Associations between Vehicle Exhaust Particles and Ozone at Home Address and Birth Weight
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Population
2.2. Individual Characteristics
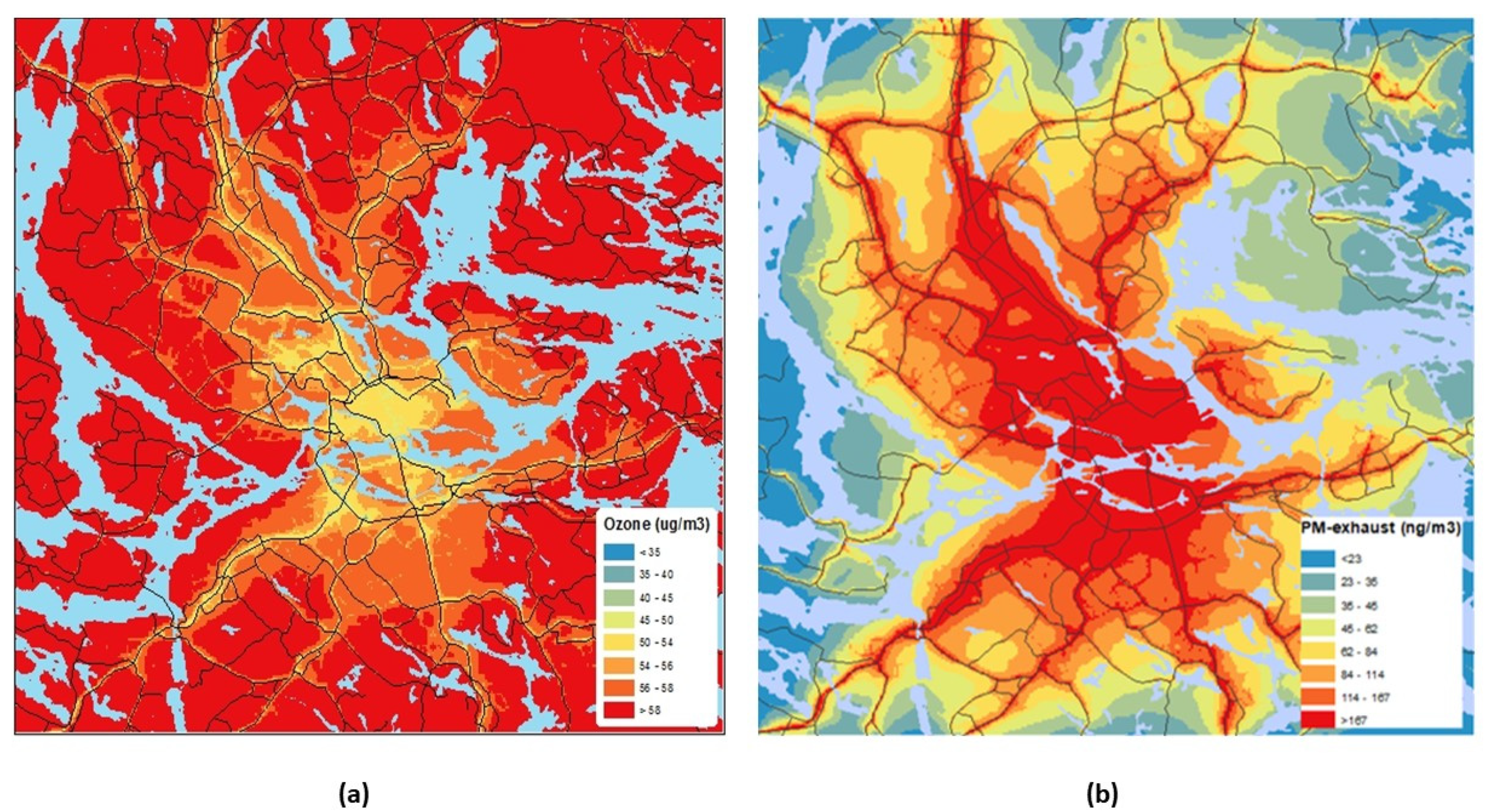
2.3. Exposure Assessment
2.4. Outcome Definition
2.5. Variable Definitions
2.6. Statistical Analysis
3. Results
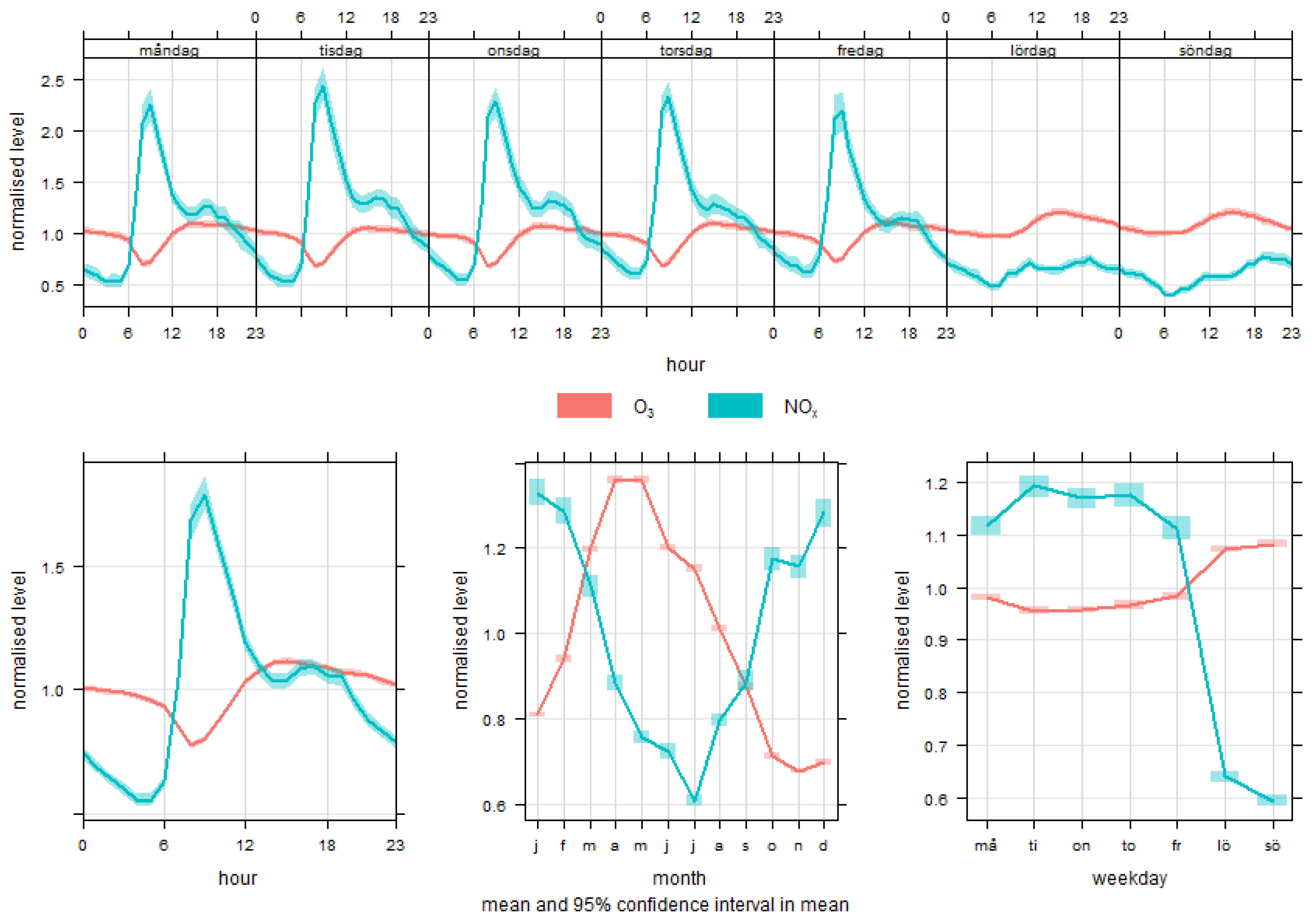
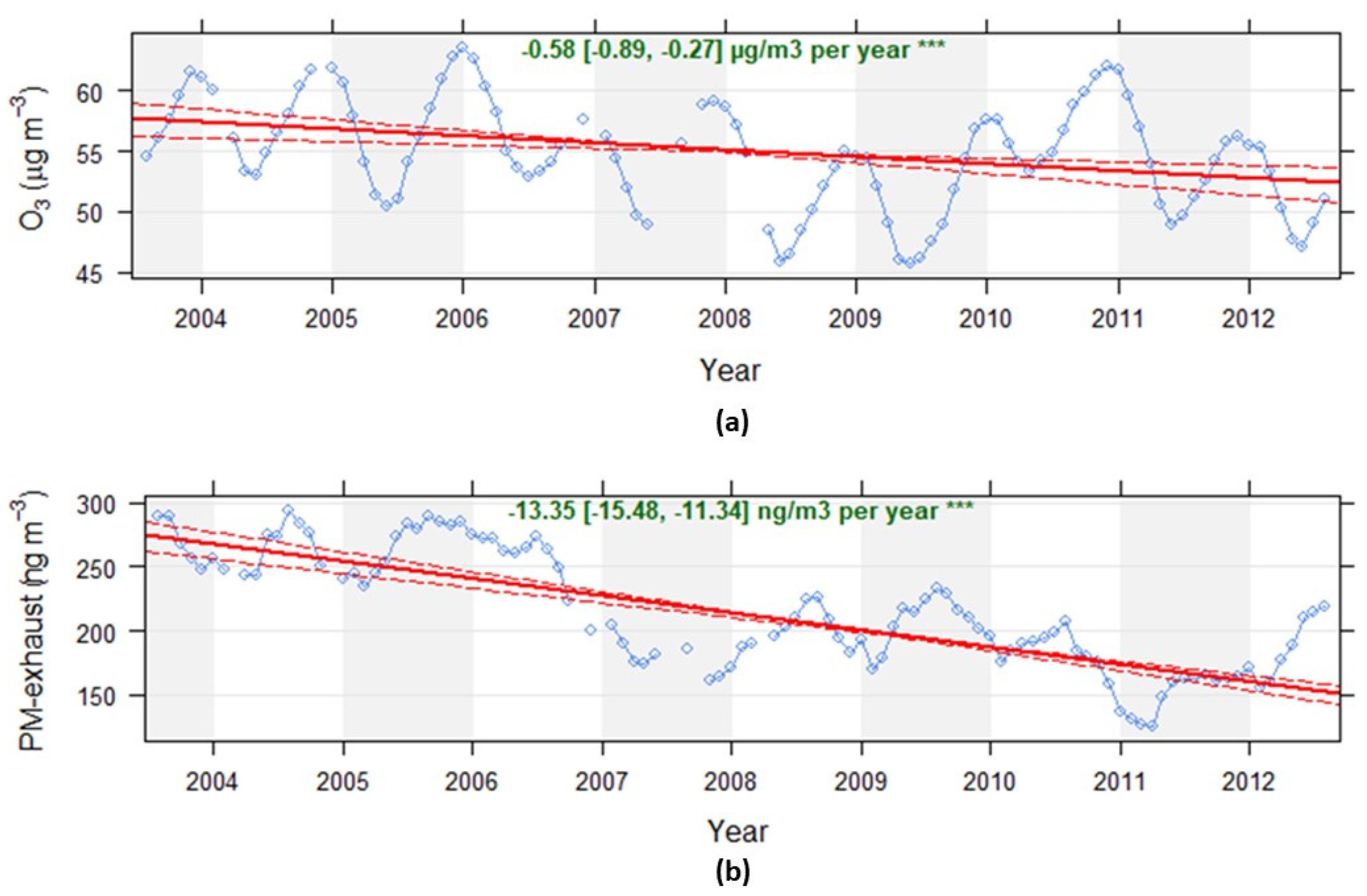
3.1. Temporal and Spatial Variations in Exposure
3.2. Socioeconomic Indicators and Exposure
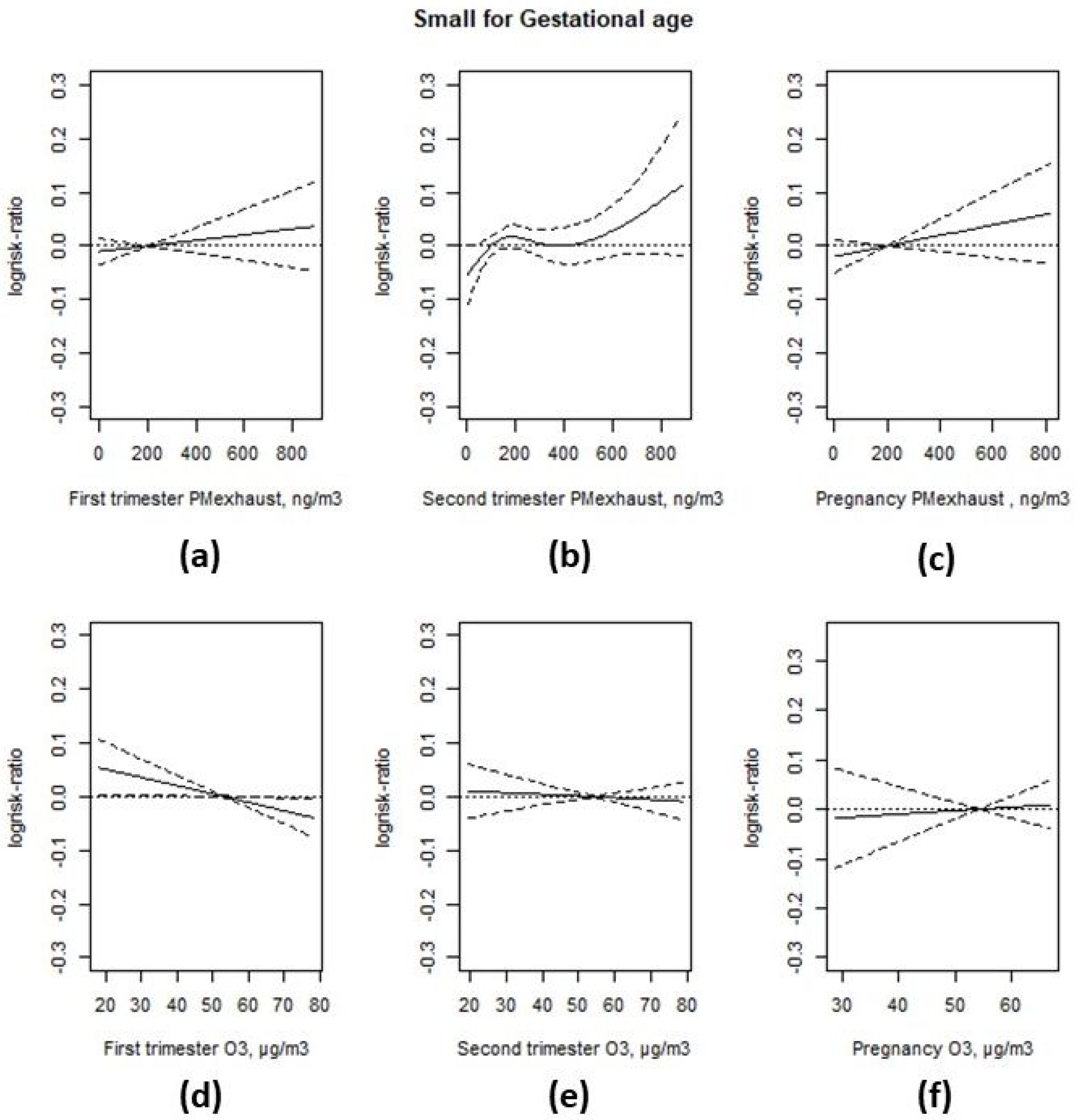
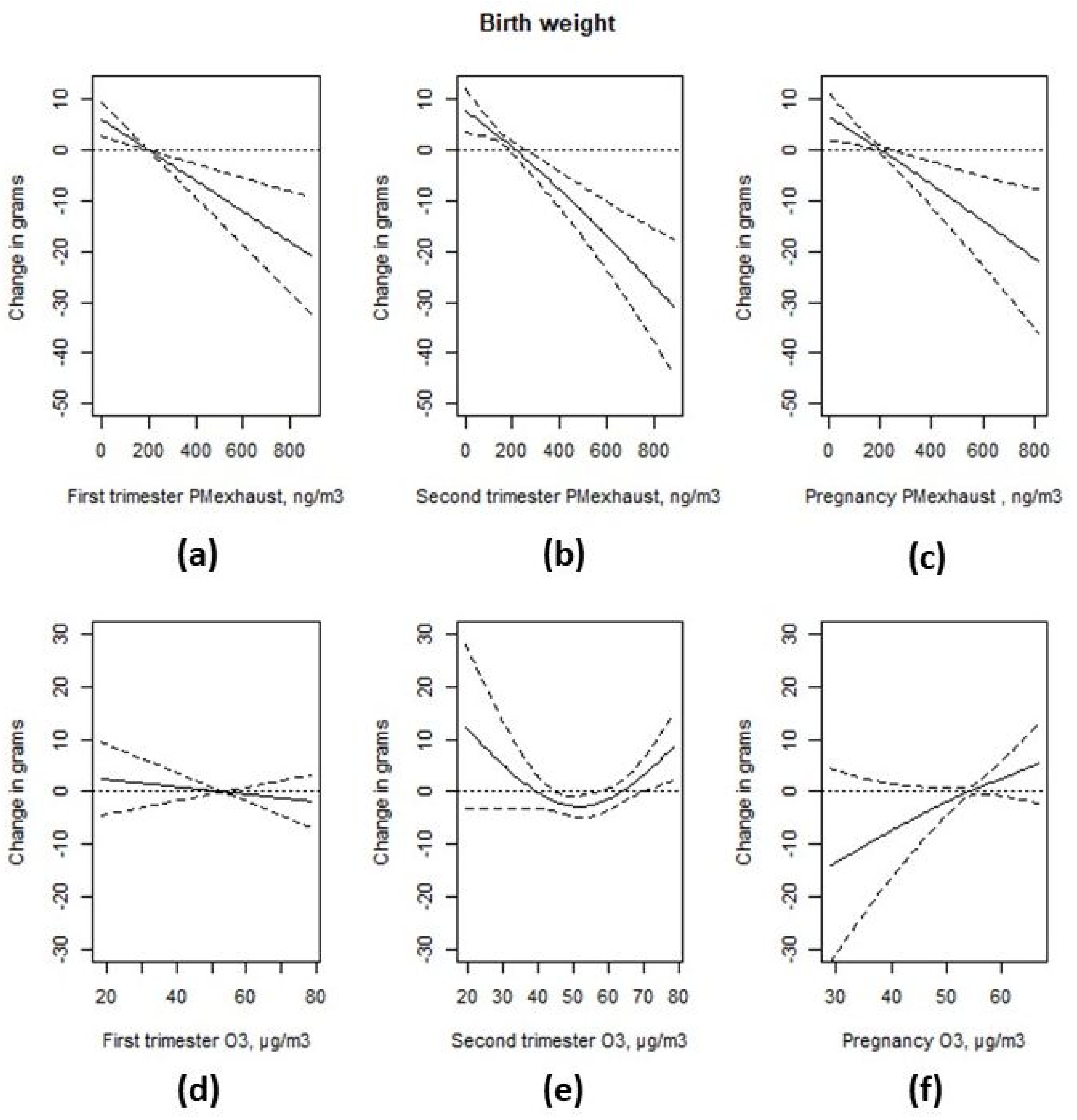
3.3. Associations with Birth Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
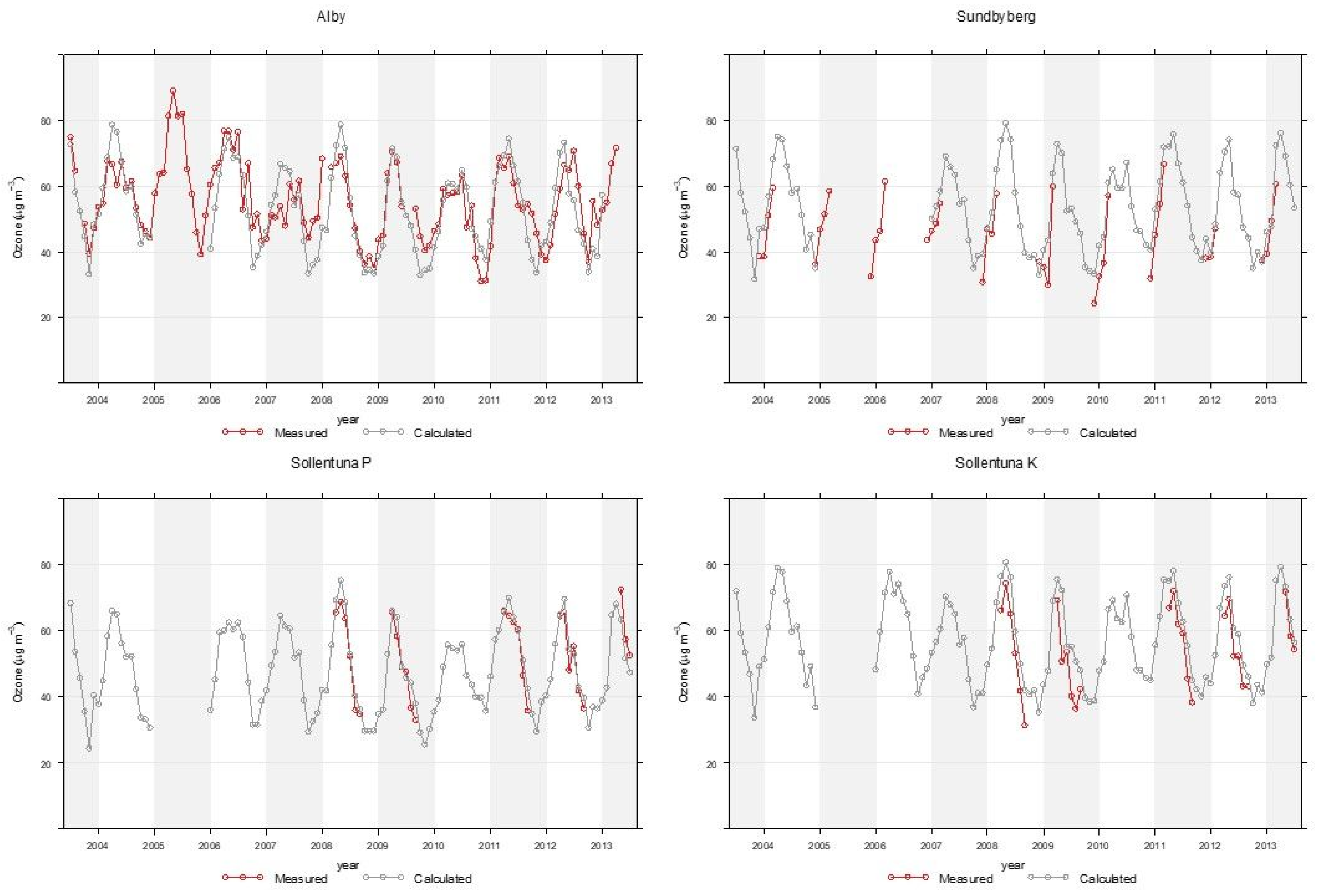
Appendix A. Modelling of Trimester Mean Exposure to Ozone and Comparison with Measurements
| Site | N | Measured Mean ± 95% CI | Model Mean ± 95% CI | R2 |
|---|---|---|---|---|
| Alby | 81 | 51.6 ± 2.9 | 54.0 ± 2.5 | 0.71 |
| Sollentuna P | 26 | 52.7 ± 4.7 | 54.5 ± 4.4 | 0.89 |
| Sollentuna K | 27 | 54.1 ± 4.8 | 61.7 ± 4.6 | 0.88 |
| Sundbyberg | 31 | 44.5 ± 3.9 | 49.7 ± 4.1 | 0.85 |
| All | 165 | 51.0 ± 1.9 | 54.7 ± 1.8 | 0.77 |
Appendix B
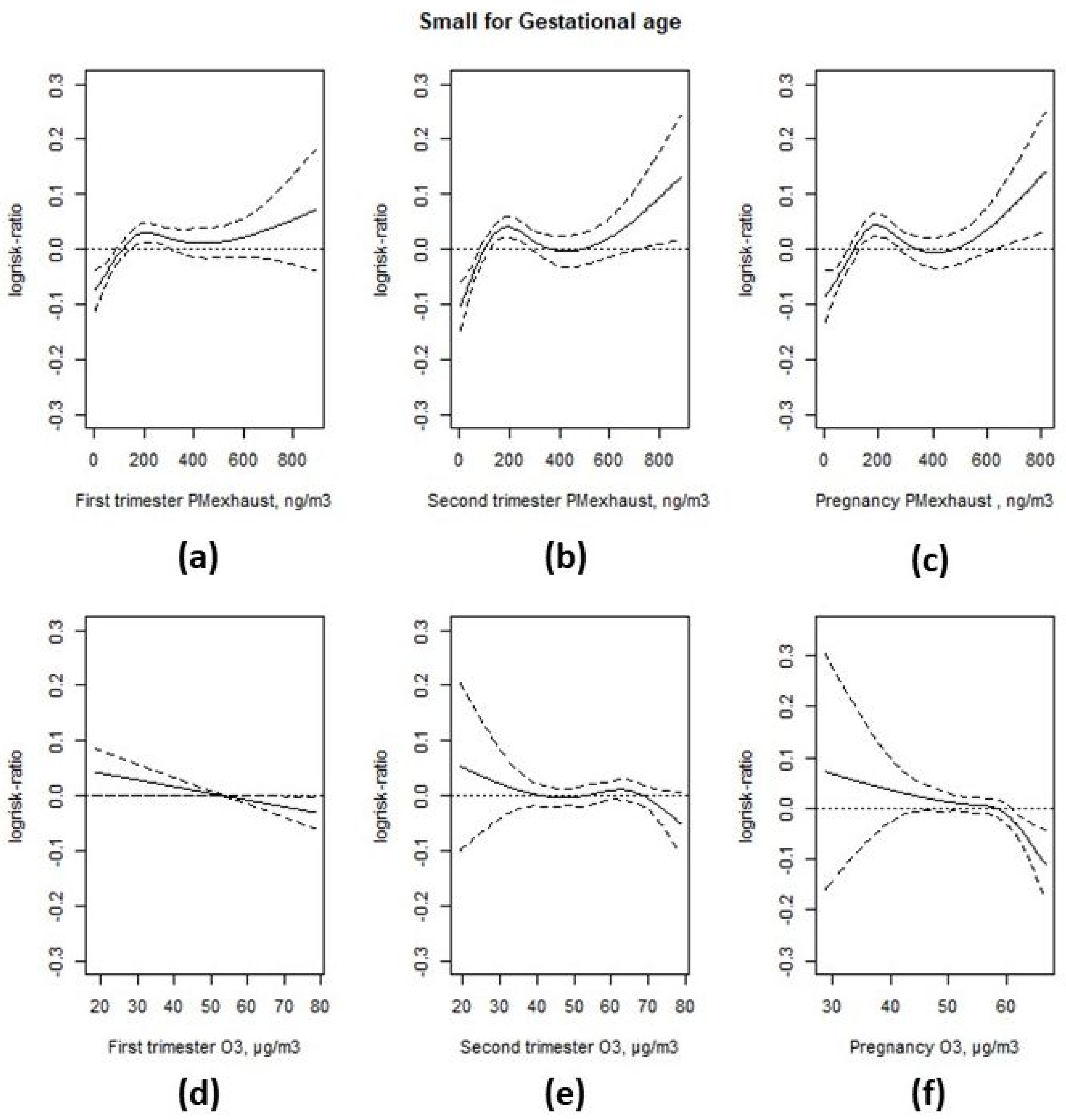
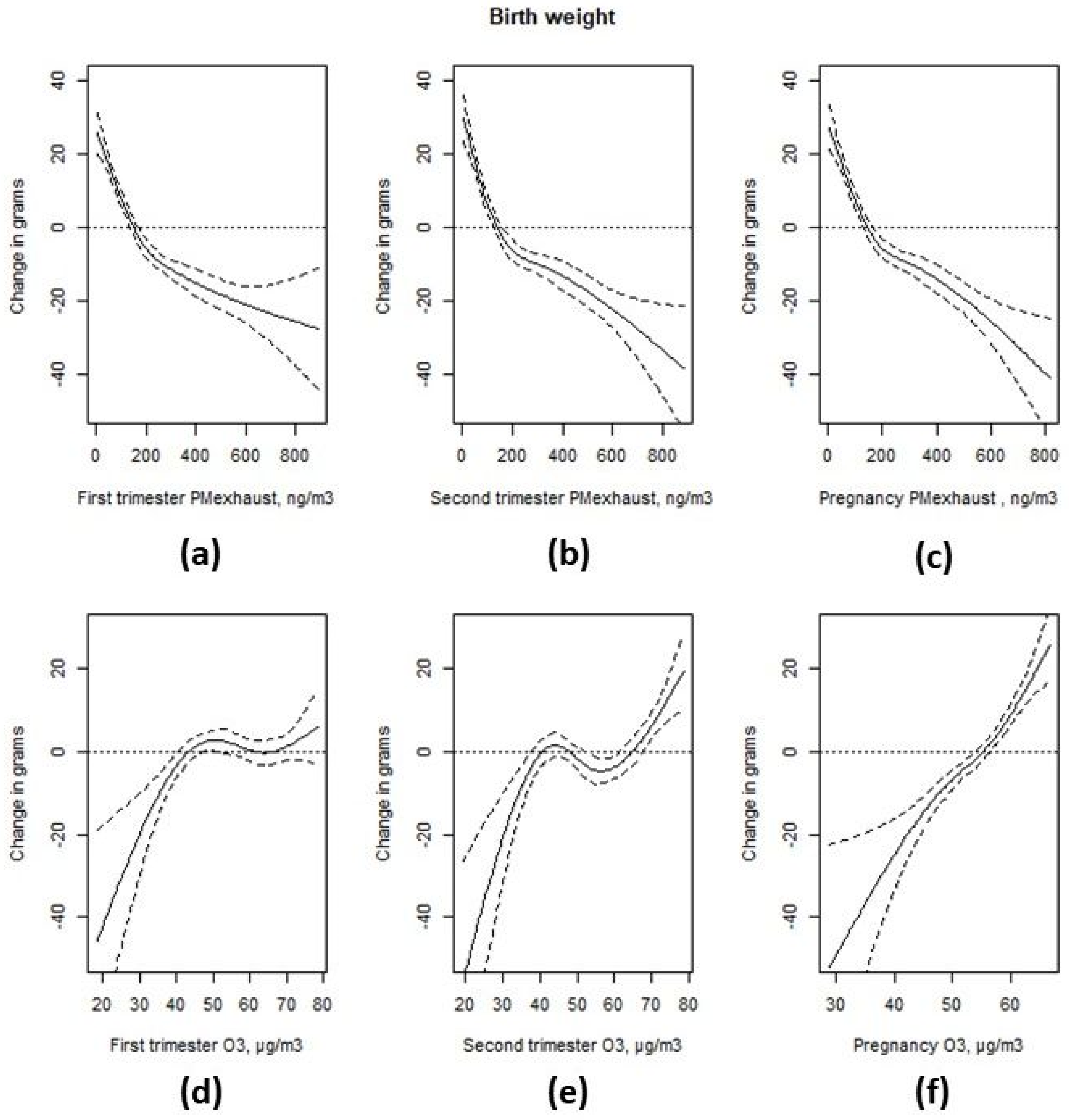
