Effect of Different Running Exercise Modalities on Post-Exercise Oxidative Stress Markers in Trained Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
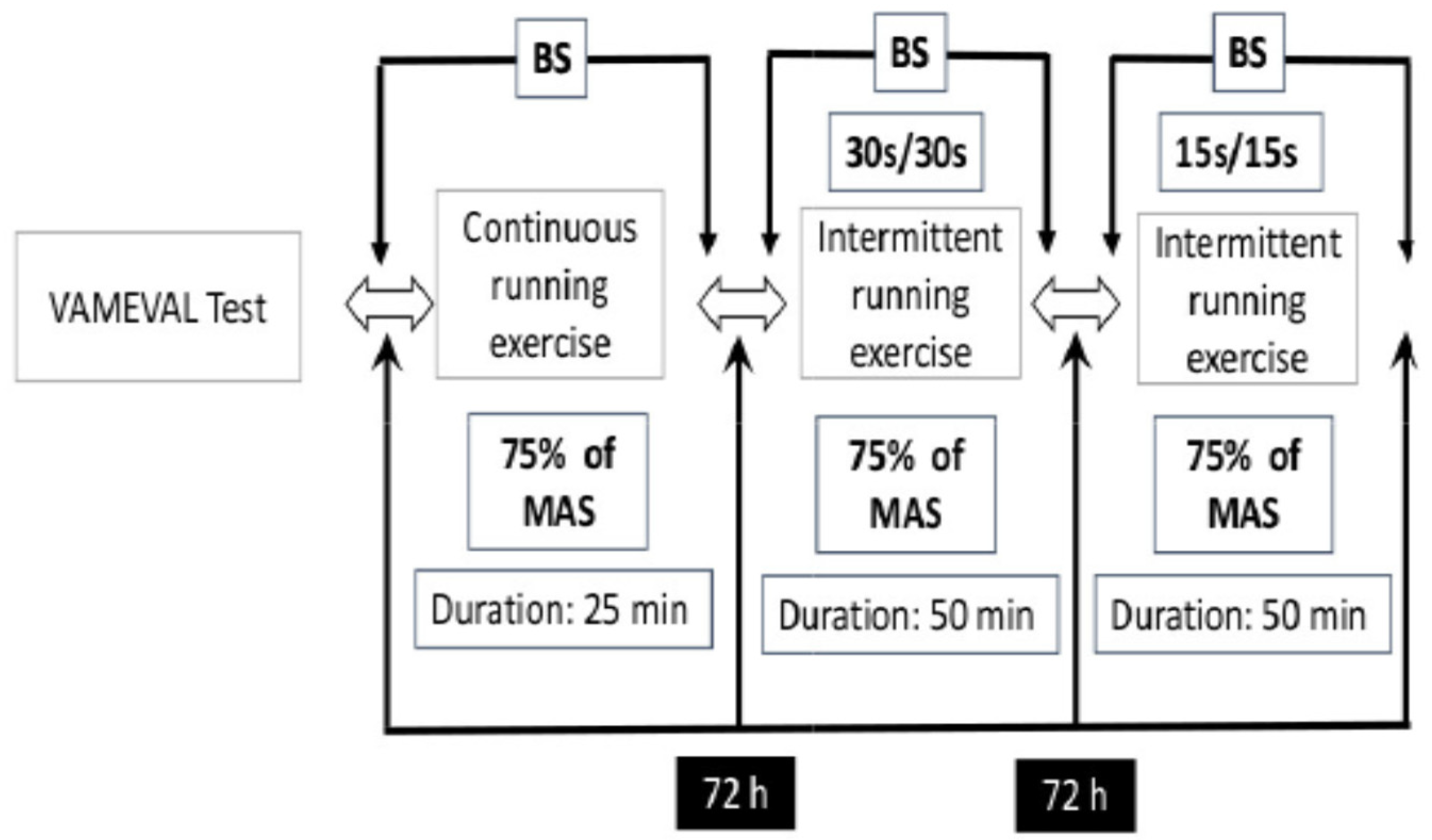
2.2. Experimental Protocol
2.3. VAMEVAL Test
2.4. Dietary Records
2.5. Blood Sampling and Analysis
2.6. Protein Rate Determination
2.7. Antioxidants Measurement (SOD)
2.8. GPX
2.9. Markers of Radical Damages (MDA)
2.10. AOPP
2.11. Statistical Analysis
3. Results
3.1. Physiological Parameters
3.2. Dietary Intake
3.3. MDA Level
3.4. AOPP Level
3.5. SOD Activity
3.6. GPX Activity
4. Discussion
Experimental Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2011, 2, 1143–1211. [Google Scholar]
- Pedisic, Z.; Shrestha, N.; Kovalchik, S.; Stamatakis, E.; Liangruenrom, N.; Grgic, J.; Titze, S.; Biddle, S.; Bauman, A.E.; Oja, P. Is running associated with a lower risk of all-cause, cardiovascular and cancer mortality, and is the more the better? A systematic review and meta-analysis. Br. J. Sports Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E.; Vollaard, N.B.; Choueiri, T.; Wilson, M.T. Exercise, free radicals and oxidative stress. Biochem. Soc. Trans. 2002, 30, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Smuder, A.J.; Kavazis, A.N.; Hudson, M.B. Experimental guidelines for studies designed to investigate the impact of antioxidant supplementation on exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 2–14. [Google Scholar] [CrossRef]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Martínez, A.; Santangelo, G.; Pallardó, F.V.; Sastre, J.; Vina, J. Oxidative stress in marathon runners: Interest of antioxidant supplementation. Br. J. Nutr. 2006, 96, S31–S33. [Google Scholar] [CrossRef]
- Knez, W.L.; Jenkins, D.G.; Coombes, J.S. Oxidative stress in half and full Ironman triathletes. Med. Sci. Sports Exerc. 2007, 39, 283. [Google Scholar] [CrossRef]
- Lenn, J.; Uhl, T.; Mattacola, C.; Boissonneault, G.; Yates, J.; Ibrahim, W.; Bruckner, G. The effects of fish oil and isoflavones on delayed onset muscle soreness. Med. Sci. Sports Exerc. 2002, 34, 1605–1613. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Hansen, P.A.; Holloszy, J.O.; Heinecke, J.W. Oxidized amino acids in the urine of aging rats: Potential markers for assessing oxidative stress in vivo. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1999, 276, R128–R135. [Google Scholar] [CrossRef]
- Inal, M.; AkyÜz, F.; Turgut, A.; Getsfrid, W.M. Effect of aerobic and anaerobic metabolism on free radical generation swimmers. Med. Sci. Sports Exerc. 2001, 33, 564–567. [Google Scholar] [CrossRef]
