The Cost-Effectiveness of Mobile Health (mHealth) Interventions for Older Adults: Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
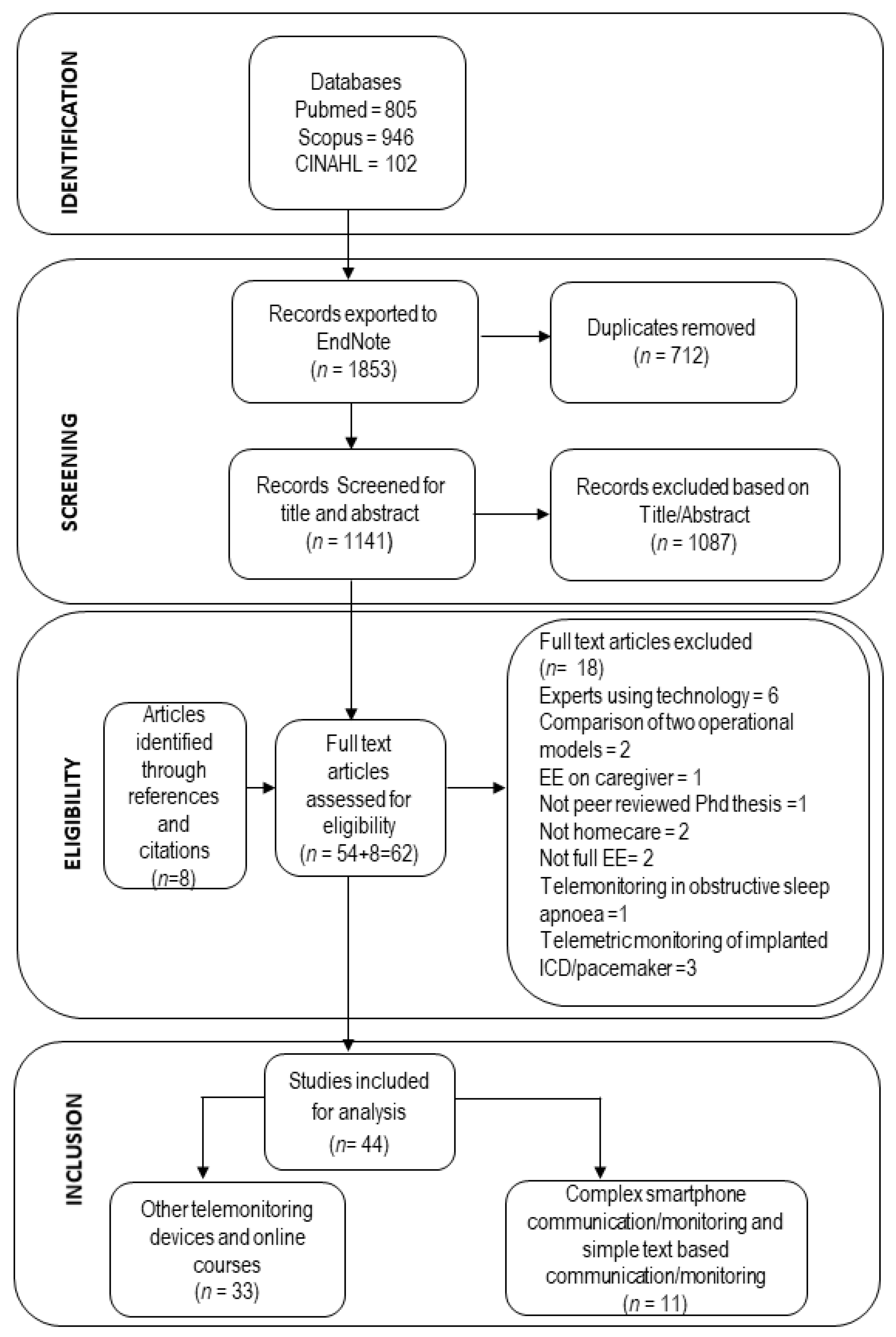
2.4. Study Selection Process and Data Extraction
2.5. Assessment of Quality of Reporting
3. Results
3.1. Search Results
3.2. Overview of Included Studies
3.3. Results of Included Studies
3.3.1. Complex Smartphone Communication
3.3.2. Simple Text-Based Communication
4. Discussion
4.1. Adherence to CHEERs List
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kwon, S. Gerontechnology: Research, Practice, and Principles in the Field of Technology and Aging; Springer Publishing Company, LLC: New York, NY, USA, 2017. [Google Scholar]
- Lupton, D. Digital Health: Critical and Cross-Disciplinary Perspectives; Routledge: London, UK, 2018; pp. 1–170. [Google Scholar]
- WHO Global Observatory for eHealth. mHealth: New Horizons for Health through Mobile Technologies; WHO Press, World Health Organization: Geneva, Switzerland, 2011; pp. 1–112. [Google Scholar]
- Iribarren, S.J.; Cato, K.; Falzon, L.; Stone, P.W. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS ONE 2017, 12, e0170581. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, C.; Stolee, P.; Juzwishin, D.; Husereau, D. Economic evaluations of eHealth technologies: A systematic review. PLoS ONE 2018, 13, e0198112. [Google Scholar] [CrossRef]
- Thielen, F.W.; Van Mastrigt, G.; Burgers, L.T.; Bramer, W.M.; Majoie, H.J.M.; Evers, S.; Kleijnen, J. How to prepare a systematic review of economic evaluations for clinical practice guidelines: Database selection and search strategy development (part 2/3). Expert Rev. Pharm. Outcomes Res. 2016, 16, 705–721. [Google Scholar] [CrossRef] [PubMed]
- mHealth. Available online: https://innovatemedtec.com/digital-health/mhealth (accessed on 8 June 2018).
- Free, C.; Phillips, G.; Felix, L.; Galli, L.; Patel, V.; Edwards, P. The effectiveness of M-health technologies for improving health and health services: A systematic review protocol. Bmc Res. Notes 2010, 3, 250. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.C. How much are Americans willing to pay for a quality-adjusted life year? Med. Care 2008, 46, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—Explanation and elaboration: A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health J. Int. Soc. Pharm. Outcomes Res. 2013, 16, 231–250. [Google Scholar] [CrossRef]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes, 4th ed.; Oxford University Press: Oxford, UK, 2015; pp. 65–75. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS ONE 2009, 6, e1000097. [Google Scholar]
- Barnett, T.E.C.; Chumbler, N.R.; Vogel, W.B.; Beyth, R.J.; Ryan, P.; Figueroa, S. The cost-utility of a care coordination/home telehealth programme for veterans with diabetes. J. Telemed. Telecare 2007, 13, 318–321. [Google Scholar] [CrossRef]