| Interquartile Range | Prevalence Rate Ratio | 95% CI | Change in Grams | 95% CI | |
|---|---|---|---|---|---|
| SGA 1 | Birth weight 2 | ||||
| First trimester exhaust particles | 214 ng/m3 | 1.01 | (0.99; 1.04) | −6.5 | (−10.0; −3.0) |
| Second trimester exhaust particles | 213 ng/m3 | −8.7 | (−12.3; −5.0) | ||
| Pregnancy exhaust particles | 209 ng/m3 | 1.02 | (0.99; 1.05) | −7.5 | (−12.0; −2.9) |
| First trimester ozone | 19.4 µg/m3 | 0.97 | (0.94; 1.00) | −1.4 | (−5.2; 2.4) |
| Second trimester ozone | 20.2 µg/m3 | 0.99 | (0.97; 1.02) | ||
| Pregnancy ozone | 8.0 µg/m3 | 1.01 | (0.98; 1.04) | 3.7 | (−0.9; 8.2) |
| Second Trimester Exhaust Particles | Prevalence Rate Ratio | 95% CI |
|---|---|---|
| exhaust particles ≤ 61 ng/m3 (ref) | 1.00 | |
| 61 < exhaust particles ≤ 124 ng/m3 | 1.02 | (0.95; 1.09) |
| 124 < exhaust particles ≤ 201 ng/m3 | 1.05 | (0.98; 1.12) |
| 201 < exhaust particles ≤ 332 ng/m3 | 1.09 | (1.01; 1.17) |
| exhaust particles > 332 ng/m3 | 1.07 | (0.99; 1.15) |
| Second Trimester Ozone | Change in Grams | 95% CI |
|---|---|---|
| ozone ≤ 42.8 µg/m3 | 9.4 | (2.6; 16.3) |
| 42.8 < ozone ≤ 49.7 µg/m3 | 7.0 | (0.5; 13.5) |
| 49.7 < ozone ≤ 42.8 µg/m3 (ref) | ||
| 57.9 < ozone ≤ 66.6 µg/m3 | 9.7 | (3.1; 16.3) |
| ozone > 66.6 µg/m3 | 11.2 | (4.6; 17.8) |
