Potential Application of Whole Body Vibration Exercise for Improving the Clinical Conditions of COVID-19 Infected Individuals: A Narrative Review from the World Association of Vibration Exercise Experts (WAVex) Panel
Abstract
1. Introduction
2. Effects of the WBV Exercises That Could Be Relevant to the Management of Individuals Infected with COVID-19
3. Reduction of the Fatigue and the Risk of Dyspnea
4. Anti-Inflammatory Biomarkers Responses to WBV
5. Immune and Myokine Responses to WBV
6. WBV Exercise in Bed-Bound and ICU-Bound Subjects
7. Practical Implementation of WBV Exercise for ICU-Bound Subjects: Monitoring and Adaptation of Training Intensity
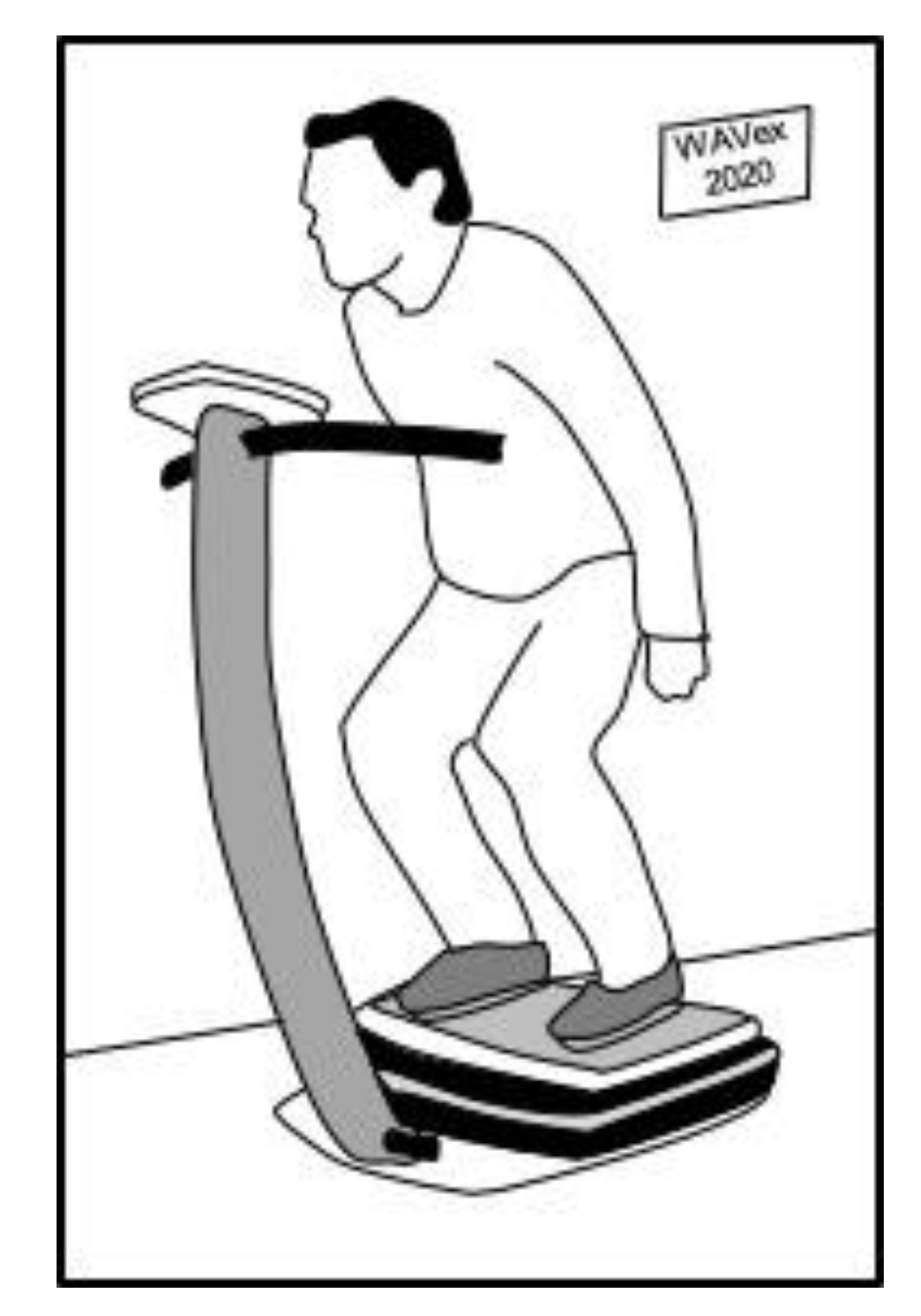
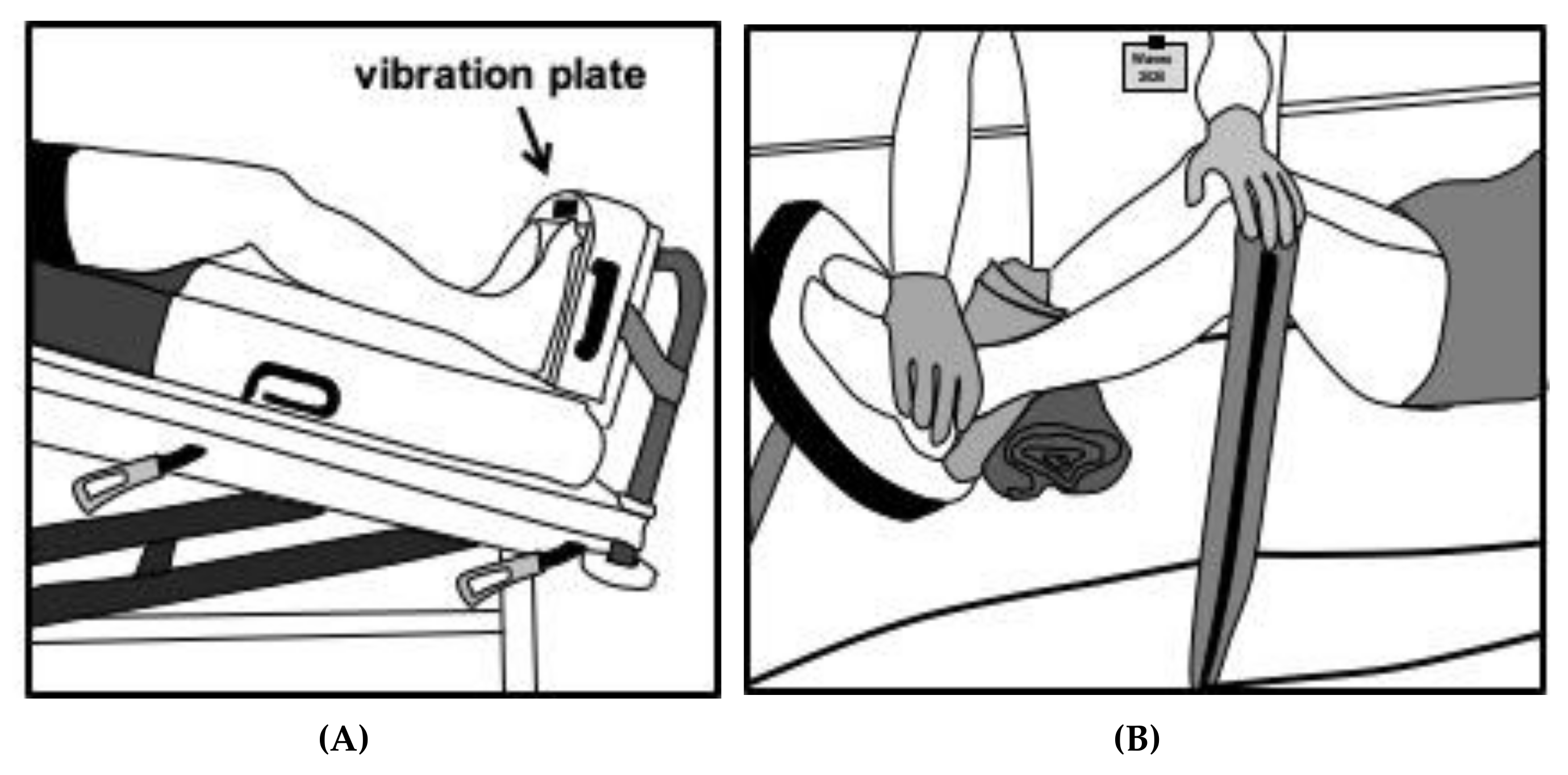
7.1. Application of WBV
7.2. Adjusting Training Intensity
8. Effects of WBV on Quality of Life
9. Effects of WBV on Mental Conditions in COVID-19 Patients
10. Limitations
11. Practical Applications
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (covid-19) outbreak in china: Summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to covid-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Huang, Y.; Ma, W.; Xue, Z.; Zhang, J.; Gong, Y. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chin. Med. Sci. J. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Dai, S.-M.; Tong, Q. Covid-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Qie, S.; Liu, Z.; Ren, J.; Li, K.; Xi, J. Clinical characteristics of hospitalized patients with sars-cov-2 infection: A single arm meta-analysis. J. Med. Virol. 2020, 92, 612–617. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical features of 69 cases with coronavirus disease 2019 in wuhan, china. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Cai, L.; Cheng, Z.-S.; Cheng, H.; Deng, T.; Fan, Y.-P.; Fang, C.; Huang, D.; Huang, L.-Q.; Huang, Q. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-ncov) infected pneumonia (standard version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef]
- Chen, P.; Mao, L.; Nassis, G.P.; Harmer, P.; Ainsworth, B.E.; Li, F. Coronavirus disease (covid-19): The need to maintain regular physical activity while taking precautions. J. Sport Health Sci. 2020, 9, 103–104. [Google Scholar] [CrossRef]
- Haskell, W.L.; Lee, I.-M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the american college of sports medicine and the american heart association. Med. Sci. Sports Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.M.; Davison, G.W.; McClean, C.M.; Murphy, M.H. A systematic review of the acute effects of exercise on immune and inflammatory indices in untrained adults. Sports Med. -Open 2015, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Wollersheim, T.; Haas, K.; Wolf, S.; Mai, K.; Spies, C.; Steinhagen-Thiessen, E.; Wernecke, K.-D.; Spranger, J.; Weber-Carstens, S. Whole-body vibration to prevent intensive care unit-acquired weakness: Safety, feasibility, and metabolic response. Crit. Care 2017, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Bidonde, J.; Busch, A.J.; van der Spuy, I.; Tupper, S.; Kim, S.Y.; Boden, C. Whole body vibration exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-F.; Lin, P.-C.; Yang, R.-S.; Yang, R.-J. The preliminary effect of whole-body vibration intervention on improving the skeletal muscle mass index, physical fitness, and quality of life among older people with sarcopenia. BMC Geriatr. 2018, 18, 17. [Google Scholar] [CrossRef]
- Murthy, S.; Gomersall, C.D.; Fowler, R.A. Care for critically ill patients with covid-19. JAMA 2020, 323, 1499–1500. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. Jama Intern. Med. 2020. [Google Scholar] [CrossRef]
- Wujtewicz, M.; Dylczyk-Sommer, A.; Aszkiełowicz, A.; Zdanowski, S.; Piwowarczyk, S.; Owczuk, R. Covid-19-what should anaethesiologists and intensivists know about it? Anaesthesiol. Intensive Ther. 2020, 52, 34–41. [Google Scholar] [CrossRef]
- Neufeld, K.J.; Leoutsakos, J.-M.S.; Yan, H.; Lin, S.; Zabinski, J.S.; Dinglas, V.D.; Hosey, M.M.; Parker, A.M.; Hopkins, R.O.; Needham, D.M. Fatigue symptoms during the first year after ards. Chest 2020. [Google Scholar] [CrossRef] [PubMed]
- Goërtz, Y.M.; Spruit, M.A.; Van ‘t Hul, A.J.; Peters, J.B.; Van Herck, M.; Nakken, N.; Djamin, R.S.; Burtin, C.; Thong, M.S.; Coors, A. Fatigue is highly prevalent in patients with copd and correlates poorly with the degree of airflow limitation. Ther. Adv. Respir. Dis. 2019, 13, 1753466619878128. [Google Scholar] [CrossRef]
- Spadaro, S.; Capuzzo, M.; Valpiani, G.; Bertacchini, S.; Ragazzi, R.; Dalla Corte, F.; Terranova, S.; Marangoni, E.; Volta, C.A. Fatigue in intensive care survivors one year after discharge. Health Qual. Life Outcomes 2016, 14, 148. [Google Scholar] [CrossRef]
