Loughlin, J.; Rehm, J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health 2009, 9, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Hitsman, B.; Papandonatos, G.D.; McChargue, D.E.; Demott, A.; Herrera, M.J.; Spring, B.; Borrelli, B.; Niaura, R. Past major depression and smoking cessation outcome: A systematic review and meta-analysis update. Addiction 2013, 108, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Grenard, J.L.; Munjas, B.A.; Adams, J.L.; Suttorp, M.; Maglione, M.; McGlynn, E.A.; Gellad, W.F. Depression and medication adherence in the treatment of chronic diseases in the United States: A meta-analysis. J. Gen. Intern. Med. 2011, 26, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Caruso, R.; Da Ronch, C.; Härter, M.; Schulz, H.; Volkert, J.; Dehoust, M.; Sehner, S.; Suling, A.; Wegscheider, K.; et al. Quality of life, level of functioning, and its relationship with mental and physical disorders in the elderly: Results from the MentDis_ICF65+ study. Health Qual. Life Outcomes 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Da Ronch, C.; Canuto, A.; Volkert, J.; Massarenti, S.; Weber, K.; Dehoust, M.C.; Nanni, M.G.; Andreas, S.; Sehner, S.; Schulz, H.; et al. Association of television viewing with mental health and mild cognitive impairment in the elderly in three European countries, data from the MentDis-ICF65+ project. Ment. Health Phys. Act. 2015, 8, 8–14. [Google Scholar] [CrossRef]
- Papasavvas, T.; Bonow, R.O.; Alhashemi, M.; Micklewright, D. Depression Symptom Severity and Cardiorespiratory Fitness in Healthy and Depressed Adults: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 219–230. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.C.; Martínez-Vizcaíno, V. Muscular Strength as a Predictor of All-Cause Mortality in an Apparently Healthy Population: A Systematic Review and Meta-Analysis of Data From Approximately 2 Million Men and Women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. J. Am. Med. Assoc. 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- Chekroud, S.R.; Gueorguieva, R.; Zheutlin, A.B.; Paulus, M.; Krumholz, H.M.; Krystal, J.H.; Chekroud, A.M. Association between physical exercise and mental health in 12 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatr. 2018, 5, 739–746. [Google Scholar] [CrossRef]
- Salman, A.; Sellami, M.; Al-Mohannadi, A.S.; Chun, S. The associations between mental well-being and adherence to physical activity guidelines in patients with cardiovascular disease: Results from the scottish health survey. Int. J. Environ. Res. Public Health 2019, 16, 3596. [Google Scholar] [CrossRef]
- Heath, G.W.; Parra, D.C.; Sarmiento, O.L.; Andersen, L.B.; Owen, N.; Goenka, S.; Montes, F.; Brownson, R.C.; Alkandari, J.R.; Bauman, A.E.; et al. Evidence-based intervention in physical activity: Lessons from around the world. Lancet 2012, 380, 272–281. [Google Scholar] [CrossRef]
- Sallis, J.F.; Bull, F.; Guthold, R.; Heath, G.W.; Inoue, S.; Kelly, P.; Oyeyemi, A.L.; Perez, L.G.; Richards, J.; Hallal, P.C. Progress in physical activity over the Olympic quadrennium. Lancet 2016, 388, 1325–1336. [Google Scholar] [CrossRef]
- Ekkekakis, P. Routledge Handbook of Physical Activity and Mental Health; Routledge: Abingdon-on-Thames, UK, 2013. [Google Scholar]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Holsboer-Trachsler, E.; Pühse, U.; Brand, S. Exercise is medicine for patients with major depressive disorders: But only if the “pill” is taken! Neuropsychiatr. Dis. Treat. 2016, 12, 1977. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical Activity, Exercise and Physical Fitness Definitions for Health-Related Research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- De Carvalho Souza Vieira, M.; Boing, L.; Leitão, A.E.; Vieira, G.; Coutinho de Azevedo Guimarães, A. Effect of physical exercise on the cardiorespiratory fitness of men—A systematic review and meta-analysis. Maturitas 2018, 115, 23–30. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef]
