Anticholinergic Burden and Safety Outcomes in Older Patients with Chronic Hepatitis C: A Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Variables
2.2. Comorbidities
2.3. Exposure to Polypharmacy and Anticholinergic Medication
2.4. Assessing Clinical Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Anticholinergic Burden and Associated Risk Factors
3.3. Safety Outcomes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Nair, N.P.; Chalmers, L.; Peterson, G.M.; Bereznicki, B.J.; Castelino, R.L.; Bereznicki, L.R. Hospitalization in older patients due to adverse drug reactions—The need for a prediction tool. Clin. Interv. Aging 2016, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.L.; Hart, L.A.; Perera, S.; Semla, T.P.; Schmader, K.E.; Hanlon, J.T. Meta-analysis of Interventions to Reduce Adverse Drug Reactions in Older Adults. J. Am. Geriatr. Soc. 2017, 66, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, F.; Laino, I.; Pedone, C.; Corica, F.; Maltese, G.; Salerno, G.; Garasto, S.; Corsonello, A.; Incalzi, R.A. Geriatric conditions and adverse drug reactions in elderly hospitalized patients. J. Am. Med. Dir. Assoc. 2012, 13, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. Tools for Assessment of the Appropriateness of Prescribing and Association with Patient-Related Outcomes: A Systematic Review. Drugs Aging 2018, 35, 43–60. [Google Scholar] [CrossRef]
- World Health Organization Medication Safety in Polypharmacy. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/325454/WHO-UHC-SDS-2019.11-eng.pdf (accessed on 26 May 2020).
- Pazan, F.; Kather, J.; Wehling, M. A systematic review and novel classification of listing tools to improve medication in older people. Eur. J. Clin. Pharmacol. 2019, 75, 619–625. [Google Scholar] [CrossRef]
- Petrovic, M.; van der Cammen, T.; Onder, G. Adverse drug reactions in older people: Detection and prevention. Drugs Aging 2012, 29, 453–462. [Google Scholar] [CrossRef]
- Wang-Hansen, M.S.; Wyller, T.B.; Hvidsten, L.T.; Kersten, H. Can screening tools for potentially inappropriate prescriptions in older adults prevent serious adverse drug events? Eur. J. Clin. Pharmacol. 2019, 75, 627–637. [Google Scholar] [CrossRef]
- O’Shaughnessy, K.M. Cholinergic and antimuscarinic (anticholinergic) mechanisms and drugs. In Clinical Pharmacology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 372–381. [Google Scholar]
- Lozano-Ortega, G.; Johnston, K.M.; Cheung, A.; Wagg, A.; Campbell, N.L.; Dmochowski, R.R.; Ng, D.B. A review of published anticholinergic scales and measures and their applicability in database analyses. Arch. Gerontol. Geriatr. 2019, 87, 103885. [Google Scholar] [CrossRef]