- Whitehead, L. The measurement of fatigue in chronic illness: A systematic review of unidimensional and multidimensional fatigue measures. J. Pain Symptom Manag. 2009, 37, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Alentorn-Geli, E.; Padilla, J.; Moras, G.; Haro, C.L.; Fernández-Solà, J. Six weeks of whole-body vibration exercise improves pain and fatigue in women with fibromyalgia. J. Altern. Complementary Med. 2008, 14, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Corbianco, S.; Cavallini, G.; Baldereschi, G.; Carboncini, M.C.; Fiamingo, F.L.; Bongioanni, P.; Dini, M. Whole body vibration and treadmill training in Parkinson’s disease rehabilitation: Effects on energy cost and recovery phases. Neurol. Sci. 2018, 39, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Uribe, S.; Hochsprung, A.; Heredia-Camacho, B.; Izquierdo-Ayuso, G. Effect of training exercises incorporating mechanical devices on fatigue and gait pattern in persons with relapsing-remitting multiple sclerosis. Physiother. Can. 2017, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Pahl, A.; Wehrle, A.; Kneis, S.; Gollhofer, A.; Bertz, H. Whole body vibration training during allogeneic hematopoietic cell transplantation—the effects on patients’ physical capacity. Ann. Hematol. 2020, 99, 1–14. [Google Scholar] [CrossRef]
- Prioreschi, A.; Makda, M.A.; Tikly, M.; McVeigh, J.A. In patients with established ra, positive effects of a randomised three month wbv therapy intervention on functional ability, bone mineral density and fatigue are sustained for up to six months. PLoS ONE 2016, 11, e0153470. [Google Scholar] [CrossRef]
- Furness, T.; Joseph, C.; Welsh, L.; Naughton, G.; Lorenzen, C. Whole-body vibration as a mode of dyspnoea free physical activity: A community-based proof-of-concept trial. BMC Res. Notes 2013, 6, 452. [Google Scholar] [CrossRef]
- Gloeckl, R.; Richter, P.; Winterkamp, S.; Pfeifer, M.; Nell, C.; Christle, J.W.; Kenn, K. Cardiopulmonary response during whole-body vibration training in patients with severe copd. ERJ Open Res. 2017, 3. [Google Scholar] [CrossRef]
- Furness, T.; Joseph, C.; Naughton, G.; Welsh, L.; Lorenzen, C. Benefits of whole-body vibration to people with copd: A community-based efficacy trial. BMC Pulm. Med. 2014, 14, 38. [Google Scholar] [CrossRef]
- Jawed, Y.; Beli, E.; March, K.; Kaleth, A.; Loghmani, M.T. Whole-body vibration training increases stem/progenitor cell circulation levels and may attenuate inflammation. Mil. Med. 2020, 185, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Neves, C.D.; Lacerda, A.C.R.; Lage, V.K.; Soares, A.A.; Chaves, M.G.A.; Lima, L.P.; Silva, T.J.; Vieira, É.L.; Teixeira, A.L.; Leite, H.R. Whole body vibration training increases physical measures and quality of life without altering inflammatory-oxidative biomarkers in patients with moderate copd. J. Appl. Physiol. 2018, 125, 520–528. [Google Scholar] [CrossRef]
- Ribeiro, V.; Mendonça, V.; Souza, A.; Fonseca, S.; Camargos, A.; Lage, V.; Neves, C.; Santos, J.; Teixeira, L.; Vieira, E. Inflammatory biomarkers responses after acute whole body vibration in fibromyalgia. Braz. J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.P.; Avelar, N.C.; Tossige-Gomes, R.; Neves, C.D.; Mendonça, V.A.; Miranda, A.S.; Teixeira, M.M.; Teixeira, A.L.; Andrade, A.P.; Coimbra, C.C. Functional performance and inflammatory cytokines after squat exercises and whole-body vibration in elderly individuals with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2012, 93, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Liu, X.; Feng, Q.; Xu, M.; Lan, X.; Li, M.; Liu, R.; Li, C.; Dong, T.; Wang, D. Whole body vibration triggers a change in the mutual shaping state of intestinal microbiota and body’s immunity. Front. Bioeng. Biotechnol. 2019, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Blanks, A.M.; Rodriguez-Miguelez, P.; Looney, J.; Tucker, M.A.; Jeong, J.; Thomas, J.; Blackburn, M.; Stepp, D.W.; Weintraub, N.J.; Harris, R.A. Whole body vibration elicits differential immune and metabolic responses in obese and normal weight individuals. Brainbehaviorimmunity-Health 2020, 1, 100011. [Google Scholar] [CrossRef]
- Tossige-Gomes, R.; Avelar, N.; Simão, A.; Neves, C.; Brito-Melo, G.; Coimbra, C.; Rocha-Vieira, E.; Lacerda, A. Whole-body vibration decreases the proliferativeb response of tcd4+ cells in elderly individuals with knee osteoarthritis. Braz. J. Med. Biol. Res. 2012, 45, 1262–1268. [Google Scholar] [CrossRef]
- Rittweger, J.; Beller, G.; Armbrecht, G.; Mulder, E.; Buehring, B.; Gast, U.; Dimeo, F.; Schubert, H.; De Haan, A.; Stegeman, D.F. Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise. Bone 2010, 46, 137–147. [Google Scholar] [CrossRef]
- Greulich, T.; Nell, C.; Koepke, J.; Fechtel, J.; Franke, M.; Schmeck, B.; Haid, D.; Apelt, S.; Filipovic, S.; Kenn, K. Benefits of whole body vibration training in patients hospitalised for copd exacerbations-a randomized clinical trial. BMC Pulm. Med. 2014, 14, 60. [Google Scholar] [CrossRef]