References
- Class, Q.A.; Rickert, M.E.; Lichtenstein, P.; D’Onofrio, B.M. Birth weight, physical morbidity, and mortality: A population-based sibling-comparison study. Am. J. Epidemiol. 2014, 179, 550–558. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, Y.; Gao, Y.; Tian, Y. Maternal fine particulate matter (PM2.5) exposure and adverse birth outcomes: An updated systematic review based on cohort studies. Environ. Sci. Pollut. Res. Int. 2019, 26, 13963–13983. [Google Scholar] [CrossRef] [PubMed]
- Klepac, P.; Locatelli, I.; Korosec, S.; Kunzli, N.; Kukec, A. Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environ. Res. 2018, 167, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, S.; Jiao, A.; Yang, X.; Yun, J.; Wang, Y.; Xue, X.; Chu, Y.; Liu, F.; Liu, Y.; et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environ. Pollut. 2017, 227, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Gehring, U.; Beelen, R.; Wang, M.; Giorgis-Allemand, L.; Andersen, A.M.; Basagana, X.; Bernard, C.; Cirach, M.; Forastiere, F.; et al. Elemental Constituents of Particulate Matter and Newborn’s Size in Eight European Cohorts. Environ. Health Perspect 2016, 124, 141–150. [Google Scholar] [CrossRef]
