COVID-19 and Dentistry: Prevention in Dental Practice, a Literature Review
Abstract
1. Introduction
1.1. SARS-CoV-2: Characteristics and Mechanism of Action
1.2. Epidemiology of the Coronavirus Pandemic
1.3. Dentistry and SARS-CoV-2: Clinical Aspects
1.4. Aim of the Study
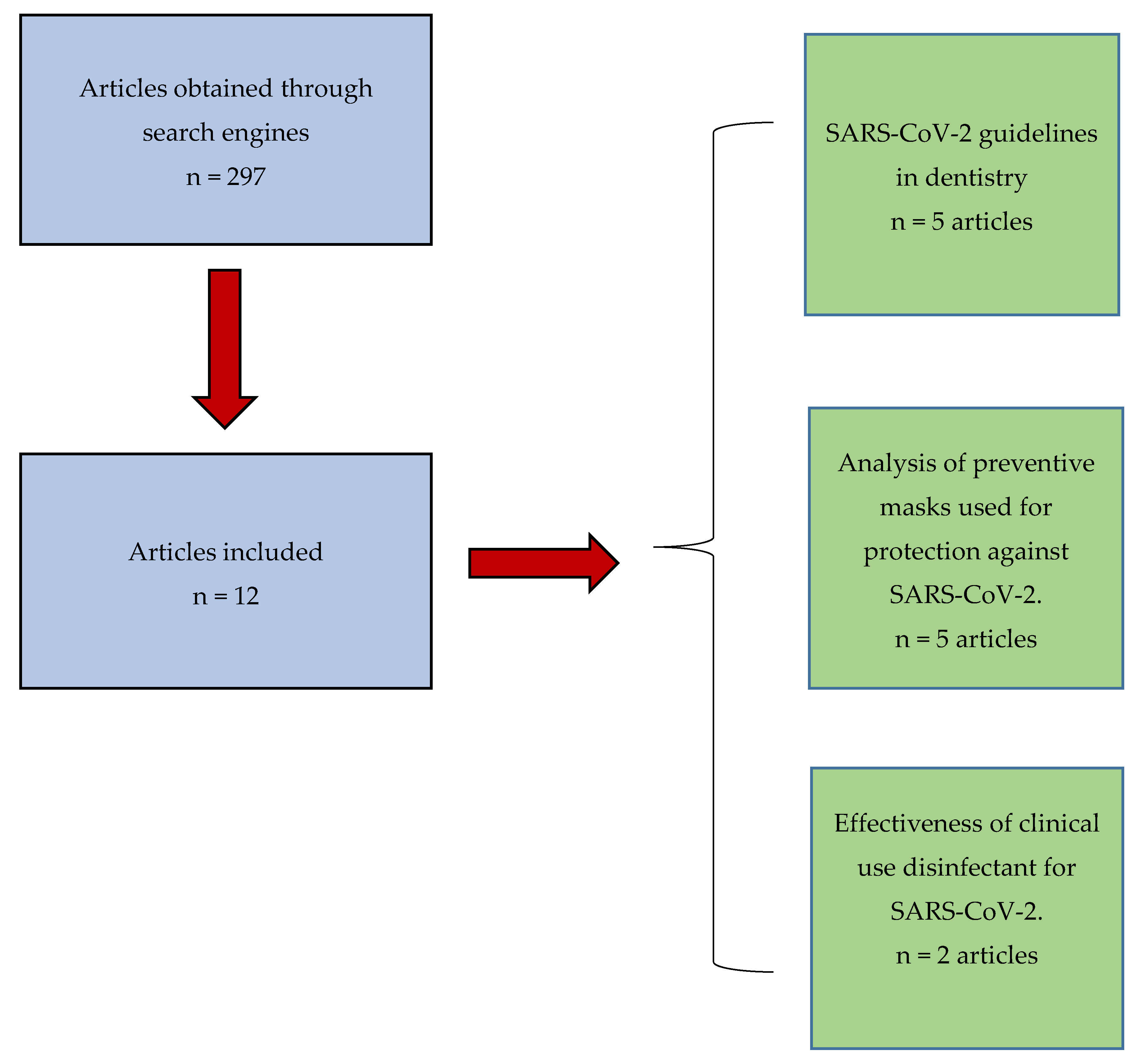
2. Materials and Methods
3. Results
4. Discussion
4.1. Preventive Measures against COVID-19 in Dental Practice
4.2. Efficacy of Respirators and Surgical Masks against Viral Respiratory Infections
4.3. Pragmatic and Technical Recommendations during Dental Treatment in the COVID-19 Era
4.4. Importance of Disinfectants in the Sterilization of the Dental Office
4.5. Looking toward the Future. A New Approach to the Dental Profession
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cantas, L.; Suer, K. Review: The important bacterial zoonoses in “One Health” concept. Front. Public Health 2014, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.S.; Mazet, J.A.K.; Woolhouse, M.; Parris, C.R.; Carroll, D.; Karesh, W.B.; Zambrana-Torrelio, C.; Lipkin, W.I.; Daszak, P. Prediction and prevention of next pandemic zoonosis. Lancet 2012, 380, 1956–1965. [Google Scholar] [CrossRef]
- Bidaisee, S.; Macpherson, C. Zoonoses and One Health: A Review of Literature. J. Paras. Res. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Furuse, Y.; Suzuki, A.; Oshitani, H. Origin of measles virus: Divergence from rinderpest virus between the 11th and 12th centuries. Virol. J. 2010, 7, 1–4. [Google Scholar] [CrossRef]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Chen, S.D.; Jin, H.G.; Tan, K.S.; Wand, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Durmus, S.; Ulgen, K.O. Comparative interactomics for virus–human protein–protein interactions: DNA viruses versus RNA viruses. FERBS Open Bio 2017, 7, 96–107. [Google Scholar] [CrossRef]
- Fehr, A.; Perlman, S. Coronaviruses: An Overview of Their Replication and Pathogenesis. In Coronaviruses: Methods and Protocols; Mayer, H.J., Bickerton, E., Britton, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; p. 282. [Google Scholar]