- Stark, C.; Herkenrath, P.; Hollmann, H.; Waltz, S.; Becker, I.; Hoebing, L.; Semler, O.; Hoyer-Kuhn, H.; Duran, I.; Hero, B. Early vibration assisted physiotherapy in toddlers with cerebral palsy–a randomized controlled pilot trial. J. Musculoskelet. Neuronal Interact. 2016, 16, 183. [Google Scholar]
- Gloeckl, R.; Jarosch, I.; Bengsch, U.; Claus, M.; Schneeberger, T.; Andrianopoulos, V.; Christle, J.W.; Hitzl, W.; Kenn, K. What’s the secret behind the benefits of whole-body vibration training in patients with copd? A randomized, controlled trial. Respir. Med. 2017, 126, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rittweger, J.; Schiessl, H.; Felsenberg, D. Oxygen uptake during whole-body vibration exercise: Comparison with squatting as a slow voluntary movement. Eur. J. Appl. Physiol. 2001, 86, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Thomas, G.W.; DeGuire, J.R.; Lemon, P.W. Vertical whole-body vibration does not increase cardiovascular stress to static semi-squat exercise. Eur. J. Appl. Physiol. 2008, 104, 903. [Google Scholar] [CrossRef] [PubMed]
- Boeselt, T.; Nell, C.; Kehr, K.; Holland, A.; Dresel, M.; Greulich, T.; Tackenberg, B.; Kenn, K.; Boeder, J.; Klapdor, B. Whole-body vibration therapy in intensive care patients: A feasibility and safety study. J. Rehabil. Med. 2016, 48, 316–321. [Google Scholar] [CrossRef]
- Kim, J.-H.; Seo, H.-J. Influence of pelvic position and vibration frequency on muscle activation during whole body vibration in quiet standing. J. Phys. Ther. Sci. 2015, 27, 1055–1058. [Google Scholar] [CrossRef]
- Ritzmann, R.; Gollhofer, A.; Kramer, A. The influence of vibration type, frequency, body position and additional load on the neuromuscular activity during whole body vibration. Eur. J. Appl. Physiol. 2013, 113, 1–11. [Google Scholar] [CrossRef]
- Eckhardt, H.; Wollny, R.; Müller, H.; Bärtsch, P.; Friedmann-Bette, B. Enhanced myofiber recruitment during exhaustive squatting performed as whole-body vibration exercise. J. Strength Cond. Res. 2011, 25, 1120–1125. [Google Scholar] [CrossRef]
- Abercromby, A.F.; Amonette, W.E.; Layne, C.S.; McFarlin, B.K.; Hinman, M.R.; Paloski, W.H. Vibration exposure and biodynamic responses during whole-body vibration training. Med. Sci. Sports Exerc. 2007, 39, 1794–1800. [Google Scholar] [CrossRef]
- Rohlmann, A.; Schmidt, H.; Gast, U.; Kutzner, I.; Damm, P.; Bergmann, G. In vivo measurements of the effect of whole body vibration on spinal loads. Eur. Spine J. 2014, 23, 666–672. [Google Scholar] [CrossRef][Green Version]
- Pollock, R.D.; Woledge, R.C.; Mills, K.R.; Martin, F.C.; Newham, D.J. Muscle activity and acceleration during whole body vibration: Effect of frequency and amplitude. Clin. Biomech. 2010, 25, 840–846. [Google Scholar] [CrossRef]
- Braz Júnior, D.S.; de Andrade, A.D.; Teixeira, A.S.; Cavalcanti, C.A.; Morais, A.B.; Marinho, P.E. Whole-body vibration improves functional capacity and quality of life in patients with severe chronic obstructive pulmonary disease (copd): A pilot study. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 125. [Google Scholar]
- Gloeckl, R.; Heinzelmann, I.; Baeuerle, S.; Damm, E.; Schwedhelm, A.-L.; Diril, M.; Buhrow, D.; Jerrentrup, A.; Kenn, K. Effects of whole body vibration in patients with chronic obstructive pulmonary disease–a randomized controlled trial. Respir. Med. 2012, 106, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Boerema, A.S.; Heesterbeek, M.; Boersma, S.A.; Schoemaker, R.; de Vries, E.F.; van Heuvelen, M.J.; Van der Zee, E.A. Beneficial effects of whole body vibration on brain functions in mice and humans. Dose-Response 2018, 16, 1559325818811756. [Google Scholar] [CrossRef]
- Regterschot, G.R.H.; Van Heuvelen, M.J.; Zeinstra, E.B.; Fuermaier, A.B.; Tucha, L.; Koerts, J.; Tucha, O.; Van Der Zee, E.A. Whole body vibration improves cognition in healthy young adults. PLoS ONE 2014, 9, e100506. [Google Scholar] [CrossRef]
- Choi, D.-S.; Lee, H.-J.; Shin, Y.-I.; Lee, A.; Kim, H.-G.; Kim, Y.-H. Modulation of cortical activity by high-frequency whole-body vibration exercise: An fnirs study. J. Sport Rehabil. 2019, 28, 665–670. [Google Scholar] [CrossRef]
- Heesterbeek, M.; Jentsch, M.; Roemers, P.; Keijser, J.N.; Toth, K.; Nyakas, C.; Schoemaker, R.G.; van Heuvelen, M.; van der Zee, E. Whole body vibration enhances choline acetyltransferase-immunoreactivity in cortex and amygdale. J. Neurol. Transl. Neurosci. 2017, 5, 1079. [Google Scholar]
- Zhao, L.; He, L.; Huang, S.; Gong, L.; Lv, Y.; Qian, Z. Protection of dopamine neurons by vibration training and up-regulation of brain-derived neurotrophic factor in a mptp mouse model of Parkinson’s disease. Physiol. Res. 2014, 63, 649–657. [Google Scholar]
- Leitch, A.; Duffin, R.; Haslett, C.; Rossi, A. Relevance of granulocyte apoptosis to resolution of inflammation at the respiratory mucosa. Mucosal Immunol. 2008, 1, 350–363. [Google Scholar] [CrossRef]