- Guo, P.; Chen, Y.; Wu, H.; Zeng, J.; Zeng, Z.; Li, W.; Zhang, Q.; Huo, X.; Feng, W.; Lin, J.; et al. Ambient air pollution and markers of fetal growth: A retrospective population-based cohort study of 2.57 million term singleton births in China. Environ. Int. 2020, 135, 105410. [Google Scholar] [CrossRef]
- Olstrup, H.; Forsberg, B.; Orru, H.; Spanne, M.; Nguyen, H.; Molnar, P.; Johansson, C. Trends in air pollutants and health impacts in three Swedish cities over the past three decades. Atmos. Chem. Phys. 2018, 18, 15705–15723. [Google Scholar] [CrossRef]
- Kannan, S.; Misra, D.P.; Dvonch, J.T.; Krishnakumar, A. Exposures to airborne particulate matter and adverse perinatal outcomes: A biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ. Health Perspect 2006, 114, 1636–1642. [Google Scholar] [CrossRef]
- Nachman, R.M.; Mao, G.; Zhang, X.; Hong, X.; Chen, Z.; Soria, C.S.; He, H.; Wang, G.; Caruso, D.; Pearson, C.; et al. Intrauterine Inflammation and Maternal Exposure to Ambient PM2.5 during Preconception and Specific Periods of Pregnancy: The Boston Birth Cohort. Environ. Health Perspect 2016, 124, 1608–1615. [Google Scholar] [CrossRef]