- Bouzid, M.A.; Filaire, E.; Matran, R.; Robin, S.; Fabre, C. Lifelong voluntary exercise modulates age-related changes in oxidative stress. Inter. J. Sports Med. 2018, 40, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bessa, A.L.; Oliveira, V.N.; Agostini, G.G.; Oliveira, R.J.; Oliveira, A.C.; White, G.E.; Wells, G.D.; Teixeira, D.N.; Espindola, F.S. Exercise intensity and recovery: Biomarkers of injury, inflammation, and oxidative stress. J. Strenght Cond. Res. 2016, 30, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Accattato, F.; Greco, M.; Pullano, S.A.; Carè, I.; Fiorillo, A.S.; Pujia, A.; Montalcini, T.; Foti, D.P.; Brunetti, A.; Gulletta, E. Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS ONE 2017, 12, e0178900. [Google Scholar] [CrossRef] [PubMed]
- González-Bartholin, R.; Mackay, K.; Valladares, D.; Zbinden-Foncea, H.; Nosaka, K.; Peñailillo, L. Changes in oxidative stress, inflammation and muscle damage markers following eccentric versus concentric cycling in older adults. Eur. J. Appl. Physiol. 2019, 119, 2301–2312. [Google Scholar] [CrossRef]
- Combes, A.; Dekerle, J.; Webborn, N.; Watt, P.; Bougault, V.; Daussin, F.N. Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle. Physiol. Rep. 2015, 3, e12462. [Google Scholar] [CrossRef]
- Combes, A.; Dekerle, J.; Bougault, V.; Daussin, F.N. Effect of work: Rest cycle duration on fluctuations during intermittent exercise. J. Sports Sci. 2017, 35, 7–13. [Google Scholar] [CrossRef]
- Vezzoli, A.; Pugliese, L.; Marzorati, M.; Serpiello, F.R.; La Torre, A.; Porcelli, S. Time-course changes of oxidative stress response to high-intensity discontinuous training versus moderate-intensity continuous training in masters runners. PLoS ONE 2014, 9, e87506. [Google Scholar] [CrossRef]
- Souza, A.V.; Giolo, J.S.; Teixeira, R.R.; Vilela, D.D.; Peixoto, L.G.; Justino, A.B.; Caixeta, D.C.; Puga, G.M.; Espindola, F.S. Salivary and Plasmatic Antioxidant Profile following Continuous, Resistance, and High-Intensity Interval Exercise: Preliminary Study. Oxid. Med. Cell. Longev. 2019, 2019, 5425021. [Google Scholar] [CrossRef]
- Brown, M.; McClean, C.M.; Davison, G.W.; Brown, J.C.; Murphy, M.H. The acute effects of walking exercise intensity on systemic cytokines and oxidative stress. Eur. J. Appl. Physiol. 2018, 118, 2111–2120. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Léger, L.; Boucher, R. An indirect continuous running multistage field test: The Universite de Montreal track test. Can. J. Appl. Sport. Sci. 1980, 5, 77–84. [Google Scholar] [PubMed]
- El Abed, K.; Ammar, A.; Boukhris, O.; Trabelsi, K.; Masmoudi, L.; Bailey, S.; Hakim, A.; Luigi Bragazzi, N. Independent and combined effects of all-out sprint and low-intensity continuous exercise on plasma oxidative stress biomarkers in trained judokas. Front. Physiol. 2019, 10, 842. [Google Scholar] [CrossRef]