- Brusselle, G.; Bracke, K. Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2014, 11, S322–S328. [Google Scholar] [CrossRef]
- Wong, J.; Magun, B.E.; Wood, L.J. Lung inflammation caused by inhaled toxicants: A review. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1391. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista Jr, M.L. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta 2010, 411, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Mizuhara, H.; O’Neill, E.; Seki, N.; Ogawa, T.; Kusunoki, C.; Otsuka, K.; Satoh, S.; Niwa, M.; Senoh, H.; Fujiwara, H. T cell activation-associated hepatic injury: Mediation by tumor necrosis factors and protection by interleukin 6. J. Exp. Med. 1994, 179, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Møller, K.; Pedersen, B.K. Il-6 enhances plasma il-1ra, il-10, and cortisol in humans. Am. J. Physiol. -Endocrinol. Metab. 2003, 285, E433–E437. [Google Scholar] [CrossRef]
- Murakami, S.; Kurihara, S.; Titchenal, C.A.; Ohtani, M. Suppression of exercise-induced neutrophilia and lymphopenia in athletes by cystine/theanine intake: A randomized, double-blind, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2010, 7, 23. [Google Scholar] [CrossRef]
- Furuncuoğlu, Y.; Tulgar, S.; Dogan, A.; Cakar, S.; Tulgar, Y.; Cakiroglu, B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1300–1306. [Google Scholar]
- Wärnberg, J.; Cunningham, K.; Romeo, J.; Marcos, A. Physical activity, exercise and low-grade systemic inflammation. Proc. Nutr. Soc. 2010, 69, 400–406. [Google Scholar] [CrossRef]
- Thomas, S.; Mehrholz, J.; Bodechtel, U.; Elsner, B. Effect of physiotherapy on regaining independent walking in patients with intensive-care-unit-acquired muscle weakness: A cohort study. J. Rehabil. Med. 2019, 51, 797–804. [Google Scholar] [CrossRef]
- Capri, M.; Morsiani, C.; Santoro, A.; Moriggi, M.; Conte, M.; Martucci, M.; Bellavista, E.; Fabbri, C.; Giampieri, E.; Albracht, K. Recovery from 6-month spaceflight at the international space station: Muscle-related stress into a proinflammatory setting. FASEB J. 2019, 33, 5168–5180. [Google Scholar] [CrossRef]
- Buehring, B.; Belavý, D.L.; Michaelis, I.; Gast, U.; Felsenberg, D.; Rittweger, J. Changes in lower extremity muscle function after 56 days of bed rest. J. Appl. Physiol. 2011, 111, 87–94. [Google Scholar] [CrossRef]
- National Strength & Conditioning Association. Strength Training; Human Kinetics, Incorporated: Champaign, IL, USA, 2016. [Google Scholar]
- Toigo, M. Muskelrevolution: Konzepte und Rezepte zum Muskel- und Kraftaufbau; Springer: Berlin, Germany, 2019. [Google Scholar]
- Zhou, J.; Pang, L.; Chen, N.; Wang, Z.; Wang, C.; Hai, Y.; Lyu, M.; Lai, H.; Lin, F. Whole-body vibration training–better care for copd patients: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3243. [Google Scholar] [CrossRef] [PubMed]
- Sá-Caputo, D.; Gonçalves, C.R.; Morel, D.S.; Marconi, E.M.; Fróes, P.; Rufino, R.; Costa, C.H.; Lopes, A.J.; Arnóbio, A.; Asad, N.R. Benefits of whole-body vibration, as a component of the pulmonary rehabilitation, in patients with chronic obstructive pulmonary disease: A narrative review with a suitable approach. Evid. -Based Complementary Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Van der Zee, E.A.; Heesterbeek, M.; Tucha, O.; Fuermaier, A.B.; van Heuvelen, M.J. Whole body vibration, cognition, and the brain. In Whole Body Vibrations; CRC Press: Boca Raton, FL, USA, 2018; pp. 151–170. [Google Scholar]
- Girard, T.D.; Self, W.H.; Edwards, K.M.; Grijalva, C.G.; Zhu, Y.; Williams, D.J.; Jain, S.; Jackson, J.C. Long-term cognitive impairment after hospitalization for community-acquired pneumonia: A prospective cohort study. J. Gen. Intern. Med. 2018, 33, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Davydow, D.S.; Hough, C.L.; Levine, D.A.; Langa, K.M.; Iwashyna, T.J. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am. J. Med. 2013, 126, 615–624.e5. [Google Scholar] [CrossRef]
- McCoy, J.G.; McKenna, J.T.; Connolly, N.P.; Poeta, D.L.; Ling, L.; McCarley, R.W.; Strecker, R.E. One week of exposure to intermittent hypoxia impairs attentional set-shifting in rats. Behav. Brain Res. 2010, 210, 123–126. [Google Scholar] [CrossRef][Green Version]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011, 306, 613–619. [Google Scholar] [CrossRef]
- Carmeli, E.; Reznick, A.Z. The physiology and biochemistry of skeletal muscle atrophy as a function of age. Proc. Soc. Exp. Biol. Med. 1994, 206, 103–113. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Hall, J. Icu-acquired weakness. Chest 2007, 131, 1541–1549. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- Kellum, J.A.; Kong, L.; Fink, M.P.; Weissfeld, L.A.; Yealy, D.M.; Pinsky, M.R.; Fine, J.; Krichevsky, A.; Delude, R.L.; Angus, D.C. Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the genetic and inflammatory markers of sepsis (genims) study. Arch. Intern. Med. 2007, 167, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Moffitt, T.E.; Pariante, C.M.; Ambler, A.; Poulton, R.; Caspi, A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry 2008, 65, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef] [PubMed]