- Dowd, F.J. 6—Cholinergic Agonists and Muscarinic Receptor Antagonists. In Pharmacology and Therapeutics for Dentistry; Dowd, F.J., Johnson, B.S., Mariotti, A.J.B.T.-P., Seventh, E., Eds.; Mosby: St. Louis, MO, USA, 2017; pp. 82–97. ISBN 978-0-323-39307-2. [Google Scholar]
- Graves-Morris, K.; Stewart, C.; Soiza, R.L.; Taylor-Rowan, M.; Quinn, T.J.; Loke, Y.K.; Myint, P.K. The Prognostic Value of Anticholinergic Burden Measures in Relation to Mortality in Older Individuals: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 570. [Google Scholar] [CrossRef]
- Corsonello, A.; Cozza, A.; D’Alia, S.; Onder, G.; Volpato, S.; Ruggiero, C.; Cherubini, A.; Di Rosa, M.; Fabbietti, P.; Lattanzio, F. The excess mortality risk associated with anticholinergic burden among older patients discharged from acute care hospital with depressive symptoms. Eur. J. Intern. Med. 2019, 61, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Vermehren, J.; Peiffer, K.-H.; Welsch, C.; Grammatikos, G.; Welker, M.-W.; Weiler, N.; Zeuzem, S.; Welzel, T.M.; Sarrazin, C. The efficacy and safety of direct acting antiviral treatment and clinical significance of drug-drug interactions in elderly patients with chronic hepatitis C virus infection. Aliment. Pharmacol. Ther. 2016, 44, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Brillanti, S.; Buonfiglioli, F.; Vukotic, R.; Morelli, M.C.; Lalanne, C.; Massari, M.; Foschi, F.G.; Bernabucci, V.; Serio, I.; et al. Safety and efficacy of direct-acting antivirals for the treatment of chronic hepatitis C in a real-world population aged 65 years and older. J. Viral Hepat. 2017, 24, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, M.A.; Manne, V.; Kowdley, K.V. Chronic Hepatitis C in Elderly Patients: Current Evidence with Direct-Acting Antivirals. Drugs Aging 2018, 35, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Mazzarelli, C.; Considine, A.; Childs, K.; Carey, I.; Manini, M.A.; Suddle, A.; Dusheiko, G.; Agarwal, K.; Cannon, M.D. Efficacy and Tolerability of Direct-Acting Antivirals for Hepatitis C in Older Adults. J. Am. Geriatr. Soc. 2018, 66, 1339–1345. [Google Scholar] [CrossRef]
- Ozono, Y.; Nagata, K.; Hasuike, S.; Iwakiri, H.; Nakamura, K.; Tsuchimochi, M.; Yamada, Y.; Takaishi, Y.; Sueta, M.; Miike, T.; et al. Efficacy and safety of sofosbuvir and ledipasvir in Japanese patients aged 75 years or over with hepatitis C genotype 1. World J. Hepatol. 2017, 9, 1340. [Google Scholar] [CrossRef]
- Trifan, A.; Stanciu, C.; Gheorghe, L.; Iacob, S.; Curescu, M.; Cijevschi Prelipcean, C.; Stefanescu, G.; Girleanu, I.; Chiriac, S.; Mihai, C.; et al. Efficacy and safety of paritaprevir/ritonavir, ombitasvir, and dasabuvir with ribavirin for the treatment of HCV genotype 1b compensated cirrhosis in patients aged 70 years or older. Medicine 2017, 96, e9271. [Google Scholar] [CrossRef]