- Whittaker, F.W.; Wade, V. The costs and benefits of technology-enabled, home-based cardiac rehabilitation measured in a randomised controlled trial. J. Telemed. Telecare 2014, 20, 419–422. [Google Scholar] [CrossRef]
- Burn, E.N.; Nghiem, S.H.; Jan, S.; Redfern, J.; Rodgers, A.; Thiagalingam, A.; Graves, N.; Chow, C.K. Cost-effectiveness of a text message programme for the prevention of recurrent cardiovascular events. Heart 2017, 103, 923–930. [Google Scholar] [CrossRef]
- Cui, Y.D.; Doupe, M.; Katz, A.; Nyhof, P.; Forget, E.L. Economic evaluation of Manitoba Health Lines in the management of congestive heart failure. Healthc. N.A. = Polit. Sante 2013, 9, 36–50. [Google Scholar] [CrossRef]
- Maddison, R.; Pfaeffli, L.; Whittaker, R.; Stewart, R.; Kerr, A.; Jiang, Y.; Kira, G.; Leung, W.; Dalleck, L.; Carter, K.; et al. A mobile phone intervention increases physical activity in people with cardiovascular disease: Results from the HEART randomized controlled trial. Eur. J. Prev. Cardiol. 2015, 22, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, A.v.d.P.; Pinnock, H.; Hanley, J.; McCloughan, L.; Todd, A.; Krishan, A.; McKinstry, B. Telemonitoring for chronic obstructive pulmonary disease: A cost and cost-utility analysis of a randomised controlled trial. J. Telemed. Telecare 2015, 21, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Udsen, F.W.L.; Lilholt, P.H.; Hejlesen, O.; Ehlers, L. Cost-effectiveness of telehealthcare to patients with chronic obstructive pulmonary disease: Results from the Danish TeleCare North’ cluster-randomised trial. Bmj Open 2017, 7. [Google Scholar]
- Katalenich, B.S.; Liu, S.; Shao, H.; McDuffie, R.; Carpio, G.; Thethi, T.; Fonseca, V. Evaluation of a Remote Monitoring System for Diabetes Control. Clin. Ther. 2015, 37, 1216–1225. [Google Scholar] [CrossRef]
- Gordon, L.G.; Bird, D.; Oldenburg, B.; Friedman, R.H.; Russell, A.W.; Scuffham, P.A. A cost-effectiveness analysis of a telephone-linked care intervention for individuals with Type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 104, 103–111. [Google Scholar] [CrossRef]
- Cubo, E.M.; Solano, B.; Becerra, V.; Armesto, D.; Calvo, S.; Arribas, J.; Seco, J.; Martinez, A.; Zorrilla, L.; Heldman, D. Prospective study on cost-effectiveness of home-based motor assessment in Parkinson’s disease. J Telemed. Telecare 2016, 23, 328–338. [Google Scholar] [CrossRef]
- Choi Yoo, S.J.N.; Nyman, J.A.; Cheville, A.L.; Kroenke, K. Cost effectiveness of telecare management for pain and depression in patients with cancer: Results from a randomized trial. Gen. Hosp. Psychiatry 2014, 36, 599–606. [Google Scholar] [CrossRef]
- McCabe, C.; Claxton, K.; Culyer, A.J. The NICE Cost-Effectiveness Threshold. Pharm. Econ. 2008, 26, 733–744. [Google Scholar] [CrossRef]
- Taylor, C.J.; Jan, S. Economic evaluations of medicines. Aust. Prescr. 2007, 40, 76–78. [Google Scholar] [CrossRef]