| Author | Participants and Age (Years/Months/Weeks) ± SD or [SE] or (Min–Max) | Condition | Study Design | Frequency (Hz) | Amplitude or PPD (mm) | Peak Acceleration (m/s2 or g) | Vibration Type/Device | Position/Exercises | Session Protocol | Intervention | Footwear |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wollersheim 2017 [13] | EG1: n = 12 EG2: n = 7 54 (52–59) years | Immobilized ICU patients | Clinical trial with longitudinal analysis (before, during, and after intervention) | EG1: 26 EG2: 24 | 2–5 | No information | EG1: Synchronous vibration (Vibrosphere®, Promedvi: Sweden) EG2: side alternating vibration (Galileo, home-ICU®. Novotec Medical GmbH, Pforzheim, Germany) | Supine position with knees flexed at about 20° | One session | EG1: 9 × 1 min, 45 s rest EG2: 3 × 3 min | Socks |
| Chang 2018 [15] | n = 17 82.1 ± 8.2 years | Older people | Quasi-experimental, single-group, pretest-posttest design | 12 | 3 | No information | Vertical synchronous vibration (i-vib6050 model; Bodygreen, Changhua, Taiwan) | Stand on position | 3-Month period, 3 sessions/week | 10 × 60 s, 30 s rest | No information |
| Alentorn-Geli 2008 [23] | EG1: n = 11, 55.2 [3.4] years EG2: n = 12, 53.7 [2.7] years CG: n = 10, 59.3 [2.3] years | Fibromyalgia | RCT (2-factor mixed experimental design) | EG1: 30 | EG1: 2 | No information | Synchronous vibration (PowerPlate®, Power Plate North America, Inc., Northbrook, IL) | Static and dynamic lower extremities tasks (static and dynamic squat; ankle plantar-flexion with legs in extension; flexo-extension of the right leg or of the left leg; squat shifting the body weight from 1 leg to the other) | 6-Week period, 2 sessions/week | 3–6 × 4–18 min, 3 min rest | No information |
| Corbianco 2018 [24] | EG1: n = 10, 58.8 ± 3.9 years EG2: n = 10 56.9 ± 4.7 years | Parkinson’s disease | RCT | EG1: 26 | EG1: 4 | EG1: 106.64 m/s2 | Side alternating vibration (Galileo, Med L2000, Novotec Medical GmbH, Pforzheim, Germany) | Isometric protocol in semi squat position with normalized workload (20–100% patient’s body weight, progressive increase of 5% body weight was added every week) | 4-Week period, 4 sessions/week | 20 × 1 min, 1 min rest | No information |
| Pahl 2020 [26] | EG: n = 18, 55 (50–63) years CG: n = 26, 56 (32–63) years | Allogeneic hematopoietic cell transplantation | RCT (subjects randomly allocated 1:1 to two parallel groups) | EG: 20–27 | EG: 0–3 | No information | Side alternating vibration (Galileo, Med L2000, Novotec Medical GmbH, Pforzheim, Germany) | Standing position: five exercises from a repertoire of 16 exercises for lower limbs, especially the knee extensors and flexors | 180-Day period, 5 sessions/week | ~20 min/session | Barefoot |
| Prioreschi 2016 [27] | EG: n = 16 CG: n = 15 EG: 51 ±10 yrs CG: 52 ±12 years | Reumatoid Arthitis Female | RCT | 30 | 3 | No information | Vertical synchronous vibration ((DKN XG 5.0, DKN Technology, California, USA) | Standing position holding on to the handlebars with knees slightly bent | 12 weeks 2 sessions/week 15 min/session | EG: WBV 10 × 60 s, 30 s rest CG: normal activities | Barefoot |
| Furness 2013 [28] | n = 17 69 ± 8 years | COPD | Non-randomised, cross-over design to sham | 25 | 2 | 24.7 m/s2 | Side alternating vibration platform (Amazing Super Health, Melbourne, AUS) | Static squatting position with knees flexed at about 20° | One session | 5 × 1 min, 1 min rest | Flat soled shoes |
| Gloeckl 2017 [29] | n = 10 62 ± 8 years | COPD | RCT cross-over study | 26 | 5 | No information | Side alternating vibration (Galileo, Novotec Medical, Pforzheim, Germany) | Dynamic squatting position with knees and hips at about 90–100° | One session | 6 × 3 min, 10 repetitions per minute (to bend their knees 2 s concentric, 2 s eccentric, 2 s standing between each repetition) | Flat soled shoes |
| Furness 2014 [30] | n = 16 72 ± 7 years | COPD | non-randomized, cross-over design to sham | 25 | 2 | ~24.7 m/s2 | Side alternating vibration platform (Amazing Super Health, Melbourne, AUS) | 53° knee flexion | 6-Week period, 2 sessions/week | No information | Flat soled shoes. |
| Jawed 2020 [31] | n = 11 24 ± 1 (6-Young) 55 ± 3 (5-old) years | Healthy male subjects | Single site, within subjects, pre and post-test design, cross-over | 35 | 4 | No information | Power Plate my3 (Power Plate North America, Northbrook, IL) | EG1: standing platform vibration; EG2: repetitive leg squat exercise (no vibration); and EG3: EG1 plus EG2 (with vibration) | 2 to 3-week period, one session | EG1: 8 bouts (WBV) × 60 s × 120 (rest), knees slightly bent; EG2: 8 bouts (WBV) × 60 s × 120 (rest), 90° knee flexion, 120 total repetitions of leg squats; EG3: same EG2 | Barefoot |