- Slama, R.; Darrow, L.; Parker, J.; Woodruff, T.J.; Strickland, M.; Nieuwenhuijsen, M.; Glinianaia, S.; Hoggatt, K.J.; Kannan, S.; Hurley, F.; et al. Meeting report: Atmospheric pollution and human reproduction. Environ. Health Perspect 2008, 116, 791–798. [Google Scholar] [CrossRef]
- Olsson, D.; Ekstrom, M.; Forsberg, B. Temporal variation in air pollution concentrations and preterm birth-a population based epidemiological study. Int. J. Environ. Res. Public Health 2012, 9, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Olsson, D.; Mogren, I.; Forsberg, B. Air pollution exposure in early pregnancy and adverse pregnancy outcomes: A register-based cohort study. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Olsson, D.; Mogren, I.; Eneroth, K.; Forsberg, B. Traffic pollution at the home address and pregnancy outcomes in Stockholm, Sweden. BMJ Open 2015, 5, e007034. [Google Scholar] [CrossRef] [PubMed]
- Cnattingius, S.; Ericson, A.; Gunnarskog, J.; Kallen, B. A quality study of a medical birth registry. Scand. J. Soc. Med. 1990, 18, 143–148. [Google Scholar] [CrossRef]
- Gruzieva, O.; Merid, S.K.; Gref, A.; Gajulapuri, A.; Lemonnier, N.; Ballereau, S.; Gigante, B.; Kere, J.; Auffray, C.; Melen, E.; et al. Exposure to Traffic-Related Air Pollution and Serum Inflammatory Cytokines in Children. Environ. Health Perspect 2017, 125, 067007. [Google Scholar] [CrossRef]