- Hurst, C.; Weston, K.L.; McLaren, S.J.; Weston, M. The effects of same-session combined exercise training on cardiorespiratory and functional fitness in older adults: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2019, 31, 1701–1717. [Google Scholar] [CrossRef]
- Magutah, K.; Thairu, K.; Patel, N. Effect of short moderate intensity exercise bouts on cardiovascular function and maximal oxygen consumption in sedentary older adults. BMJ Open Sport Exerc. Med. 2020, 6, e000672. [Google Scholar] [CrossRef]
- Shaw, K.; Gennat, H.; O’Rourke, P.; Del Mar, C. Exercise for overweight or obesity. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Vazou, S.; Bixby, W.R.; Georgiadis, E. The mysterious case of the public health guideline that is (almost) entirely ignored: Call for a research agenda on the causes of the extreme avoidance of physical activity in obesity. Obes. Rev. 2016, 17, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Fock, K.M.; Khoo, J. Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 2013, 28, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Marson, E.C.; Delevatti, R.S.; Prado, A.K.G.; Netto, N.; Kruel, L.F.M. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: A systematic review and meta-analysis. Prev. Med. 2016, 93, 211–218. [Google Scholar] [CrossRef]
- Verheggen, R.J.H.M.; Maessen, M.F.H.; Green, D.J.; Hermus, A.R.M.M.; Hopman, M.T.E.; Thijssen, D.H.T. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: Distinct effects on body weight and visceral adipose tissue. Obes. Rev. 2016, 17, 664–690. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Sabapathy, S.; Leveritt, M.; Desbrow, B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014, 44, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.; Chan, E.; Giallauria, F.; Graham, P.L.; Smart, N.A. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 37. [Google Scholar] [CrossRef]
- Umpierre, D.; Ribeiro, P.A.B.; Kramer, C.K.; Leitão, C.B.; Zucatti, A.T.N.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef]
- Salman, A.; Ukwaja, K.N.; Alkhatib, A. Factors associated with meeting current recommendation for physical activity in scottish adults with diabetes. Int. J. Environ. Res. Public Health 2019, 16, 3857. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef]
- Ashton, R.E.; Tew, G.A.; Aning, J.J.; Gilbert, S.E.; Lewis, L.; Saxton, J.M. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: Systematic review with meta-analysis. Br. J. Sports Med. 2018, 54, 341–348. [Google Scholar]
- Naci, H.; Salcher-Konrad, M.; Dias, S.; Blum, M.R.; Sahoo, S.A.; Nunan, D.; Ioannidis, J.P.A. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br. J. Sports Med. 2019, 53, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Hayashino, Y.; Jackson, J.L.; Hirata, T.; Fukumori, N.; Nakamura, F.; Fukuhara, S.; Tsujii, S.; Ishii, H. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Metabolism 2014, 63, 431–440. [Google Scholar] [CrossRef]
- Swardfager, W.; Herrmann, N.; Cornish, S.; Mazereeuw, G.; Marzolini, S.; Sham, L.; Lanctôt, K.L. Exercise intervention and inflammatory markers in coronary artery disease: A meta-analysis. Am. Heart J. 2012, 163, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Villafaina, S.; Collado-Mateo, D.; Fuentes, J.P.; Merellano-Navarro, E.; Gusi, N. Physical Exercise Improves Heart Rate Variability in Patients with Type 2 Diabetes: A Systematic Review. Curr. Diab. Rep. 2017, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.J.; Smart, N.A. Exercise therapy and autonomic function in heart failure patients: A systematic review and meta-analysis. Heart Fail. Rev. 2018, 23, 91–108. [Google Scholar] [CrossRef]