| Neves 2018 [32] | EG: n = 10, 63.5 ± 7.8 years CG: n = 10, 63.8 ± 8.1 years | COPD | Single-blind trial with a controlled parallel design | EG: 30–40 | EG: 2 | EG: 1.45–2.25 g | Synchronous vibration (Fitvibe Excel Pro C, Bilzen, Belgium) | Static squatting position with knees flexed at about 30° | 12-Week period, 3 sessions/week | 6 × 30 s, 60 s rest | Barefoot |
| Ribeiro 2018 [33] | EG: n = 19, 52.1 [1.8] years CG: n = 19, 51.0 [1.9] years | Fibromyalgia | CT 1:1 case-control paired study (variables assessed before and immediately after one session) | EG: 40 | EG: 4 | No information | Synchronous vibration (Fitvibe Excel Pro C, Bilzen, Belgium) | Dynamic squatting position with knees flexed at about 10° to 60° | One session | 8 × 40 s, 40 s rest (to bend their knees to 60° angle for 3 s and then to 10° angle for 3 s, over the 40 s of each series) | Barefoot |
| Simão 2012 [34] | EG1: n = 10, 75 ± 7.4 years EG2: n = 10, 69 ± 3.7 years CG: n = 11, 71 ± 5.3 years | Knee osteoarthritis | Clinical, prospective, randomized, single-blinded study | EG1: 35–40 | EG1: 4 | EG1: 2.00-2.61 g | Synchronous vibration (Fitvibe Excel Pro C, Bilzen, Belgium) | Dynamic squatting position with knees flexed at about 10° to 60° | 12-Week period, 3 sessions/week | 6–8 × 20–40 s, 20–40 s rest (to bend their knees to 60° angle for 3 s and then to 10° angle for 3 s, over each series) | Barefoot |
| Song 2019 [35] | EG1: n = 11 (hum), 22–27 years EG2: n = 10(mice), 6 wks | EG1: healthy individuals EG2: old C57BL/6 mice | Non-randomized study | EG1: 21 EG2: 13 e 17 | No information | No information | Vertical vibration (Weibutexun, Jinan, China) | EG1: standing body vibration and seated for 10min in each position; EG2: no information | 4-week period, 7 sessions/week | EG1: 10 min (WBV); EG2: 30 min (WBV) | No information |
| Blanks 2020 [36] | EG1: n = 11, 33 ± 4 years EG2: n = 10, 28 ± 8 years | EG1: normal weight EG2: obese | Non-randomized study | 14 | 2.5 | 20.19 m/s2 | Side alternating whole body vibration platform (RS3000, Rock Solid Wholesale, Atlantic Beach, FL, USA) | Static squat position, knee flexion (~60°) with a stable non-flexed trunk. | One session | 10 bouts × 60s (WBV) × 30 s (rest) | Barefoot |
| Tossige-Gomes 2012 [37] | EG1: n = 8, 75 ± 7 years EG2: n = 10, 71 ± 4 years CG: n = 8, 72 ± 6 years | Knee osteoarthritis | Randomized controlled trial (variables assessed before and after training) | EG1: 35–40 | EG1: 4 | EG1: 2.78–3.26 g | Synchronous vibration (Fitvibe Excel Pro C, Bilzen, Belgium) | Dynamic squatting position with knees flexed at about 10° to 60° | 12-Week period, 3 sessions/week | 6–8 × 20–40 s, 20–40 s rest (to bend their knees to 60° angle for 3 s and then to 10° angle for 3 s, over each serie) | Barefoot |
| Rittweger 2010 [38] | CG: n = 10, 33.4 ± 6.6 years EG: n = 10, 32.6 ± 4.8 years | Healthy male | Randomized controlled trial | 19–30 | No information | No information | Side alternating vibration (Galileo Space, Novotec Medical, Pforzheim, Germany) | EG: squating exercise, heel raises, toe raises and kicks | 8-week, twice daily (except for Wednesday afternoons and Sundays) | Exercises were performed rhythmically at a repetition rate of 1 in 6 s, and kicks (explosive squats with 10 s rest insertion) | No information |
| Greulich 2014 [39] | CG: n = 20, 70.4 ± 10.1 years EG: n = 20, 66.4 ± 9.93 years | COPD | Clinical trial | 12–26 | 1.5; 2; and 3 | No information | Side alternating vibration Galileo®, Novotec Medical, Pforzheim, Germany) | CG: physiotherapy program, EG: physiotherapy program plus WBV (bended knees on the Platform) | No information | 3 × 2 min/day | No information |
| Stark 2016 [40] | EG1: n = 12, 8.6 ± 3.2 months EG2: n = 12, 19.4 ± 3.2 months | Cerebral palsy | Prospective, evaluator-blinded, monocenter, randomized waiting-control design with follow-up | 12 or 22 | 2.5 | 0.72 g or 2.43 g | Side alternating vibration Galileo® system combined with a tilt table (Novotec Medical GmbH, Pforzheim, Germany) | Standing still or alternately squatting and standing up (using tilt table); sitting on the platform; four-point position | 14-week, twice daily (10 times per week) | Ten 9-minute (3 × 3) min Feet or hands were placed at equal distance from the center of the platform | If possible the children trained without shoes, but with socks |
| Gloeckl 2017 [41] | CG: n = 37, 63 ± 9 years EG: n=37, 65 ± 8 years | COPD | Randomized controlled trial | 24–26 | 5 PPD | No information | Side-alternating vibration platform Galileo® (Novotec Medical GmbH, Pforzheim, Germany) | Dynamic squat training, 90° and 120° Knee and hip flexion during each squat movement without holding on to anything | 3-week, 3 times a week (non-consecutive days) | 4 bouts × 120 s (WBV) | Flat soled shoes |