- Ljungman, P.L.S.; Andersson, N.; Stockfelt, L.; Andersson, E.M.; Nilsson Sommar, J.; Eneroth, K.; Gidhagen, L.; Johansson, C.; Lager, A.; Leander, K.; et al. Long-Term Exposure to Particulate Air Pollution, Black Carbon, and Their Source Components in Relation to Ischemic Heart Disease and Stroke. Environ. Health Perspect 2019, 127, 107012. [Google Scholar] [CrossRef]
- Segersson, D.; Eneroth, K.; Gidhagen, L.; Johansson, C.; Omstedt, G.; Nylen, A.E.; Forsberg, B. Health Impact of PM10, PM2.5 and Black Carbon Exposure Due to Different Source Sectors in Stockholm, Gothenburg and Umea, Sweden. Int. J. Environ. Res. Public Health 2017, 14, 742. [Google Scholar] [CrossRef]
- Johansson, C.; Hadenius, A.; Johansson, P.Å.; Jonson, T. The Stockholm Study on Health effects of Air Pollution and Its Economic Consequences. Part I. NO2 and Particulate Matter in Stockholm. In AQMA Report 6: 98; Stockholm Environment and Health Protection Administration: Stockholm, Sweden, 1999. [Google Scholar]
- Eneroth, K.; Johansson, C.; Bellander, T. EXPOSURE—Comparison between Measurments and Calculations Based on Exposure Modelling; SLB-analys: Stockholm, Sweden, 2006; Available online: http://slb.nu/slb/rapporter/pdf8/lvf2006_012.pdf (accessed on 28 May 2020).