- Ampuero, J.; Jimeno, C.; Quiles, R.; Rosales, J.M.; Llerena, S.; Palomo, N.; Cordero, P.; Serrano, F.J.; Urquijo, J.J.; Moreno-Planas, J.M.; et al. Impact of comorbidities on patient outcomes after interferon-free therapy-induced viral eradication in hepatitis C. J. Hepatol. 2018, 68, 940–948. [Google Scholar] [CrossRef]
- Lens, S.; Fernández, I.; Rodríguez-Tajes, S.; Hontangas, V.; Vergara, M.; Forné, M.; Calleja, J.L.; Diago, M.; Llaneras, J.; Llerena, S.; et al. Interferon-Free Therapy in Elderly Patients with Advanced Liver Disease. Am. J. Gastroenterol. 2017, 112, 1400–1409. [Google Scholar] [CrossRef]
- Amoros-Reboredo, P.; Sotoca, J.M.; Mariño, Z.; Rodríguez-Tajes, S.; Pocurull, A.; Soy, D.; Forns, X.; Lens, S. Efficacy and safety of direct-acting antivirals in older patients with cirrhosis and high comorbidity index. Eur. J. Gastroenterol. Hepatol. 2019, 32, 389–394. [Google Scholar] [CrossRef]
- Villani, R.; Donatiello, I.; Barone, F.; Cavallone, F.; Fioravanti, G.; Di Cosimo, F.; Bellanti, F.; Sollitto, F.; Vendemiale, G.; Serviddio, G. Efficacy and safety of direct-acting antivirals in elderly with chronic hepatitis C: Results from a retrospective cohort study. J. Gerontol. Geriatr. 2018, 66, 46–55. [Google Scholar]
- Villani, R.; Monami, M.; Di Cosimo, F.; Fioravanti, G.; Mannucci, E.; Vendemiale, G.; Serviddio, G. Direct-acting antivirals for HCV treatment in older patients: A systematic review and meta-analysis. J. Viral Hepat. 2019, 26, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, A.M.; Iacobellis, A.; Milella, M.; Conti, F.; Messina, V.; Valvano, M.R.; Niro, G.A.; Morisco, F.; Barone, M.; Termite, A.P.; et al. Hepatitis C Virus Clearance in Older Adults. J. Am. Geriatr. Soc. 2017, 66, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, A.; Cortesi, P.A.; Bellelli, G.; Rota, M.; Conti, S.; Okolicsanyi, S.; Rota, M.; Cesana, G.; Mantovani, L.G.; Annoni, G.; et al. Direct-acting antivirals combination for elderly patients with chronic hepatitis C: A cost-effectiveness analysis. Liver Int. 2017, 37, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Rheem, J.; Sundaram, V. Hepatitis C Infection in the Elderly. Dig. Dis. Sci. 2015, 60, 3170–3180. [Google Scholar] [CrossRef] [PubMed]
- Green, A.R.; Reifler, L.M.; Bayliss, E.A.; Weffald, L.A.; Boyd, C.M. Drugs Contributing to Anticholinergic Burden and Risk of Fall or Fall-Related Injury among Older Adults with Mild Cognitive Impairment, Dementia and Multiple Chronic Conditions: A Retrospective Cohort Study. Drugs Aging 2019, 36, 289–297. [Google Scholar] [CrossRef]
- Welsh, T.J.; van der Wardt, V.; Ojo, G.; Gordon, A.L.; Gladman, J.R.F. Anticholinergic Drug Burden Tools/Scales and Adverse Outcomes in Different Clinical Settings: A Systematic Review of Reviews. Drugs Aging 2018, 35, 523–538. [Google Scholar] [CrossRef]