| Rittweger 2001 [42] | n = 12, 25.2 years | Healthy individuals | Non-randomized study | 26 | 6 | No information | Side alterning vibration Galileo, 2000 (Novotec Medical GmbH, Pforzheim, Germany) | Standing, squatting, and squatting with a load | One session | Exercises performed in randomized sequence for 3 min each | No information |
| Hazell 2008 [43] | EG1: n = 8, 25 ± 3.4 years EG2: n = 8, 25 ± 2.6 years | Healthy RA men | Non-randomized study | 45 | 2 | No information | Vertical vibration WAVE platform (Whole-body Advanced Vibration Exercise, Windsor, Canada) | EG1: seated next to the WBV device (passive, unloaded), 90º knee flexion EG2: semi-squat (static, loaded), 120° knee flexion | One session | EG1 and EG2: 15 repetitions of 1 min (WBV) × 1 min (rest) and 10 min of recovery (40 min of total time) | Barefoot |
| Boeselt 2016 [44] | EG1: n = 12, 41.8 ± 19.7 years EG2: n = 12, 31.3 ± 6.6 years | EG1: ICU patients EG2: healthy individuals | Non-randomized study | 24 | No information | No information | Side alternating vibration Galileo® (Novotec Medical, Pforzheim, Germany) | EG1 and EG2: WBV alone and WBV with a dumbbell | One session | EG1 and EG2: 3 min (WBV) × 1 min (rest) × 3 min (WBV + dumbbell) | Barefoot |
| Kim 2015 [45] | Males: n = 9, 29 ± 3.9 years Females: n = 9, 25.6 ± 3.5 years | Healthy individuals | Single-group, repeated-measure, cross-study | 0, 10, 20 | No information | No information | Side alternating vibration Galileo® (Novotec Medical, Pforzheim, Germany) | Three pelvic positions (neutral, anterior tilt, posterior tilt) | One session | 3 × 10 s (WBV) × 10 s (rest) in each position | No information |
| Ritzmann 2013 [46] | EG1 and EG2: n = 18, 25 ± 4 years | Healthy individuals | Single-group, repeated measures, crossed-study | EG1 and EG2: 5, 10, 15, 20, 25, 30 | EG 1: 2 and 4 EG2: 2 | No information | EG1: Novotec Medical (Pforzheim, Germany); EG2: Power Plate. (Germany, Frankfurt am Main, Germany) | One session | EG1: side alternating vibration and EG2: synchronous vibration: 10 s (WBV) × 30 s (rest) | Barefoot | |
| Eckhardt 2011 [47] | n = 14 26.0 ± 4.5 years | Physically active men | Randomizedcross-over | 22 | Mean 4 (feet at shoulder width) | No information | Side-alternating Galileo 900 (Novotec, Pforzheim, Germany) | Squat exercise knee bending angle 80° and additional load 10RM applied by barbell | One session | EG: WBV 5 sets of 10 squats within 30 s per set. 3 min rest between sets CC: same procedure on floor | Shoes |
| Albercromby 2007 [48] | n = 9 male 32.7± 7.0 years n = 7 female 32.7 ±8.3 years | Healthy adults | Single-group repeated measures | 30 | 2 | No information | Vertical: Powerplate Power Plate North America, Inc., Northbrook, IL) and side-alternating: Galileo 2000 (Novotec Medical, Pforzheim, Germany) | Slow dynamic squatting movement from 5° to 40° knee flexion for several | One session | Two trials for in max 15 s per condition. 60s rest between trials, 5 min rest between vibration directions | Sport socks |
| Rohlmann 2014 [49] | n = 3, 62,63,66 years | Patients fractured lumbar vertebral body, male | Repeated measures | 5–25 | 1, 2, 4 | No information | Vertical: Powerplate Pro 5. (Power Plate North America, Inc., Northbrook, IL). Side-alternating: Galileo advanced (Novotec Medical, Pforzheim, Germany) | 4 postures: knees straight, knees slightly bent, knees bent at 60° and on the forefeet | One session | 8 WBV trials on each plate, 12–15 s per trial, One trial 60 s. Breaks between trials 10-30s, break 5 min when changing device | No information |
| Pollock 2010 [50] | EG1: n = 12 31.3 ± 12.4 years EG2: n = 15 36 ± 12.1 years | Healthy adults | Single group repeated measures Randomized order | 5–30 | 5.5 and 2.5 | 0.2–9 g | Side-alternating Galileo 2000 (Novotec Medical GmBH, Pforzheim, Germany) | Standing straight legs, without locking knees, resulting in 15.1 ± 4.8° knee flexion | One session | EC: WBV 7s for each condition (6 frequencies x 2 amplitudes) Rest 30s | Barefoot |
| Braz Júnior 2015 [51] | EG + CG: n = 11 62.91 ± 8.82 | COPD, 72.7% male | Cross-over RCT | 35 | 2 or 4 (wk1–4: 2 wk2–12: 4) | No information | Vibrating platform (MY3; Power Plate, London, UK) | Static work of the lower limbs, semi squatting position at an angle of 120°–130° with the upper limbs lightly flexed in support | 12 weeks 3 sessions/week Wk 1–4: 10 min/session Wk 5–8: 15 min/session Wk 9–12: 20 min/session | EG: 1–4 wks (10 min; 30 s WBV × 60 s rest); 5-8 wks (15 min); 9-12 wks (20 min; 60 s WBV × 30 s rest) CG: no intervention | No information |
| Gloeckl 2012 [52] | EG: n = 42 CG: n = 40 EG: 64 ± 11 years CG: 65 ± 7 years | COPD, 51% female | RCT | 24–26 | 3 | No information | Side-alternating Galileo® (Novotec Medical GmbH, Pforzheim, Germany) | Squat exercises | 3 weeks 3 sessions per week 3 × 3 min/session | EG: WBV 3 × 3 min CG: same exercises on floor | No information |