- Krecl, P.; Johansson, C.; Targino, A.C.; Ström, J.; Burman, L. Trends in black carbon and size-resolved particle number concentrations and vehicle emission factors under real-world conditions. Atmos. Environ. 2017, 165, 155–168. [Google Scholar] [CrossRef]
- Fong, K.C.; Di, Q.; Kloog, I.; Laden, F.; Coull, B.A.; Koutrakis, P.; Schwartz, J.D. Relative toxicities of major particulate matter constituents on birthweight in Massachusetts. Environ. Epidemiol. 2019, 3, e047. [Google Scholar] [CrossRef]
- Strickland, M.J.; Lin, Y.; Darrow, L.A.; Warren, J.L.; Mulholland, J.A.; Chang, H.H. Associations Between Ambient Air Pollutant Concentrations and Birth Weight: A Quantile Regression Analysis. Epidemiology 2019, 30, 624–632. [Google Scholar] [CrossRef]
- Smith, R.B.; Fecht, D.; Gulliver, J.; Beevers, S.D.; Dajnak, D.; Blangiardo, M.; Ghosh, R.E.; Hansell, A.L.; Kelly, F.J.; Anderson, H.R.; et al. Impact of London’s road traffic air and noise pollution on birth weight: Retrospective population based cohort study. BMJ 2017, 359, j5299. [Google Scholar] [CrossRef] [PubMed]
- Oudin, A.; Forsberg, B.; Stromgren, M.; Beelen, R.; Modig, L. Impact of residential mobility on exposure assessment in longitudinal air pollution studies: A sensitivity analysis within the ESCAPE project. Sci. World J. 2012, 2012, 125818. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, W.; Zheng, X.; Sun, J.; Chen, L.; Deng, Q. Combined effects of ambient air pollution and home environmental factors on low birth weight. Chemosphere 2020, 240, 124836. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Liu, J.; Lu, Z.; Yue, H.; Zhang, J.; Jiang, Y. Influence of residential greenness on adverse pregnancy outcomes: A systematic review and dose-response meta-analysis. Sci. Total Environ. 2020, 718, 137420. [Google Scholar] [CrossRef] [PubMed]
| Min | Median | Max | Interquartile Range | |
|---|---|---|---|---|
| First trimester PM-exhaust (ng/m3) | 3 | 159 | 886 | 214 |
| Second trimester PM-exhaust (ng/m3) | 3 | 159 | 882 | 213 |
| Pregnancy PM-exhaust (ng/m3) | 7 | 160 | 854 | 209 |
| First trimester ozone (µg/m3) | 18.5 | 52.8 | 78.4 | 19.4 |
| Second trimester ozone (µg/m3) | 19.3 | 53.6 | 78.6 | 20.2 |
| Pregnancy ozone (µg/m3) | 28.8 | 54.9 | 66.7 | 8.0 |
| Factor Level | Subjects (%) | Small for Gestational age, % | Birth Weight, g (SD) | PM-Exhaust, ng/m3 (SD) | Ozone, µg/m3 (SD) | |
|---|---|---|---|---|---|---|
| Maternal level of education | pre-upper secondary school (<9 years) | 4021 (2.7) | 13.5 (34.2) | 3444.2 (528.1) | 145.8 (99.1) | 55.6 (5.0) |
| pre-upper secondary school (9 years) | 9981 (6.7) | 11.4 (31.8) | 3481.0 (549.5) | 150.9 (129.4) | 55.7 (5.3) | |
| upper secondary school (<3 years) | 13,571 (9.2) | 10.5 (30.7) | 3510.2 (550.0) | 165.2 (139.5) | 55.8 (5.5) | |
| upper secondary school (3 years) | 34,643 (23.4) | 9.2 (28.9) | 3547.0 (532.3) | 170.3 (149.5) | 55.2 (5.7) | |
| post-upper secondary school (<3 years) | 20,312 (13.7) | 9.7 (29.6) | 3544.8 (534.5) | 206.6 (160.6) | 54.3 (5.8) | |
| post-secondary school (3 years) | 63,605 (42.9) | 9.0 (28.6) | 3563.0 (522.8) | 233.7 (165.1) | 53.4 (5.9) | |
| postgraduate education | 2103 (1.4) | 9.4 (29.2) | 3552.8 (524.4) | 244.7 (166.4) | 53.1 (6.0) | |
| Paternal level of education | pre-upper secondary school (<9 years) | 3589 (2.4) | 14.7 (35.4) | 3426.3 (530.9) | 137.9 (92.3) | 55.7 (4.9) |
| pre-upper secondary school (9 years) | 13,365 (9.0) | 10.8 (31) | 3507.6 (538) | 151.8 (132.2) | 55.6 (5.4) | |
| upper secondary school (<3 years) | 21,049 (14.2) | 10.2 (30.2) | 3530.5 (551) | 163.5 (139.4) | 55.7 (5.5) | |
| upper secondary school (3 years) | 33,891 (22.9) | 9.1 (28.7) | 3545.0 (532.1) | 176.7 (151.6) | 54.9 (5.7) | |
| post-upper secondary school (<3 years) | 23,358 (15.8) | 9.6 (29.5) | 3550.0 (531.4) | 212.0 (161.5) | 54.2 (5.8) | |
| post-secondary school (3 years) | 50,052 (33.8) | 9.0 (28.6) | 3560.7 (520.5) | 243.2 (166.2) | 53.2 (6.0) | |
| postgraduate education | 2932 (2.0) | 8.9 (28.5) | 3559.1 (532.8) | 249.2 (167.1) | 53.1 (6.0) | |