- Vinayachandran, D.; Balasubramanian, S. Salivary diagnostics in COVID-19: Future research implications. J. Dent. Sci. 2020. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklov, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Meng, S.; Wu, Y.; Mao, Y.; Ye, R.; Wang, Q.; Sun, C.; Sylvia, S.; Rozelle, S.; Raat, H.; et al. Epidemiology, causes, clinical manifestations and diagnosis, prevention and control of coronavirus disease (COVID19) during the early outbreak period: A scoping review. Infect. Dis. Poverty 2020, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.M.; Bolotin, S.; Lim, G.; Heffernan, J.; Deeks, S.L.; Li, Y.; Crowcroft, N.S. The basic reproduction number (R0) of a measles: A systematic review. Lancet 2017, 17, 420–428. [Google Scholar] [CrossRef]
- Ireland’s Health Services. Health Care Worker Information. Available online: https://www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter23.pdf (accessed on 1 April 2020).
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Jain, A. COVID-19 and lung pathology. Indian J. Pathol. Microbiol. 2020, 63, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Armocida, B.; Formenti, B.; Ussai, S.; Palestra, F.; Missoni, E. The Italian health system and the COVID-19 challenge. Lancet Public Health 2020. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.; Holbrook, M.; Gamble, A.; Williamson, B.; Tamin, A.; Harcourt, J.L.; Thornburg, J.N.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from a Symptomatic Patient. JAMA 2020. [Google Scholar] [CrossRef]
- Bin, S.Y.; Heo, J.Y.; Song, M.-S.; Lee, J.; Kim, E.-H.; Park, S.-J.; Kwon, H.I.; Kim, S.M.; Kim, Y.I.; Si, Y.-J.; et al. Environmental Contamination and Viral Shedding in MERS Patients During MERS-CoV Outbreak in South Korea. Clin. Infect. Dis. 2016, 62, 755–760. [Google Scholar] [CrossRef]
- Ming, W.K.; Huang, J.; Zhang, C. Breaking down of healthcare system: Mathematical modelling for controlling the novel coronavirus (2019-nCoV) outbreak in Wuhan, China. BioRxiv 2020. [Google Scholar] [CrossRef]
- Villa, M. La Letalità in Italia: Tra Apparenza e Realtà. Available online: https://www.ispionline.it/it/pubblicazione/coronavirus-la-letalita-italia-tra-apparenza-e-realta-25563 (accessed on 1 May 2020).