- Bertram, M.Y.; Lauer, J.A.; De Joncheere, K.; Edejer, T.; Hutubessy, R.; Kieny, M.-P.; Hill, S.R. Cost-effectiveness thresholds: Pros and cons. Bull. World Health Organ. 2016, 94, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, E.; Byford, S. A guide to cost-effectiveness acceptability curves. Br. J. Psychiatry J. Ment. Sci. 2005, 187, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Dakin, H.; Wordsworth, S. Cost-minimisation analysis versus cost-effectiveness analysis, revisited. Health Econ. 2013, 22, 22–34. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year, Country | Effectiveness Measure | Reported | Evaluation | Remarks on Cost-Effectiveness |
|---|---|---|---|---|
| Complex Smartphone Communication | ||||
| Cubo E, 2016, Spain. [22] | UPDRS (I II III IV), QALY | Cost-effective in terms of UPDRS (II III IV) | Not reported if UPDRS (I II III IV), QALY and costs differ between alternatives. | Unknown due to lack of information |
| Gordon LG, 2014, Australia. [21] | QALY | dominant | Insignificant mean SF-6D score and mean annual total healthcare cost. Probabilistic sensitivity analysis showed 55.4% probability to be cost-effective at WTP threshold of AUS$50,000 (£33,000)/QALY | Not cost-effective |
| Stoddart A 2015, UK [18] | QALY | Not cost-effective | No significant differences in costs or QALY gain was observed for telemonitoring. CEAC showed 10.1% or 14.9% probability to be cost-effective at NICE threshold of £20,000 or £30,000, respectively. | Not cost-effective |
| Udsen FW, 2017, Denmark. [19] | QALY | Not cost-effective | No significant differences in costs or QALY gain was observed for tele-healthare. CEAC showed 50% probability of cost-effectiveness at €55,000 WTP for QALY. | Not cost-effective |
| Whittaker F, 2014, Australia. [14] | Cost savings | Cost-effective | Outcome was assumed to be equal between treatment alternatives. Not reported if costs differ between treatment alternatives. | Unknown due to lack of information |
| Simple Text-Based Communication | ||||
| Barnett T, 2007, USA. [13] | QALY | Cost-effective | Not reported if QALY and costs differ between alternatives. | Unknown due to lack of information |
| Burn E, 2017, Australia. [15] | QALY | Dominant | Significant differences were observed in costs and effects for Text me. | Cost-effective (Dominant) |
| Choi Yoo SJ, 2014, USA. [23] | DFD, QALY based on i)DFD, ii) SF-6D, and iii) modified EQ-5D | Cost-effective for DFD, QALY based on i) DFD, and ii) modified EQ-5D | Significant differences were observed in DFD, QALY based on (i) DFD, (ii) SF-6D and (iii) EQ-5D. Not reported if costs differ between alternatives. | Unknown due to lack of information |
| Cui Y, 2013, Canada. [16] | QALY | HLM not cost-effective | Simulation results showed that cost differences were not significant but QALY differences were significant for HL CEAC showed 95.4% probability to be cost-effective at $100,000 WTP for HL and 85.8 % probability to be cost-effective at threshold of CAD 50,000/QALY. | HLM not cost-effective |