- Nolan, R.P.; Jong, P.; Barry-Bianchi, S.M.; Tanaka, T.H.; Floras, J.S. Effects of drug, biobehavioral and exercise therapies on heart rate variability in coronary artery disease: A systematic review. Eur. J. Prev. Cardiol. 2008, 15, 386–396. [Google Scholar] [CrossRef]
- Besnier, F.; Labrunée, M.; Pathak, A.; Pavy-Le Traon, A.; Galès, C.; Sénard, J.M.; Guiraud, T. Exercise training-induced modification in autonomic nervous system: An update for cardiac patients. Ann. Phys. Rehabil. Med. 2017, 60, 27–35. [Google Scholar] [CrossRef]
- Gerber, M.; Ludyga, S.; Mücke, M.; Colledge, F.; Brand, S.; Pühse, U. Low vigorous physical activity is associated with increased adrenocortical reactivity to psychosocial stress in students with high stress perceptions. Psychoneuroendocrinology 2017, 80, 104–113. [Google Scholar] [CrossRef]
- Klaperski, S.; von Dawans, B.; Heinrichs, M.; Fuchs, R. Effects of a 12-week endurance training program on the physiological response to psychosocial stress in men: A randomized controlled trial. J. Behav. Med. 2014, 37, 1118–1133. [Google Scholar] [CrossRef]
- Chen, C.; Nakagawa, S.; An, Y.; Ito, K.; Kitaichi, Y.; Kusumi, I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocrinol. 2017, 44, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, M.; Vancampfort, D.; Giesen, E.S.; Lundin, A.; Stubbs, B. Exercise as treatment for alcohol use disorders: Systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Klinsophon, T.; Thaveeratitham, P.; Sitthipornvorakul, E.; Janwantanakul, P. Effect of exercise type on smoking cessation: A meta-analysis of randomized controlled trials. BMC Res. Notes 2017, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- De Vries, N.M.; van Ravensberg, C.D.; Hobbelen, J.S.M.; Olde Rikkert, M.G.M.; Staal, J.B.; Nijhuis-van der Sanden, M.W.G. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: A meta-analysis. Ageing Res. Rev. 2012, 11, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Melanson, E.L. The effect of exercise on non-exercise physical activity and sedentary behavior in adults. Obes. Rev. 2017, 18, 40–49. [Google Scholar] [CrossRef]
- Ruotsalainen, H.; Kyngäs, H.; Tammelin, T.; Kääriäinen, M. Systematic review of physical activity and exercise interventions on body mass indices, subsequent physical activity and psychological symptoms in overweight and obese adolescents. J. Adv. Nurs. 2015, 71, 2461–2477. [Google Scholar] [CrossRef]
- Anderson, L.; Thompson, D.R.; Oldridge, N.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Price, K.J.; Gordon, B.A.; Bird, S.R.; Benson, A.C. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? Eur. J. Prev. Cardiol. 2016, 23, 1715–1733. [Google Scholar] [CrossRef]
- Kraus, W.E.; Powell, K.E.; Haskell, W.L.; Janz, K.F.; Campbell, W.W.; Jakicic, J.M.; Troiano, R.P.; Sprow, K.; Torres, A.; Piercy, K.L. Physical Activity, All-Cause and Cardiovascular Mortality, and Cardiovascular Disease. Med. Sci. Sports Exerc. 2019, 51, 1270. [Google Scholar] [CrossRef]
- Saeidifard, F.; Medina-Inojosa, J.R.; West, C.P.; Olson, T.P.; Somers, V.K.; Bonikowske, A.R.; Prokop, L.J.; Vinciguerra, M.; Lopez-Jimenez, F. The association of resistance training with mortality: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 1647–1665. [Google Scholar] [CrossRef]