- Dill, D.B.; Costill, D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974, 37, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.B.W.; Kruger, N.J. The bradford method for protein quantitation. In New Protein Techniques; Humana Press: Totowa, NJ, USA, 1988; pp. 25–32. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Kayali, R.; Çakatay, U.; Akçay, T.; Altuğ, T. Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem. Funct. 2006, 24, 79–85. [Google Scholar] [CrossRef]
- Canale, R.E.; Farney, T.M.; McCarthy, C.G.; Bloomer, R.J. Influence of acute exercise of varying intensity and duration on postprandial oxidative stress. Eur. J. Appl. Physiol. 2014, 114, 1913–1924. [Google Scholar] [CrossRef]
- Park, S.Y.; Kwak, Y.S. Impact of aerobic and anaerobic exercise training on oxidative stress and antioxidant defense in athletes. J. Exerc. Rehabil. 2016, 12, 113–117. [Google Scholar] [CrossRef]
- Ashton, T.; Rowlands, C.C.; Jones, E.; Young, I.S.; Jackson, S.K.; Davies, B.; Peters, J.R. Electron spin resonance spectroscopic detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 498–502. [Google Scholar] [CrossRef]
- Bailey, D.M.; Davies, B.; Young, I.S. Intermittent hypoxic training: Implications for lipid peroxidation induced by acute normoxic exercise in active men. Clin. Sci. 2001, 101, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, I.; Tessier, F.; Richard, M.J.; Marconnet, P. No evidence of oxidative stress after a triathlon race in highly trained competitors. Int. J. Sports Med. 1997, 18, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Goldfarb, A.H.; Wideman, L.; McKenzie, M.J.; Consitt, L.A. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J. Strength Cond. Res. 2005, 19, 276–285. [Google Scholar] [PubMed]
- Välimäki, I.A.; Vuorimaa, T.; Ahotupa, M.; Vasankari, T. Effect of Continuous and Intermittent Exercises on Oxidised HDL and LDL Lipids in Runners. Int. J. Sports Med. 2016, 37, 1103–1109. [Google Scholar] [CrossRef]
- Kabasakalis, A.; Tsalis, G.; Zafrana, E.; Loupos, D.; Mougios, V. Effects of endurance and high-intensity swimming exercise on the redox status of adolescent male and female swimmers. J. Sports Sci. 2014, 32, 747–756. [Google Scholar] [CrossRef]
- Berzosa, C.; Cebrian, I.; Fuentes-Broto, L.; Gomez-Trullen, E.; Piedrafita, E.; Martinez-Ballarin, E.; Lopez-Pingarron, L.; Reiter, R.J.; Garcia, J.J. Acute exercise increases plasma total antioxidant status and antioxidant enzyme activities in untrained men. BioMed Res. Int. 2011, 2011, 540458. [Google Scholar] [CrossRef]