| Katalenic B, 2015, USA. [20] | QALY | Cost-effective | QALY differences are not statistically or clinically significant (data not shown) Costs are significantly lower for DRMS. | Cost-effective |
| Maddison R, 2015, New Zealand. [17] | QALY, MET-hour of walking, leisure activity | Not cost-effective for QALY, cost-effective for both MET-hour of walking and leisure activity. | Significant differences were observed in MET-hour of walking and leisure activity in favor of the Heart intervention. No significant differences were observed in QALYs. Not reported if costs differ between alternatives. There would be a 72% and 90% probability of this intervention being cost-effective if WTP of the decision maker is NZD20,000 (€10,600) and NZD 50,000(€26,500). | Unknown due to lack of information for MET-hour of walking and leisure activity. Cost-effective for QALY based on threshold of USD 50 000 used in this study. |
| Scenarios | Cost | Outcome | Interpretation |
|---|---|---|---|
| 1 | ↑ | ↑ | Cost-effective if WTP exceeds the ICER |
| 2 | ↓ | ↑ | Cost-effective (intervention dominates the comparator) |
| 3 | ≈ | ↑ | Cost-effective (intervention dominates the comparator) |
| 4 | ↑ | ↓ | Not cost-effective (comparator dominates the intervention) |
| 5 | ↓ | ↓ | Cost-effective if willingness-to-accept exceeds the ICER |
| 6 | ≈ | ↓ | Not cost-effective (comparator dominates the intervention) |
| 7 | ↑ | ≈ | Not cost-effective (comparator dominates the intervention) |
| 8 | ↓ | ≈ | Cost-effective (intervention dominates the comparator i.e. cost-saving) |
| 9 | ≈ | ≈ | Not cost-effective (intervention and comparator are equal) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghani, Z.; Jarl, J.; Sanmartin Berglund, J.; Andersson, M.; Anderberg, P. The Cost-Effectiveness of Mobile Health (mHealth) Interventions for Older Adults: Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 5290. https://doi.org/10.3390/ijerph17155290
Ghani Z, Jarl J, Sanmartin Berglund J, Andersson M, Anderberg P. The Cost-Effectiveness of Mobile Health (mHealth) Interventions for Older Adults: Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(15):5290. https://doi.org/10.3390/ijerph17155290
Chicago/Turabian StyleGhani, Zartashia, Johan Jarl, Johan Sanmartin Berglund, Martin Andersson, and Peter Anderberg. 2020. "The Cost-Effectiveness of Mobile Health (mHealth) Interventions for Older Adults: Systematic Review" International Journal of Environmental Research and Public Health 17, no. 15: 5290. https://doi.org/10.3390/ijerph17155290
APA StyleGhani, Z., Jarl, J., Sanmartin Berglund, J., Andersson, M., & Anderberg, P. (2020). The Cost-Effectiveness of Mobile Health (mHealth) Interventions for Older Adults: Systematic Review. International Journal of Environmental Research and Public Health, 17(15), 5290. https://doi.org/10.3390/ijerph17155290