| Boerema 2018 [53] | EG + CG: n = 20 15 weeks | C57BI/6 mice, males | RCT | 30 | 0.0537 | 0.098 g | Synchronous, 3D LEVELL R.C. Oscillator (Levell Electronics Ltd, Barnet, GB) with Shaker power amplifier | Free choice | 5 weeks 5 session/week 10 minutes/session | EG: WBV CG: same procedures but without WBV | No information |
| Regterschot 2014 [54] | n = 133 20.5 ± 2.2 years | Healthy young adults, 84% female | Cross-over Short-term effects | 30 | 0.5 | No information | Vertical/Vibe 300 (Tonic Vibe, Nantes, France) with chair | Sitting | One session | EG: WBV 6 × 2 min CC: rest 6 × 2 min | Socks |
| Choi 2019 [55] | n = 18 25.3 ± 2.4 years | Healthy young male adults | Cross-over Acute effects | 10, 20, 27 | 4 | No information | Side-alternating/Galileo® Advanced Plus (Novotec Medical GmbH, Pforzheim, Germany) | Static half squat 30° flexion Standing | One session | EG: WBV 3 conditions × 2 tasks each 5 × 30 s CG: same position without WBV 3 min rest between conditions | No information |
| Heesterbeek 2017 [56] | EG + CG: n = 14 2 months | Young C57BI/6J mice, males | RCT | 30 | 0.0537 | 0.098 g | Synchronous, 3D LEVELL R.C. Oscillator (Levell Electronics Ltd, Barnet, GB) with Shaker power amplifier | Free choice | 5 weeks 5 session/week 10 minutes/session | EG: WBV 1 × 10 min CG: same procedures but without WBV | No information |
| Zhao 2014 [57] | EG + CG: n = 25 Body weight 25–30 g | mouse model of Parkinson’s disease C57BL mice | RCT | 10 and 30 | 5 | No information | Synchronous Platform (Columbus instruments, OH, USA) | Free choice | 4 weeks 5 sessions/week 15 × 1 min/session | EG1: 5 mm/10 Hz: 15 × 1 min WBV, rest 1min EG2: 5 mm/30 Hz: 15 × 1 min WBV, rest 1 min CG1+2: same procedures without WBV | No information |
| Parameter | Value |
|---|---|
| Heart rate | <40 or >180 BPM |
| Systolic blood pressure | <80 mmHg or >200 mmHg; |
| Mean arterial blood pressure | <60 mmHg or >120 mmHg |
| Increase in intracerebral pressure | >20 mmHg |
| Oxygen saturation (SpO2) | <88% |
| Potassium levels | <3.0 mmol/L or >5.5 mmol/L |
| Tilt Angle | % Load |
|---|---|
| 10° | 17% BW |
| 20° | 34% BW |
| 30° | 50% BW |
| 60° | 87% BW |
| 80° | 97% BW |
| Parameter | Value |
|---|---|
| 1 to 2 | |
| Times per day | Standard ICU bed (severe cases): |
| 0° tilt + 20° knee angle | |
| 30° tilt + bent knees | |
| Tilt Angle | Special Tilt-Table (less severe cases): |
| 30° to 90° | |
| Standing device (further increase of intensity): 90° (standing) | |
| Frequency | Side-alternating WBV: 20 to 27 Hz Vertical WBV: 25 to 35 Hz |
| Duration | 1 to 3 min |
| Number of sets | 1 to 4 |
| Amplitude (peak-to peak) | Side-alternating WBV: 1–2.5 mm (2–5 mm) Vertical WBV: 1 mm (2 mm) |
| Further increase of intensity by additional exercise tasks | Squatting (hip & thigh muscles) |
| Heel-raises (calf muscles) | |
| Toe-raises (shin-muscles) | |
| Pelvis lifting (thigh muscles & trunk) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sañudo, B.; Seixas, A.; Gloeckl, R.; Rittweger, J.; Rawer, R.; Taiar, R.; van der Zee, E.A.; van Heuvelen, M.J.G.; Lacerda, A.C.; Sartorio, A.; et al. Potential Application of Whole Body Vibration Exercise for Improving the Clinical Conditions of COVID-19 Infected Individuals: A Narrative Review from the World Association of Vibration Exercise Experts (WAVex) Panel. Int. J. Environ. Res. Public Health 2020, 17, 3650. https://doi.org/10.3390/ijerph17103650
Sañudo B, Seixas A, Gloeckl R, Rittweger J, Rawer R, Taiar R, van der Zee EA, van Heuvelen MJG, Lacerda AC, Sartorio A, et al. Potential Application of Whole Body Vibration Exercise for Improving the Clinical Conditions of COVID-19 Infected Individuals: A Narrative Review from the World Association of Vibration Exercise Experts (WAVex) Panel. International Journal of Environmental Research and Public Health. 2020; 17(10):3650. https://doi.org/10.3390/ijerph17103650
Chicago/Turabian StyleSañudo, Borja, Adérito Seixas, Rainer Gloeckl, Jörn Rittweger, Rainer Rawer, Redha Taiar, Eddy A. van der Zee, Marieke J.G. van Heuvelen, Ana Cristina Lacerda, Alessandro Sartorio, and et al. 2020. "Potential Application of Whole Body Vibration Exercise for Improving the Clinical Conditions of COVID-19 Infected Individuals: A Narrative Review from the World Association of Vibration Exercise Experts (WAVex) Panel" International Journal of Environmental Research and Public Health 17, no. 10: 3650. https://doi.org/10.3390/ijerph17103650
APA StyleSañudo, B., Seixas, A., Gloeckl, R., Rittweger, J., Rawer, R., Taiar, R., van der Zee, E. A., van Heuvelen, M. J. G., Lacerda, A. C., Sartorio, A., Bemben, M., Cochrane, D., Furness, T., de Sá-Caputo, D., & Bernardo-Filho, M. (2020). Potential Application of Whole Body Vibration Exercise for Improving the Clinical Conditions of COVID-19 Infected Individuals: A Narrative Review from the World Association of Vibration Exercise Experts (WAVex) Panel. International Journal of Environmental Research and Public Health, 17(10), 3650. https://doi.org/10.3390/ijerph17103650