- Groussard, C.; Rannou-Bekono, F.; Machefer, G.; Chevanne, M.; Vincent, S.; Sergent, O.; Cillard, J.; Gratas-Delamarche, A. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur. J. Appl. Physiol. 2003, 89, 14–20. [Google Scholar] [CrossRef]
- Kiyici, F.; Kishali, N.F. Acute effect of intense exercises on serum superoxide dismutase, catalase and malondialdehyde levels in soccer players. J. Sports Med. Phys. Fit. 2012, 52, 107–111. [Google Scholar]
- Jimenez, A.; Creissen, G.; Kular, B.; Firmin, J.; Robinson, S.; Verhoeyen, M.; Mullineaux, P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 2002, 214, 751–758. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]




| Continuous Running | 15/15 Intermittent Running | 30/30 Intermittent Running | |
|---|---|---|---|
| Energy intake (Mj/day) | 12.4 ± 1.7 | 13.2 ± 2.1 | 11.9 ± 2.3 |
| Carbohydrates (g/day) | 328.4 ± 77 | 320 ± 32.1 | 294.7 ± 49.3 |
| Proteins (g/kg(BW)/day) | 2.16 ± 0.3 | 2.1 ± 0.3 | 1.9 ± 0.9 |
| Lipids (g/day) | 108.5 ± 37.5 | 109 ± 33.1 | 111.2 ± 24.3 |
| Vitamin C (mg/day) | 103.2 ± 17.8 | 109 ± 20.1 | 101.4 ± 27.1 |
| Vitamin E (mg/day) | 11.8 ± 2.2 | 12.9 ± 2.1 | 10.5 ± 3.6 |
| Selenium (µg/day) | 69.1 ± 19.5 | 75.1 ± 6.6 | 74.1 ± 19.2 |
| Zinc (mg/day) | 13.1 ± 2.7 | 13.0 ± 2.8 | 11.6 ± 4.1 |
| Copper (mg/day) | 1.5± 0.2 | 1.9 ± 0.3 | 1.1 ± 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souissi, W.; Bouzid, M.A.; Farjallah, M.A.; Ben Mahmoud, L.; Boudaya, M.; Engel, F.A.; Sahnoun, Z. Effect of Different Running Exercise Modalities on Post-Exercise Oxidative Stress Markers in Trained Athletes. Int. J. Environ. Res. Public Health 2020, 17, 3729. https://doi.org/10.3390/ijerph17103729
Souissi W, Bouzid MA, Farjallah MA, Ben Mahmoud L, Boudaya M, Engel FA, Sahnoun Z. Effect of Different Running Exercise Modalities on Post-Exercise Oxidative Stress Markers in Trained Athletes. International Journal of Environmental Research and Public Health. 2020; 17(10):3729. https://doi.org/10.3390/ijerph17103729
Chicago/Turabian StyleSouissi, Wajdi, Mohamed Amine Bouzid, Mohamed Amine Farjallah, Lobna Ben Mahmoud, Mariem Boudaya, Florian A. Engel, and Zouheir Sahnoun. 2020. "Effect of Different Running Exercise Modalities on Post-Exercise Oxidative Stress Markers in Trained Athletes" International Journal of Environmental Research and Public Health 17, no. 10: 3729. https://doi.org/10.3390/ijerph17103729
APA StyleSouissi, W., Bouzid, M. A., Farjallah, M. A., Ben Mahmoud, L., Boudaya, M., Engel, F. A., & Sahnoun, Z. (2020). Effect of Different Running Exercise Modalities on Post-Exercise Oxidative Stress Markers in Trained Athletes. International Journal of Environmental Research and Public Health, 17(10), 3729. https://doi.org/10.3390/ijerph17103729