- Ministero della Salute. Covid-19- Situazione in Italia. Available online: http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?area=nuovoCoronavirus&id=5351&lingua=italiano&menu=vuoto (accessed on 4 May 2020).
- Istituto Superiore di Sanità. Characteristics of COVID-19 Patients Dying in Italy Report Based on Available Data on March 20th. 2020. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20_marzo_eng.pdf (accessed on 2 April 2020).
- Setti, L.; Passarini, F.; de Gennaro, G.; Di Gilio, A.; Palmisani, J.; Buono, P.; Fornari, G.; Perrone, M.G.; Piazzalunga, A.; Barbieri, P.; et al. Relazione circa l’effetto dell’inquinamento da particolato atmosferico e la diffusione di virus nella popolazione. SIMA 2020, 1–6. [Google Scholar]
- Gamio, L. The Workers Who Face the Greatest Coronavirus Risk. New York Times, 15 March 2020. Available online: https://www.nytimes.com/interactive/2020/03/15/business/economy/coronavirus-worker-risk.html (accessed on 5 April 2020).
- Luzzi, V.; Ierardo, G.; Bossù, M.; Polimeni, A. COVID-19: Pediatric Oral Health during and after the Pandemics. Appl. Sci. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Meng, L.; Hua, F.; Bian, Z. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J. Dent. Res. 2020, 99, 481–487. [Google Scholar] [CrossRef]
- Costa Marui, V.; Silveira Souto, M.L.; Silva Rovai, E.; Romito, G.A.; Chambrone, L.; Mendes Pannuti, C. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol. A systematic review. JADA 2019, 150, 1015–1026. [Google Scholar] [CrossRef]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Soh, H.Y.; Cai, Z.G.; Peng, X.; Zhang, Y.; Guo, C.B. Experience of Diagnosing and Managing Patients in Oral Maxillofacial Surgery during the Prevention and Control Period of the New Coronavirus Pneumonia. Chin. J. Dent. Res. 2020, 23, 57–62. [Google Scholar] [CrossRef]
- Long, Y.; Hu, T.; Liu, L.; Chen, R.; Guo, Q.; Yang, L.; Cheng, Y.; Huang, J.; Du, L. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J. Evid. Based Med. 2020. [Google Scholar] [CrossRef]
- Offeddu, V.; Yung, C.F.; Fong Low, M.S.; Tam, C.C. Effectiveness of Masks and Respirators against Respiratory Infections in Healthcare Workers: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2017, 65, 1934–1942. [Google Scholar] [CrossRef]
- Radonovich, L.J., Jr.; Simberkoff, M.S.; Bessesen, M.T.; Brown, A.C.; Cummings, D.A.T.; Gaydos, C.A.; Los, J.G.; Krosche, A.E.; Gibert, C.L.; Gorse, G.J.; et al. N95 Respirators vs Medical Masks for Preventing Influenza among Health Care Personnel. J. Am. Med. Assoc. 2019, 322, 824–833. [Google Scholar] [CrossRef]
- Ma, Q.X.; Shan, H.; Zhang, H.L.; Li, G.M.; Yang, R.M.; Chen, G.M. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J. Med. Virol. 2020, 1–13. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Chughtai, A.A.; Rahman, B.; Peng, Y.; Zhang, Y.; Seale, H.; Wang, X.; Wang, Q. The efficacy of medical masks and respirators against respiratory infection in healthcare workers. Influenza Other Respir. Viruses 2017, 11, 511–517. [Google Scholar] [CrossRef]
- Rabenau, H.F.; Kampf, G.; Cinatla, J.; Doerr, H.W. Efficacy of various disinfectants against SARS coronavirus. J. Hosp. Infect. 2005, 61, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed]
- American Dental Association. Interim Guidance for Management of Emergency and Urgent Dental Care. United States of America. 2020. Available online: https://www.ada.org/~/media/CPS/Files/COVID/ADA_Int_Guidance_Mgmt_Emerg-Urg_Dental_COVID19?utm_source=adaorg&utm_medium=VanityURL&utm_content=interimguidance-flowcharts&utm_campaign=covid-19 (accessed on 4 April 2020).