- Janzon, E.; Abidi, T.; Bahtsevani, C. Can physical activity be used as a tool to reduce depression in patients after a cardiac event? What is the evidence? A systematic literature study. Scand. J. Psychol. 2015, 56, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Gellis, Z.D.; Kang-Yi, C. Meta-analysis of the effect of cardiac rehabilitation interventions on depression outcomes in adults 64 years of age and older. Am. J. Cardiol. 2012, 110, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Martland, R.; Mondelli, V.; Gaughran, F.; Stubbs, B. Can high-intensity interval training improve physical and mental health outcomes? A meta-review of 33 systematic reviews across the lifespan. J. Sports Sci. 2020, 38, 430–469. [Google Scholar] [CrossRef] [PubMed]
- Younge, J.O.; Gotink, R.A.; Baena, C.P.; Roos-Hesselink, J.W.; Hunink, M.G.M. Mind-body practices for patients with cardiac disease: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2015, 11, 1385–1398. [Google Scholar] [CrossRef]
- Puetz, T.W.; Beasman, K.M.; O’connor, P.J. The effect of cardiac rehabilitation exercise programs on feelings of energy and fatigue: A meta-analysis of research from 1945 to 2005. Eur. J. Prev. Cardiol. 2006, 13, 886–893. [Google Scholar] [CrossRef]
- Toni, G.; Belvederi Murri, M.; Piepoli, M.; Zanetidou, S.; Cabassi, A.; Squatrito, S.; Bagnoli, L.; Piras, A.; Mussi, C.; Senaldi, R.; et al. Physical Exercise for Late-Life Depression: Effects on Heart Rate Variability. Am. J. Geriatr. Psychiatr. 2016, 24, 989–997. [Google Scholar] [CrossRef]
- Kahl, K.G.; Kerling, A.; Tegtbur, U.; Gützlaff, E.; Herrmann, J.; Borchert, L.; Ates, Z.; Westhoff-Bleck, M.; Hueper, K.; Hartung, D. Effects of additional exercise training on epicardial, intra-abdominal and subcutaneous adipose tissue in major depressive disorder: A randomized pilot study. J. Affect. Disord. 2016, 192, 91–97. [Google Scholar] [CrossRef]
- Knapen, J.; Vancampfort, D.; Moriën, Y.; Marchal, Y. Exercise therapy improves both mental and physical health in patients with major depression. Disabil. Rehabil. 2015, 37, 1490–1495. [Google Scholar] [CrossRef]
- Siqueira, C.C.; Valiengo, L.L.; Carvalho, A.F.; Santos-Silva, P.R.; Missio, G.; De Sousa, R.T.; Di Natale, G.; Gattaz, W.F.; Moreno, R.A.; Machado-Vieira, R. Antidepressant efficacy of adjunctive aerobic activity and associated biomarkers in major depression: A 4-week, randomized, single-blind, controlled clinical trial. PLoS ONE 2016, 11, e0154195. [Google Scholar] [CrossRef]
- Stubbs, B.; Rosenbaum, S.; Vancampfort, D.; Ward, P.B.; Schuch, F.B. Exercise improves cardiorespiratory fitness in people with depression: A meta-analysis of randomized control trials. J. Affect. Disord. 2016, 190, 249–253. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Rosenbaum, S.; Richards, J.; Ward, P.B.; Veronese, N.; Solmi, M.; Cadore, E.L.; Stubbs, B. Exercise for depression in older adults: A meta-analysis of randomized controlled trials adjusting for publication bias. Rev. Bras. Psiquiatr. 2016, 38, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ekkekakis, P. Honey, i shrunk the pooled SMD! Guide to critical appraisal of systematic reviews and meta-analyses using the Cochrane review on exercise for depression as example. Ment. Health Phys. Act. 2015, 8, 21–36. [Google Scholar] [CrossRef]
- Stubbs, B.; Vancampfort, D.; Rosenbaum, S.; Ward, P.B.; Richards, J.; Soundy, A.; Veronese, N.; Solmi, M.; Schuch, F.B. Dropout from exercise randomized controlled trials among people with depression: A meta-analysis and meta regression. J. Affect. Disord. 2016, 190, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.A.J.; Zvolensky, M.J.; Davis, M.L.; Rosenfield, D.; Marcus, B.H.; Church, T.S.; Powers, M.B.; Frierson, G.M.; Otto, M.W.; Hopkins, L.B.; et al. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with high anxiety sensitivity: A randomized controlled trial. Psychosom. Med. 2016, 78, 354. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.A.; Bronars, C.A.; Douglas, K.S.V.; Ussher, M.H.; Levine, J.A.; Tye, S.J.; Hughes, C.A.; Brockman, T.A.; Decker, P.A.; DeJesus, R.S.; et al. Supervised, vigorous intensity exercise intervention for depressed female smokers: A pilot study. Nicot. Tob. Res. 2017, 19, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, A.M.; Blevins, C.E.; Battle, C.L.; Read, J.P.; Gordon, A.L.; Stein, M.D. Developing a Fitbit-supported lifestyle physical activity intervention for depressed alcohol dependent women. J. Subst. Abus. Treat. 2017, 80, 88–97. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wang, Y.; Li, R.; Zhou, C. Impact of physical exercise on substance use disorders: A meta-analysis. PLoS ONE 2014, 9, e110728. [Google Scholar] [CrossRef]
- Euteneuer, F.; Dannehl, K.; Del Rey, A.; Engler, H.; Schedlowski, M.; Rief, W. Immunological effects of behavioral activation with exercise in major depression: An exploratory randomized controlled trial. Transl. Psychiatr. 2017, 7, e1132. [Google Scholar] [CrossRef]
- Beserra, A.H.N.; Kameda, P.; Deslandes, A.C.; Schuch, F.B.; Laks, J.; de Moraes, H.S. Can physical exercise modulate cortisol level in subjects with depression? A systematic review and meta-analysis. Trends Psychiatr. Psychother. 2018, 40, 360–368. [Google Scholar] [CrossRef]
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef]
- Schuch, F.B.; Deslandes, A.C.; Stubbs, B.; Gosmann, N.P.; Silva, C.T.B.; da Fleck, M.P.d.A. Neurobiological effects of exercise on major depressive disorder: A systematic review. Neurosci. Biobehav. Rev. 2016, 61, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pope, B.S.; Wood, S.K. Advances in understanding mechanisms and therapeutic targets to treat comorbid depression and cardiovascular disease. Neurosci. Biobehav. Rev. 2020. [Google Scholar] [CrossRef]
- National Collaborating Centre for Mental Health. Depression: The Nice Guideline on the Treatment and Management of Depression in Adults; National Collaborating Centre for Mental Health: London, UK, 2010. [Google Scholar]
- Sarris, J.; O’Neil, A.; Coulson, C.E.; Schweitzer, I.; Berk, M. Lifestyle medicine for depression. BMC Psychiatr. 2014, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cairns, K.E.; Yap, M.B.H.; Pilkington, P.D.; Jorm, A.F. Risk and protective factors for depression that adolescents can modify: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2014, 169, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.A.; Lasarow, S.; Baron, S.H. Exercise for the treatment of depression. In Physical Exercise Interventions for Mental Health; Lam, L.C.W., Riba, M., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 26–40. [Google Scholar]
- Nyström, M.B.T.; Neely, G.; Hassmén, P.; Carlbring, P. Treating Major Depression with Physical Activity: A Systematic Overview with Recommendations. Cogn. Behav. Ther. 2015, 44, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Rethorst, C.D.; Trivedi, M.H. Evidence-based recommendations for the prescription of exercise for major depressive disorder. J. Psychiatr. Pract. 2013, 19, 204–212. [Google Scholar] [CrossRef]
- Stanton, R.; Happell, B.M. An exercise prescription primer for people with depression. Issues Ment. Health Nurs. 2013, 34, 626–630. [Google Scholar] [CrossRef]
- Stanton, R.; Reaburn, P. Exercise and the treatment of depression: A review of the exercise program variables. J. Sci. Med. Sport 2014, 17, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.B.; Ekkekakis, P.; Magagnoli, M.; Zampogna, D.; Cattedra, S.; Capobianco, L.; Serafini, G.; Calcagno, P.; Zanetidou, S.; Amore, M. Physical exercise in major depression: Reducing the mortality gap while improving clinical outcomes. Front. Psychiatr. 2019, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Searle, A.; Calnan, M.; Turner, K.M.; Lawlor, D.A.; Campbell, J.; Chalder, M.; Lewis, G. General Practitioners’ beliefs about physical activity for managing depression in primary care. Ment. Health Phys. Act. 2012, 5, 13–19. [Google Scholar] [CrossRef]