- Sevilla-Sánchez, D.; Molist-Brunet, N.; González-Bueno, J.; Solà-Bonada, N.; Espaulella-Panicot, J.; Codina-Jané, C. Prevalence, risk factors and adverse outcomes of anticholinergic burden in patients with advanced chronic conditions at hospital admission. Geriatr. Gerontol. Int. 2018, 18, 1159–1165. [Google Scholar] [CrossRef]
- Casajús-Navasal, A.; Marín-Gorricho, R.; Gallardo-Anciano, J.; Nebot-Villacampa, M.J.; Zafra-Morales, R.; González-Pérez, Y. Prevalence of the consumption of anticholinergic drugs in HIV patients. Farm. Hosp. 2018, 42, 1–4. [Google Scholar]
- Boustani, M.; Campbell, N.; Munger, S.; Maidment, I.; Fox, C. Impact of anticholinergics on the aging brain: A review and practical application. Aging Health 2008, 4, 311–320. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Salow, M.J.; Angelini, M.C.; McGlinchey, R.E. The Anticholinergic Risk Scale and Anticholinergic Adverse Effects in Older Persons. Arch. Intern. Med. 2008, 168, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Carnahan, R.M.; Lund, B.C.; Perry, P.J.; Pollock, B.G.; Gulp, K.R. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: Associations with serum anticholinergic activity. J. Clin. Pharmacol. 2006, 46, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.T. Hepatic venous pressure gradient: Clinical use in chronic liver disease. Clin. Mol. Hepatol. 2014, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Vidal, R.; López-Pisa, R.M.; Boyero-Granados, A.; Recio-Ramos, S.; Padín-Minaya, C.; Garzón-Quiñones, M.; Rodríguez-Latre, L.M. Stratification of clinical risk groups in a population over 65 years: Features and nursing assessment. Enferm. Clin. 2014, 24, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.S.; Averill, R.F.; Eisenhandler, J.; Goldfield, N.I.; Muldoon, J.; Neff, J.M.; Gay, J.C. Clinical Risk Groups (CRGs) a classification system for risk-adjusted capitation-based payment and health care management. Med. Care 2004, 42, 81–90. [Google Scholar] [CrossRef]
- ICH. Internacional Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guideline for good clinical practice-E6 (R2). ICH Harmon. Tripart. Guidel. 2016, 5, 52. [Google Scholar]
- Hernandez, M.; Mestres, C.; Junyent, J.; Costa-Tutusaus, L.; Modamio, P.; Fernandez Lastra, C.; Mariño, E.L. Effects of a multifaceted intervention in psychogeriatric patients: One-year prospective study. Eur. J. Hosp. Pharm. 2018. [Google Scholar] [CrossRef]
- Lattanzio, F.; Corica, F.; Schepisi, R.; Amantea, D.; Bruno, F.; Cozza, A.; Onder, G.; Volpato, S.; Cherubini, A.; Ruggiero, C.; et al. Anticholinergic burden and 1-year mortality among older patients discharged from acute care hospital. Geriatr. Gerontol. Int. 2018, 18, 705–713. [Google Scholar] [CrossRef]