- American Dental Association. ADA Interim Guidance for Minimizing Risk of COVID-19 Transmission. United States of America. 2020. Available online: https://www.ada.org/~/media/CPS/Files/COVID/ADA_COVID_Int_Guidance_Treat_Pts.pdf (accessed on 7 April 2020).
- International Organization for Standardization. ISO 374-5:2016. Protective Goves against Dangerous Chemicals and Micro-Organisms. Part 5: Terminology and Performance Requirements for Micro-Organisms Risks. Available online: https://www.iso.org/standard/66562.html (accessed on 3 May 2020).
- Gobierno de España. Instituto Nacional de Seguridad y Salud en el Trabajo. Notas Técnicas de Prevención no 1143. Guantes de Protección Frente a Microorganismos. 2020. Available online: https://www.insst.es/documents/94886/706209/NTP+1143+Guantes+de+protecci%C3%B3n+contra+-+A%C3%B1o+2020/e9c01d41-b7b5-4530-9826-422aa28c1453 (accessed on 3 May 2020).
- Lo Giudice, R. The Severe Acute Respiratory Syndrome Coronavirus-2 (SARS COV-2) in dentistry. Management of biological risk in dental practice. Int. J. Environ. Res. Public Health 2020, 17, 3067. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Hwang, D.C.; Li, H.Y.; Tsai, C.F.; Chen, C.W.; Chen, J.K. Particle Size-Selective Assessment of Protection of European Standard FFP Respirators and Surgical Masks against Particles-Tested with Human Subjects. J. Healthc. Eng. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Consejo Dentistas. Organización Colegial De Dentistas De España. Plan Estratégico De Acción Para El Periodo Posterior a La Crisis Creada Por El COVID-19. 2020. Available online: https://www.consejodentistas.es/comunicacion/actualidad-consejo/notas-de-prensa-consejo/item/1763-plan-estrategico-de-accion-para-el-periodo-posterior-a-la-crisis-creada-por-el-covid-19.html (accessed on 1 May 2020).
- World Health Organization. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care. 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/112656/9789241507134_eng.pdf;jsessionid=C8857696E8E052600F0BEC469D387C20?sequence=1 (accessed on 5 April 2020).
- Alharbi, A.; Alharbi, S.; Alqaid, S. Guidelines for dental care provision during the COVID-19 pandemic. Saudi Dent. J. 2020, 32, 181–186. [Google Scholar] [CrossRef]
- Associazione Italiana Odontoiatri. Linee Guida COVID19 Restart. 2020. Available online: http://www.aio.it/html/uploads/2020/04/Linee-Guida-Covid-19-Restart.pdf (accessed on 1 May 2020).
- Fallahi, H.R.; Keyhan, S.O.; Zandian, D.; Kim, S.G.; Cheshmi, B. Being a front-line dentist during the Covid19 pandemic: A literature review. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 12. [Google Scholar] [CrossRef]
- Ki, H.K.; Han, S.K.; Son, J.S.; Park, S.O. Risk of transmission via medical employees and importance of routine infection- prevention policy in a nosocomial outbreak of Middle East respiratory syndrome (MERS): A descriptive analysis from a tertiary care hospital in South Korea. BMC Pulm. Med. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Sabino-Silva, R.; Gomes Jardim, A.C.; Siqueira, W.L. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin. Oral Investig. 2020, 24, 1619–1621. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. An Overview of the Rapid Test Situation for COVID-19 Diagnosis in the EU/EEA; ECDC: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Overview-rapid-test-situation-for-COVID-19-diagnosis-EU-EEA.pdf (accessed on 1 May 2020).