- Stanton, R.; Franck, C.; Reaburn, P.; Happell, B. A Pilot Study of the Views of General Practitioners Regarding Exercise for the Treatment of Depression. Perspect. Psychiatr. Care 2014, 51, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Searle, A.; Calnan, M.; Lewis, G.; Campbell, J.; Taylor, A.; Turner, K. Patients’ views of physical activity as treatment for depression: A qualitative study. Br. J. Gen. Pract. 2011, 61, e149–e156. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Rosenbaum, S.; Stubbs, B.; Gorczynski, P.; Yung, A.R.; Vancampfort, D. Motivating factors and barriers towards exercise in severe mental illness: A systematic review and meta-analysis. Psychol. Med. 2016, 46, 2869–2881. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.E.; King, A.C.; Buchner, D.M.; Campbell, W.W.; DiPietro, L.; Erickson, K.I.; Hillman, C.H.; Jakicic, J.M.; Janz, K.F.; Katzmarzyk, P.T.; et al. The scientific foundation for the physical activity guidelines for Americans. J. Phys. Act. Health 2019, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Ladwig, M.A.; Hartman, M.E.; Ekkekakis, P. Affect-based Exercise Prescription: An Idea Whose Time Has Come? ACSMs. Health Fit. J. 2017, 21, 10–15. [Google Scholar] [CrossRef]
- Mata, J.; Hogan, C.L.; Joormann, J.; Waugh, C.E.; Gotlib, I.H. Acute exercise attenuates negative affect following repeated sad mood inductions in persons who have recovered from depression. J. Abnorm. Psychol. 2013, 122, 45–50. [Google Scholar] [CrossRef]
- Mata, J.; Thompson, R.J.; Jaeggi, S.M.; Buschkuehl, M.; Jonides, J.; Gotlib, I.H. Walk on the bright side: Physical activity and affect in major depressive disorder. J. Abnorm. Psychol. 2012, 121, 297–308. [Google Scholar] [CrossRef]
- Weinstein, A.A.; Deuster, P.A.; Francis, J.L.; Beadling, C.; Kop, W.J. The role of depression in short-term mood and fatigue responses to acute exercise. Int. J. Behav. Med. 2010, 17, 51–57. [Google Scholar] [CrossRef]
- Rethorst, C.D.; South, C.C.; Rush, A.J.; Greer, T.L.; Trivedi, M.H. Prediction of treatment outcomes to exercise in patients with nonremitted major depressive disorder. Depress. Anxiety 2017, 34, 1116–1122. [Google Scholar] [CrossRef]
- Suterwala, A.M.; Rethorst, C.D.; Carmody, T.J.; Greer, T.L.; Grannemann, B.D.; Jha, M.; Trivedi, M.H. Affect following first exercise session as a predictor of treatment response in depression. J. Clin. Psychiatr. 2016, 77, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.; Hobson-Powell, A.; Davison, K.; Stanton, R.; Craft, L.L.; Duncan, M.; Elliot, C.; Ward, P.B. The Role of Sport, Exercise, and Physical Activity in Closing the Life Expectancy Gap for People with Mental Illness: An International Consensus Statement by Exercise and Sports Science Australia, American College of Sports Medicine, British Association. Transl. J. Am. Coll. Sports Med. 2018, 3, 72–73. [Google Scholar]
- Gebreslassie, M.; Sampaio, F.; Nystrand, C.; Ssegonja, R.; Feldman, I. Economic evaluations of public health interventions for physical activity and healthy diet: A systematic review. Prev. Med. 2020, 136, 106100. [Google Scholar] [CrossRef] [PubMed]
- Park, A.L.; McDaid, D.; Weiser, P.; Von Gottberg, C.; Becker, T.; Kilian, R. Examining the cost effectiveness of interventions to promote the physical health of people with mental health problems: A systematic review. BMC Public Health 2013, 13, 787. [Google Scholar] [CrossRef] [PubMed]