- Hanlon, P.; Quinn, T.J.; Gallacher, K.I.; Myint, P.K.; Jani, B.D.; Nicholl, B.I.; Lowrie, R.; Soiza, R.L.; Neal, S.R.; Lee, D.; et al. Assessing risks of polypharmacy involving medications with anticholinergic properties. Ann. Fam. Med. 2020, 18, 148–155. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Streim, J.; Carpenter, D.; Docherty, J.P. Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients Using antipsychotic agents in older patients. J. Clin. Psychiatry 2004, 65, 5–99. [Google Scholar]
- Davidson, J.R.T.; Feltner, D.E.; Dugar, A. Management of Generalized Anxiety Disorder in Primary Care. Prim. Care Companion J. Clin. Psychiatry 2010, 12, 09r00772. [Google Scholar] [CrossRef] [PubMed]
- Evon, D.M.; Stewart, P.W.; Amador, J.; Serper, M.; Lok, A.S.; Sterling, R.K.; Sarkar, S.; Golin, C.E.; Reeve, B.B.; Nelson, D.R.; et al. A comprehensive assessment of patient reported symptom burden, medical comorbidities, and functional well being in patients initiating direct acting antiviral therapy for chronic hepatitis C: Results from a large US multi-center observational study. PLoS ONE 2018, 13, e0196908. [Google Scholar] [CrossRef] [PubMed]
- Jean-Bart, E.; Moutet, C.; Dauphinot, V.; Krolak-Salmon, P.; Mouchoux, C. Exposure to anticholinergic and sedative medicines as indicators of high-risk prescriptions in the elderly. Int. J. Clin. Pharm. 2017, 39, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.H.; Wen, Y.W.; Chen, L.K.; Hsiao, F.Y. Effect of polypharmacy, potentially inappropriate medications and anticholinergic burden on clinical outcomes: A retrospective cohort study. CMAJ 2015, 187, E130–E137. [Google Scholar] [CrossRef]
- Villalba-Moreno, A.M.; Alfaro-Lara, E.R.; Pérez-Guerrero, M.C.; Nieto-Martín, M.D.; Santos-Ramos, B. Systematic review on the use of anticholinergic scales in poly pathological patients. Arch. Gerontol. Geriatr. 2016, 62, 1–8. [Google Scholar] [CrossRef]
- Nishtala, P.S.; Salahudeen, M.S.; Hilmer, S.N. Anticholinergics: Theoretical and clinical overview. Expert Opin. Drug Saf. 2016, 15, 753–768. [Google Scholar] [CrossRef]
- Green, A.R.; Reifler, L.M.; Boyd, C.M.; Weffald, L.A.; Bayliss, E.A. Medication Profiles of Patients with Cognitive Impairment and High Anticholinergic Burden. Drugs Aging 2018, 35, 223–232. [Google Scholar] [CrossRef]
- Hsu, W.H.; Wen, Y.W.; Chen, L.K.; Hsiao, F.Y. Comparative associations between measures of anticholinergic burden and adverse clinical outcomes. Ann. Fam. Med. 2017, 15, 561–569. [Google Scholar] [CrossRef]
- Gutiérrez-Valencia, M.; Martínez-Velilla, N.; Vetrano, D.L.; Corsonello, A.; Lattanzio, F.; Ladrón-Arana, S.; Onder, G. Anticholinergic burden and health outcomes among older adults discharged from hospital: Results from the CRIME study. Eur. J. Clin. Pharmacol. 2017, 73, 1467–1474. [Google Scholar] [CrossRef]