- Ahmed, M.A.; Jouhar, R.; Ahmed, N.; Adnan, S.; Aftab, M.; Zafar, M.S. Fear and Practice Modifications among Dentists to Combat Novel Coronavirus Disease (COVID-19) Outbreak. Int. J. Environ. Res. Public Health 2020, 17, 2821. [Google Scholar] [CrossRef]
- Ren, Y.F.; Rasubala, L.; Malmstrom, H.; Eliav, E. Dental Care and Oral Health under the Clouds of COVID-19. JDR Clin. Trans. Res. 2020, 20, 1–9. [Google Scholar] [CrossRef]
| Authors/Year | Telephone Triage Questionnaire | Body Tº Measurement | Oral Rinses | PPE Hand Hygiene | Dental Handpiece | Rubber Dam | Relevant Clinical Aspects |
|---|---|---|---|---|---|---|---|
| Meng L et al. [28] Y: 2020 | YES | YES | YES | Mandatory | Avoid | YES | No Intraoral X-ray |
| Costa V et al. [29] Y: 2019 | YES | NR | YES CLX 0.12–0.2% | Mandatory | NR | NR | Aerosol Control |
| Peng X et al. [30] Y: 2020 | YES | YES Tº > 37.3 NO tmt | YES Hyd perox 1% Povidon–Iodine 0.2% | Mandatory | YES Anti-retraction | YES | Medical waste management |
| Luzzi V et al. [27] Y: 2020 | YES | YES | YES NO CLX | Mandatory | YES Anti-retraction | YES | High volume aspirators |
| Yang Y et al. [31] Y: 2020 | YES | YES | YES | Mandatory | NR | NR | Operating room disinfection |
| Authors/Year | Type of Study | Sample | Exposure | Masks Analyzed | Efficacy: Significant Differences |
|---|---|---|---|---|---|
| Long Y et al. [32] Y: 2020 | Systematic review and meta-analysis | 6 randomized controlled clinical trials | Influenza virus | N95 Surgical masks | NO |
| Offeddu V et al. [33] Y: 2017 | Systematic review and meta-analysis | 23 observational studies 6 controlled randomized trials | -Influenza virus -Non-specific respiratory infection -SARS | N95 Surgical masks | NO virus influenza YES Clinical respiratory infection (> N95) |
| Radonovich LJ et al. [34]. Y: 2019 | Randomized clinical trial | 2862 healthy workers | Influenza virus | N95 Surgical masks | NO |
| Ma QX et al. [35]. Y: 2020 | Pilot study | 3 typologies of masks | Avian influenza | N95 Surgical masks Homemade masks (4 layer of paper + polyester) | YES -N95: 99.98% -Mas. Surg: 97.14% -Home Masks: 95% PROTECTION |
| MacIntyre et al. [36]. Y: 2017 | Randomized controlled clinical trial | 3591 healthy subjects | Influenza A Influenza B | N95 Surgical masks Control Group | YES > protection with N95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villani, F.A.; Aiuto, R.; Paglia, L.; Re, D. COVID-19 and Dentistry: Prevention in Dental Practice, a Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 4609. https://doi.org/10.3390/ijerph17124609
Villani FA, Aiuto R, Paglia L, Re D. COVID-19 and Dentistry: Prevention in Dental Practice, a Literature Review. International Journal of Environmental Research and Public Health. 2020; 17(12):4609. https://doi.org/10.3390/ijerph17124609
Chicago/Turabian StyleVillani, Federico Alcide, Riccardo Aiuto, Luigi Paglia, and Dino Re. 2020. "COVID-19 and Dentistry: Prevention in Dental Practice, a Literature Review" International Journal of Environmental Research and Public Health 17, no. 12: 4609. https://doi.org/10.3390/ijerph17124609
APA StyleVillani, F. A., Aiuto, R., Paglia, L., & Re, D. (2020). COVID-19 and Dentistry: Prevention in Dental Practice, a Literature Review. International Journal of Environmental Research and Public Health, 17(12), 4609. https://doi.org/10.3390/ijerph17124609