- Shuval, K.; Leonard, T.; Drope, J.; Katz, D.L.; Patel, A.V.; Maitin-Shepard, M.; Amir, O.; Grinstein, A. Physical activity counseling in primary care: Insights from public health and behavioral economics. CA Cancer J. Clin. 2017, 67, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Zanetidou, S.; Belvederi Murri, M.; Menchetti, M.; Toni, G.; Asioli, F.; Bagnoli, L.; Zocchi, D.; Siena, M.; Assirelli, B.; Luciano, C.; et al. Physical Exercise for Late-Life Depression: Customizing an Intervention for Primary Care. J. Am. Geriatr. Soc. 2017, 65, 348–355. [Google Scholar] [CrossRef] [PubMed]
| Cardiovascular Risk Condition | Studies | Association between Depression and Risk Factor |
|---|---|---|
| Obesity and overweight | [27] | 13 prospective studies on adolescents (of which, 7 evaluating depression leading to obesity and 6 obesity leading to depression). Bi-directional relationship, stronger for depression leading to obesity. Depression or depressive symptoms in adolescents is associated with an increased risk of 70% (RR 1.70, 95% CI: 1.40; 2.07) of becoming obese, while obesity in adolescents is associated with an increased risk of 40% (RR 1.40, 95% CI: 1.26; 1.70) of becoming depressed. |
| Type II diabetes | [29] | 16 studies comparing major depressive disorder (clearly defined) to the general population in terms of the prevalence of type II diabetes. Major depression was associated with a higher risk for type II diabetes (RR 1.49; 95% CI = 1.29–1.72; p < 0.001) (when comparing age- and gender-matched populations: RR 1.36; 95% CI = 1.28–1.44; p < 0.001). |
| Metabolic profile | [31] | 18 cross-sectional studies. Higher prevalence of metabolic syndrome in depressed (30.5%) than control individuals (OR 1.54, 95% CI = 1.21–1.97, p = 0.001); higher risk for hyperglycemia (OR 1.33; 95% CI = 1.03–1.73, p = 0.03) and hypertriglyceridemia (OR 1.17, 95% CI = 1.04–1.30, p = 0.008). Controlling for confounding factors. |
| [32] | 18 cohort studies. Lower LDL (mean difference = −4.29; 95% CI = −8.19, −0.40, p = 0.03) in depression when serum LDL considered as a continuous measure. Lower depression when low LDL (OR 0.90; 95% CI = 0.80–1.01, p = 0.08) when serum LDL considered as a categorical measure. | |
| Hypertension | [30] | 9 prospective studies, 22.367 participants, mean follow-up period 9.6 years. Increased risk of hypertension incidence with adjusted RR 1.42 (95% CI = 1.09–1.86, p = 0.009). |
| Inflammation | [9] | 82 case-control studies. Elevated plasma levels of some cytokines and chemokines in depressed subjects (IL-6, TNF-α, IL-10, sIL-2R, CCL2, IL-13, IL-18, IL-12, sTINFR-2) (g = −0.477, p = 0.043). |
| Autonomic dysfunction | [7] | 29 case-control studies. Lower HRV in depressed individuals (g = −0.349; CI 95% = −0.505, −0.193, p < 0.001). |
| [36] | 18 studies. Depression is associated with a lower HRV (g = −0.301, p < 0.001); negative correlation between depression severity and HRV (r = −0.354, p < 0.001). | |
| Behavioral Factors | ||
| Unbalanced diet | [50] | 3 cross-sectional studies. 2 out of 3 studies support an association between depression and unhealthy diets. |
| Alcohol consumption | [51] | 7 studies (2 out of 7 prospective studies). Increased risk of alcohol use disorder in depressed individuals (adjusted OR 2.09; 95% CI = 1.29–3.38). |
| Tobacco smoking | [52] | 12 prospective studies. Depression predicted onset of smoking in adolescents (RR 1.41; 95% CI = 1.21–1.63, p < 0.001). |
| [53] | 42 clinical trials on smoking cessation. History of depression is associated with lower odds of short-term (OR 0.83; 95% CI = 0.72–0.95, p = 0.009) and long-term abstinence (OR 0.81; 95% CI = 0.67–0.97, p = 0.023). | |
| Compliance to therapy | [54] | 31 studies cross-sectional studies on chronic diseases. Depressed individuals are more often non-adherent to prescribed medications (OR 1.76; 95% CI = 1.22–2.57). |