- Ruxton, K.; Woodman, R.J.; Mangoni, A.A. Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2015, 80, 209–220. [Google Scholar] [CrossRef]
- Kersten, H.; Wyller, T.B. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin. Pharmacol. Toxicol. 2014, 114, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Yang, Y.M.; Yoo, J.C.; Choi, E.J. Comparative Analysis Of Anticholinergics Prescribed To Elderly Patients At A Korean Long-Term Care Facility According To Beers Criteria 2003, 2012, And 2015 And Anticholinergic-Burden Rating Scales: A Cross-Sectional Retrospective Study. Clin. Interv. Aging 2019, 14, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, X.; Tong, J.; Du, J.; Duan, R.; Yang, L.; Moore, J.H.; Tao, C.; Chen, Y. Comparing drug safety of hepatitis C therapies using post-market data. BMC Med. Inform. Decis. Mak. 2019, 19, 147. [Google Scholar] [CrossRef] [PubMed]
- Sessa, M.; Bernardi, F.F.; Vitale, A.; Schiavone, B.; Gritti, G.; Mascolo, A.; Bertini, M.; Scavone, C.; Sportiello, L.; Rossi, F.; et al. Adverse drug reactions during hepatitis C treatment with direct-acting antivirals: The role of medication errors, their impact on treatment discontinuation and their preventability. New insights from the Campania Region (Italy) spontaneous reporting system. J. Clin. Pharm. Ther. 2018, 43, 867–876. [Google Scholar] [CrossRef]
- Sargent, L.; Nalls, M.; Amella, E.J.; Mueller, M.; Lageman, S.K.; Bandinelli, S.; Colpo, M.; Slattum, P.W.; Singleton, A.; Ferrucci, L. Anticholinergic Drug Induced Cognitive and Physical Impairment: Results from the InCHIANTI Study. J. Gerontol. Ser. A 2020, 75, 995–1002. [Google Scholar] [CrossRef]
- Merle, L.; Laroche, M.-L.; Dantoine, T.; Charmes, J.-P. Predicting and Preventing Adverse Drug Reactions in the Very Old. Drugs Aging 2012, 22, 375–392. [Google Scholar] [CrossRef]
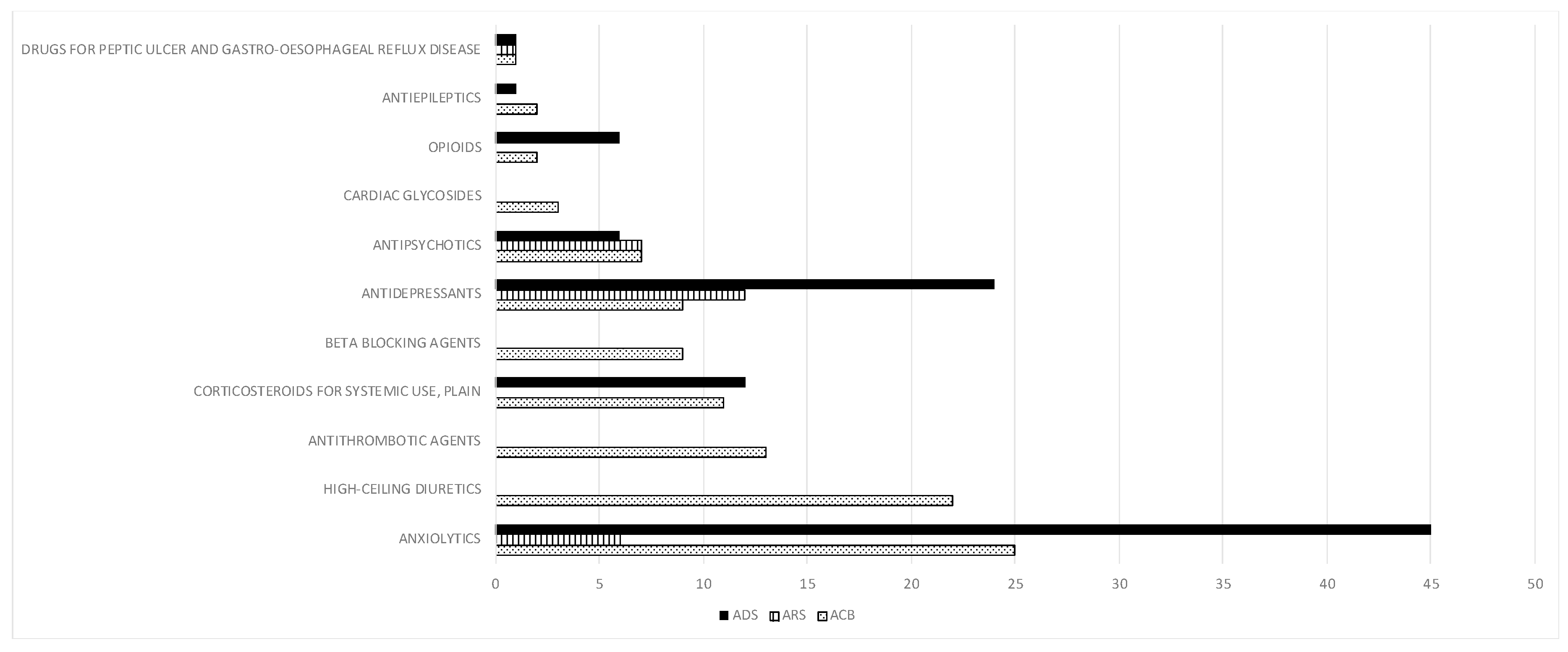
| Variable | N = 236 |
|---|---|
| Age, years | 71.7 (4.7) |
| Female gender | 147 (62.3%) |
| Fibrosi stage: | |
| f0–f1 | 6 (2.5%) |
| f2 | 16 (6.8%) |
| f3 | 41 (17.4%) |
| Cirrhosis | 173 (73.3%) |
| Previous liver transplantation | 22 (9.3%) |
| Clinical Risk Group (>06/5) | 105 (44.5%) |
| Arterial hypertension | 140 (59.3%) |
| Diabetes | 53 (22.5%) |
| Cardiopathy | 37 (15.7%) |
| Arrhythmia | 15 (6.4%) |
| COPD | 12 (5.1%) |
| Depression | 27 (11.4%) |
| Tumor | 47 (19.9%) |
| History of previous HCC | 18 (7.6%) |
| Treatment experienced | 117 (49.6%) |
| HCV genotype: | |
| 1a | 13 (5.5%) |
| 1b | 210 (89.0%) |
| 2 | 9 (3.8%) |
| 3 | 3 (1.3%) |
| 4 | 1 (0.4%) |
| Oligopharmacy | 124 (52.5%) |
| Moderate polypharmacy | 101 (42.8%) |
| Excessive polypharmacy | 11 (4.7%) |
| Patients with potential DDIs | 156 (66.1%) |