| Family income, deciles | 1st | 12,135 (8.2) | 14.0 (34.7) | 3434.5 (532.8) | 154.3 (120.4) | 54.8 (5.3) |
| 2nd | 14,744 (9.9) | 12.1 (32.6) | 3480.7 (536.2) | 162.9 (128.7) | 54.8 (5.4) | |
| 3rd | 16,036 (10.8) | 10.6 (30.7) | 3518.9 (534.2) | 169.3 (136.9) | 54.5 (5.5) | |
| 4th | 16,979 (11.5) | 8.9 (28.4) | 3550.2 (533.1) | 175.8 (145.7) | 54.5 (5.7) | |
| 5th | 16,646 (11.2) | 8.9 (28.5) | 3554.5 (539.7) | 186.5 (152.0) | 54.6 (5.8) | |
| 6th | 15,889 (10.7) | 8.9 (28.5) | 3568.7 (532.8) | 205.8 (161.4) | 54.4 (6.0) | |
| 7th | 15,212 (10.3) | 8.5 (27.9) | 3574.0 (526.5) | 219.5 (164.3) | 54.2 (6.1) | |
| 8th | 14,675 (9.9) | 8.0 (27.1) | 3579.0 (531.6) | 234.8 (169.6) | 54.1 (6.2) | |
| 9th | 13,219 (8.9) | 7.8 (26.8) | 3581.0 (514.5) | 248.1 (174.4) | 54.0 (6.1) | |
| 10th | 12,701 (8.6) | 8.7 (28.1) | 3574.9 (513.3) | 267.5 (182.1) | 53.7 (6.2) | |
| Maternal smoking | Non-smoker | 141,859 (95.7) | 9.3 (29) | 3549.6 (530.0) | 203.8 (159.2) | 54.3 (5.8) |
| <10 cigarettes/day | 4977 (3.4) | 15.6 (36.3) | 3415.1 (555.2) | 144.9 (128.0) | 56.0 (5.3) | |
| ≥10 cigarettes/day | 1400 (0.9) | 16.5 (37.1) | 3336.7 (542.9) | 131.2 (118.3) | 56.3 (5.3) | |
| Family situation | Co-habiting with father | 142,456 (96.1) | 9.4 (29.2) | 3546.1 (531.6) | 201.7 (159.0) | 54.4 (5.8) |
| Living alone | 1575 (1.1) | 13.5 (34.2) | 3459.7 (541.6) | 180.4 (134.1) | 54.6 (5.4) | |
| Other | 4205 (2.8) | 13.7 (34.4) | 3471.8 (532.6) | 189.9 (147.8) | 54.7 (5.6) | |
| Maternal BMI | Underweight | 4138 (2.8) | 17.7 (38.2) | 3321.2 (491.7) | 217.1 (166.0) | 53.9 (5.9) |
| Normal Weight | 100,208 (67.6) | 10.0 (30.0) | 3518.8 (513.6) | 215.7 (164.0) | 54.0 (5.9) | |
| Overweight | 31,681 (21.4) | 7.8 (26.8) | 3610.9 (549.5) | 173.2 (142.4) | 55.1 (5.6) | |
| Obese | 12,209 (8.2) | 8.2 (27.5) | 3641.1 (601.2) | 148.5 (124.7) | 55.6 (5.4) | |
| Maternal region of origin | Central and Southern Africa | 4556 (3.1) | 15.8 (36.5) | 3447.7 (564.9) | 169.8 (102.4) | 55.0 (5.1) |
| Oceania | 100 (0.1) | 11.0 (31.4) | 3583.3 (538.2) | 236.3 (171.1) | 53.8 (5.9) | |
| Eastern Europe | 8404 (5.7) | 11.1 (31.4) | 3509.1 (526.7) | 161.6 (125.3) | 55.3 (5.4) | |
| Middle East | 9841 (6.6) | 14.6 (35.3) | 3398.3 (507.1) | 147.4 (111.5) | 55.7 (5.1) | |
| Northern Africa | 1426 (1.0) | 11.2 (31.6) | 3496.3 (522.0) | 151.9 (106.0) | 55.4 (5.0) | |
| North America | 624 (0.4) | 9.1 (28.9) | 3552.3 (495.5) | 247.7 (170.4) | 53.5 (6.3) | |
| Other Nordic countries | 2623 (1.8) | 8.8 (28.4) | 3567.5 (534.8) | 201.4 (163.0) | 54.7 (6.0) | |
| South and Central America | 3202 (2.2) | 9.8 (29.7) | 3485.2 (551.6) | 190.9 (143.4) | 54.6 (5.6) | |
| Southeast Asia | 5317 (3.6) | 16.2 (36.9) | 3349.6 (536.5) | 194.9 (144.2) | 54.4 (5.7) | |
| Sweden | 110,176 (74.3) | 8.4 (27.7) | 3574.0 (527.8) | 210.7 (165.5) | 54.1 (5.9) | |
| Other Western European | 1967 (1.3) | 11.7 (32.2) | 3513.6 (517.6) | 225.0 (169.0) | 53.8 (6.1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olsson, D.; Johansson, C.; Forsberg, B. Associations between Vehicle Exhaust Particles and Ozone at Home Address and Birth Weight. Int. J. Environ. Res. Public Health 2020, 17, 3836. https://doi.org/10.3390/ijerph17113836
Olsson D, Johansson C, Forsberg B. Associations between Vehicle Exhaust Particles and Ozone at Home Address and Birth Weight. International Journal of Environmental Research and Public Health. 2020; 17(11):3836. https://doi.org/10.3390/ijerph17113836
Chicago/Turabian StyleOlsson, David, Christer Johansson, and Bertil Forsberg. 2020. "Associations between Vehicle Exhaust Particles and Ozone at Home Address and Birth Weight" International Journal of Environmental Research and Public Health 17, no. 11: 3836. https://doi.org/10.3390/ijerph17113836
APA StyleOlsson, D., Johansson, C., & Forsberg, B. (2020). Associations between Vehicle Exhaust Particles and Ozone at Home Address and Birth Weight. International Journal of Environmental Research and Public Health, 17(11), 3836. https://doi.org/10.3390/ijerph17113836