| Sedentary behaviors | [60] | Cross-sectional study on more than 1 million individuals in US on mental health burden and its association with physical exercise. |
| [12] | 24 cross-sectional studies. Depressed individuals tend to engage less in physical activity (standardized mean difference = −0.251; 95% CI = −0.03, 0.15, p < 0.001) and more in sedentary behavior (standardized mean difference = 0.09; 95% CI = 0.01–0.18, p = 0.02). | |
| Cardiovascular Risk Factor | Studies | Impact of Physical Exercise |
|---|---|---|
| Obesity and overweight | [76] | 117 studies. Exercise has better effects than a hypocaloric diet alone in reducing visceral adiposity (p = 0.08). However, it has less effects on total weight loss than diet alone. |
| [77] | 20 trials. Appetite-regulative hormone levels are acutely influenced by exercise. | |
| Type II diabetes | [78] | 27 prospective randomized or controlled trials of aerobic exercise training in adult subjects with type II diabetes, with a minimum duration of 2 weeks. Reduction in HbA1c% (mean difference = −0.71%; 95% CI = −1.11, −0.31, p = 0.0005) and insulin resistance (mean difference = −1.02, 95% CI = −1.77, −0.28, p = 0.007). |
| Lipid profile | [69] | 160 RCTs. Exercise reduces triglycerides (p = 0.02), and increases HDL (p < 0.001). |
| Hypertension | [81] | 93 RCTs. Reduction in systolic blood pressure and diastolic blood pressure. Different effects for different types of exercise and different blood pressure levels (greater for hypertensive patients). |
| Inflammation | [84] | 14 RCTs. Exercise reduces CRP (−14% from baseline, 95% CI = −1.09, −0.23) and IL-6 levels (−18% from baseline, 95% CI = −1.44, −0.32) in type II diabetes. |
| [85] | 23 trials. Exercise reduces CRP (SMD = −0.500; 95% CI = −0.844, −0.157, p = 0.004) and fibrinogen levels (SMD = −0.544; 95% CI = −1.058, −0.030, p = 0.038) in coronary artery disease. | |
| [86] | Exercise enhances immune competency and slows down the aging of the immune system. | |
| Autonomic dysfunction | [87] | 15 trials. Improvements in HRV in type II diabetes after at least 3 month of an exercise program. |
| [89] | 16 RCTs. Exercise training leads to an improvement in HRV in coronary artery disease. | |
| [88] | 19 studies (RCTs, quasi-RCTs and controlled trials of exercise training in adult patients with heart failure). Exercise improves HRV. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belvederi Murri, M.; Folesani, F.; Zerbinati, L.; Nanni, M.G.; Ounalli, H.; Caruso, R.; Grassi, L. Physical Activity Promotes Health and Reduces Cardiovascular Mortality in Depressed Populations: A Literature Overview. Int. J. Environ. Res. Public Health 2020, 17, 5545. https://doi.org/10.3390/ijerph17155545
Belvederi Murri M, Folesani F, Zerbinati L, Nanni MG, Ounalli H, Caruso R, Grassi L. Physical Activity Promotes Health and Reduces Cardiovascular Mortality in Depressed Populations: A Literature Overview. International Journal of Environmental Research and Public Health. 2020; 17(15):5545. https://doi.org/10.3390/ijerph17155545
Chicago/Turabian StyleBelvederi Murri, Martino, Federica Folesani, Luigi Zerbinati, Maria Giulia Nanni, Heifa Ounalli, Rosangela Caruso, and Luigi Grassi. 2020. "Physical Activity Promotes Health and Reduces Cardiovascular Mortality in Depressed Populations: A Literature Overview" International Journal of Environmental Research and Public Health 17, no. 15: 5545. https://doi.org/10.3390/ijerph17155545
APA StyleBelvederi Murri, M., Folesani, F., Zerbinati, L., Nanni, M. G., Ounalli, H., Caruso, R., & Grassi, L. (2020). Physical Activity Promotes Health and Reduces Cardiovascular Mortality in Depressed Populations: A Literature Overview. International Journal of Environmental Research and Public Health, 17(15), 5545. https://doi.org/10.3390/ijerph17155545