| Characteristics | Without Ach-Drugs | With Ach-Drugs | Comparison between Groups (p-Value) | Low ACB (1–2 Points) | High ACB (≥3 Points) | Comparison between Groups (p-Value) 2 |
|---|---|---|---|---|---|---|
| No.patients | 153 | 83 | 62 | 21 | ||
| Age, years * | 72.2 (4.8) | 70.7 (4.4) | 0.0191 | 70.5 (4.1) | 71.4 (5.1) | 0.4169 |
| Sex (female) | 91 (59.5%) | 56 (67.5%) | 0.2269 | 42 (67.7%) | 14 (66.7%) | 0.9330 |
| CRG ≥ 6/05 * | 59 (38.6%) | 46 (55.4%) | 0.0133 | 32 (51.6%) | 14 (66.7%) | 0.2317 |
| Mean drugs/patient * | 4.0 (2.5) | 5.7 (3.0) | <0.0001 | 5.5 (3.1) | 6.3 (2.6) | 0.2915 |
| Oligopharmacy | 93 (60.8%) | 31 (37.3%) | 0.0006 | 26 (41.9%) | 5 (23.8%) | 0.1407 |
| Moderate polypharmacy | 58 (37.9%) | 43 (51.8%) | 0.0397 | 29 (46.8%) | 14 (66.7%) | 0.1169 |
| Excessive polypharmacy | 2 (1.3%) | 9 (10.8%) | 0.0009 | 7 (11.3%) | 2 (9.5%) | 0.8197 |
| Potential DDIs | 95 (62.1%) | 61 (73.5%) | 0.0883 | 46 (74.2%) | 15 (71.4%) | 0.8028 |
| AEs (N = 206) | 133 (86.9%) | 73 (88%) | 0.8090 | 52 (83.9%) | 21 (100%) | 0.0513 |
| AEs per patient * | 2.9 (1.8) | 2.9 (1.9) | 1.0000 | 2.8 (1.7) | 3.3 (2.3) | 0.3093 |
| Hospital admission (N = 9) | 5 (3.3%) | 4 (4.8%) | 0.5671 | 2 (3.2%) | 2 (9.5%) | 0.2457 |
| Death (N = 4) | 3 (2.0%) | 1 (1.2%) | 0.6523 | 1 (1.6%) | 0 (0%) | 0.5622 |
| Ach events (N = 99) | 63 (41.2%) | 36 (43.4%) | 0.7442 | 25 (40.3%) | 11 (52.4%) | 0.3365 |
| Ach events per patient * | 1.3 (0.6) | 1.5 (0.8) | 0.0312 | 1.4 (0.8) | 1.8 (1.0) | 0.0671 |
| Characteristics | Without Ach-Drugs | With Ach-Drugs | Comparison between Groups (p-Value) | Low ARS (1–2 Points) | High ARS (≥3 Points) | Comparison between Groups (p-Value) 2 |
|---|---|---|---|---|---|---|
| No.patients | 211 | 25 | 15 | 10 | ||
| Age, years * | 71.8 (4.7) | 70.4 (4.8) | 0.1613 | 69.8 (3.5) | 71.4 (6.3) | 0.4221 |
| Sex (female) | 130 (61.6%) | 17 (68.0%) | 0.5333 | 9 (60.0%) | 8 (80.0%) | 0.3035 |
| CRG ≥ 6/05 | 87 (41.2%) | 18 (72.0%) | 0.0035 | 11 (73.3%) | 7 (70.0%) | 0.8600 |
| Mean drugs/patient * | 4.4 (2.8) | 6.1 (2.5) | 0.0041 | 5.7 (2.6) | 6.6 (2.3) | 0.3845 |
| Oligopharmacy | 118 (55.9%) | 6 (24.0%) | 0.0026 | 5 (33.3%) | 1 (10.0%) | 0.1903 |
| Moderate polypharmacy | 84 (39.8%) | 17 (68.0%) | 0.0072 | 9 (60.0%) | 8 (80.0%) | 0.3035 |
| Excessive polypharmacy | 9 (4.3%) | 2 (8.0%) | 0.4091 | 1 (6.7%) | 1 (10.0%) | 0.7706 |
| Potential DDIs | 138 (65.4%) | 18 (72.0%) | 0.5107 | 11 (73.3%) | 7 (70.0%) | 0.8600 |
| AEs (N = 206) | 181 (85.8%) | 25 (100%) | 0.0442 | 15 (100%) | 10 (100%) | - |
| AEs per patient * | 2.8 (1.8) | 3.3 (2.2) | 0.2071 | 2.5 (1.4) | 4.4 (2.9) | 0.0383 |
| Hospital admission (N = 9) | 7 (3.3%) | 2 (8.0%) | 0.2460 | 0 (0%) | 2 (20.0%) | 0.0768 |
| Death (N = 4) | 4 (1.9%) | 0 (0%) | 0.4879 | 0 (0%) | 0 (0%) | - |
| Ach events (N = 99) | 85 (40.3%) | 14 (56.0%) | 0.1334 | 6 (40.0%) | 8 (80.0%) | 0.0531 |
| Ach events per patient * | 1.3 (0.7) | 1.8 (0.9) | 0.0012 | 1.7 (0.5) | 1.9 (1.1) | 0.5418 |
| Characteristics | Without Ach-Drugs | With Ach-Drugs | Comparison between Groups (p-Value) | Low ADS (1–2 Points) | High ADS (≥3 Points) | Comparison between Groups (p-Value) 2 |
|---|---|---|---|---|---|---|
| No.patients | 155 | 81 | 60 | 21 | ||
| Age, years * | 71.9 (4.8) | 71.3 (4.6) | 0.3561 | 71.6 (4.4) | 70.6 (5.0) | 0.3896 |
| Sex (female) | 92 (59.4%) | 55 (67.9%) | 0.2017 | 42 (70.0%) | 13 (61.9%) | 0.4965 |
| CRG ≥ 6/05 | 63 (40.6%) | 42 (51.9%) | 0.0979 | 28 (46.7%) | 14 (66.7%) | 0.1167 |
| Mean drugs/patient * | 4 (2.7) | 5.8 (2.7) | <0.0001 | 5.7 (2.8) | 6.1 (2.4) | 0.5613 |
| Oligopharmacy | 97 (62.6%) | 27 (33.3%) | <0.0001 | 23 (38.3%) | 4 (19.0%) | 0.1084 |
| Moderate polypharmacy | 54 (34.8%) | 47 (58.0%) | 0.0006 | 31 (51.7%) | 16 (76.2%) | 0.0517 |
| Excessive polypharmacy | 4 (2.6%) | 7 (8.6%) | 0.0383 | 6 (10.0%) | 1 (4.8%) | 0.4684 |
| Potential DDIs | 99 (63.9%) | 57 (70.4%) | 0.3175 | 44 (73.3%) | 13 (61.9%) | 0.3279 |
| AEs (N = 206) | 136 (87.7%) | 70 (86.4%) | 0.7766 | 51 (85%) | 19 (90.5%) | 0.5291 |
| AEs per patient * | 2.9 (1.7) | 2.9 (2.1) | 1.0000 | 2.6 (1.9) | 3.5 (2.6) | 0.1168 |
| Hospital admission (N = 9) | 6 (3.9%) | 3 (3.7%) | 0.9396 | 1 (1.7%) | 2 (9.5%) | 0.1063 |
| Death (N = 4) | 2 (1.3%) | 2 (2.5%) | 0.5007 | 2 (3.3%) | 0 (0%) | 0.4022 |
| Ach events (N = 99) | 63 (40.6%) | 36 (44.4%) | 0.5751 | 25 (41.7%) | 11 (52.4%) | 0.3987 |
| Ach events per patient * | 1.3 (0.6) | 1.6 (0.9) | 0.0025 | 1.4 (0.9) | 1.9 (0.9) | 0.0314 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoros-Reboredo, P.; Soy, D.; Hernandez-Hernandez, M.; Lens, S.; Mestres, C. Anticholinergic Burden and Safety Outcomes in Older Patients with Chronic Hepatitis C: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 3776. https://doi.org/10.3390/ijerph17113776
Amoros-Reboredo P, Soy D, Hernandez-Hernandez M, Lens S, Mestres C. Anticholinergic Burden and Safety Outcomes in Older Patients with Chronic Hepatitis C: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2020; 17(11):3776. https://doi.org/10.3390/ijerph17113776
Chicago/Turabian StyleAmoros-Reboredo, Patricia, Dolors Soy, Marta Hernandez-Hernandez, Sabela Lens, and Conxita Mestres. 2020. "Anticholinergic Burden and Safety Outcomes in Older Patients with Chronic Hepatitis C: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 17, no. 11: 3776. https://doi.org/10.3390/ijerph17113776
APA StyleAmoros-Reboredo, P., Soy, D., Hernandez-Hernandez, M., Lens, S., & Mestres, C. (2020). Anticholinergic Burden and Safety Outcomes in Older Patients with Chronic Hepatitis C: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 17(11), 3776. https://doi.org/10.3390/ijerph17113776






