Regional Disparities in the Decline of Anemia and Remaining Challenges among Children in Tanzania: Analyses of the Tanzania Demographic and Health Survey 2004–2015
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Analysis
3. Results
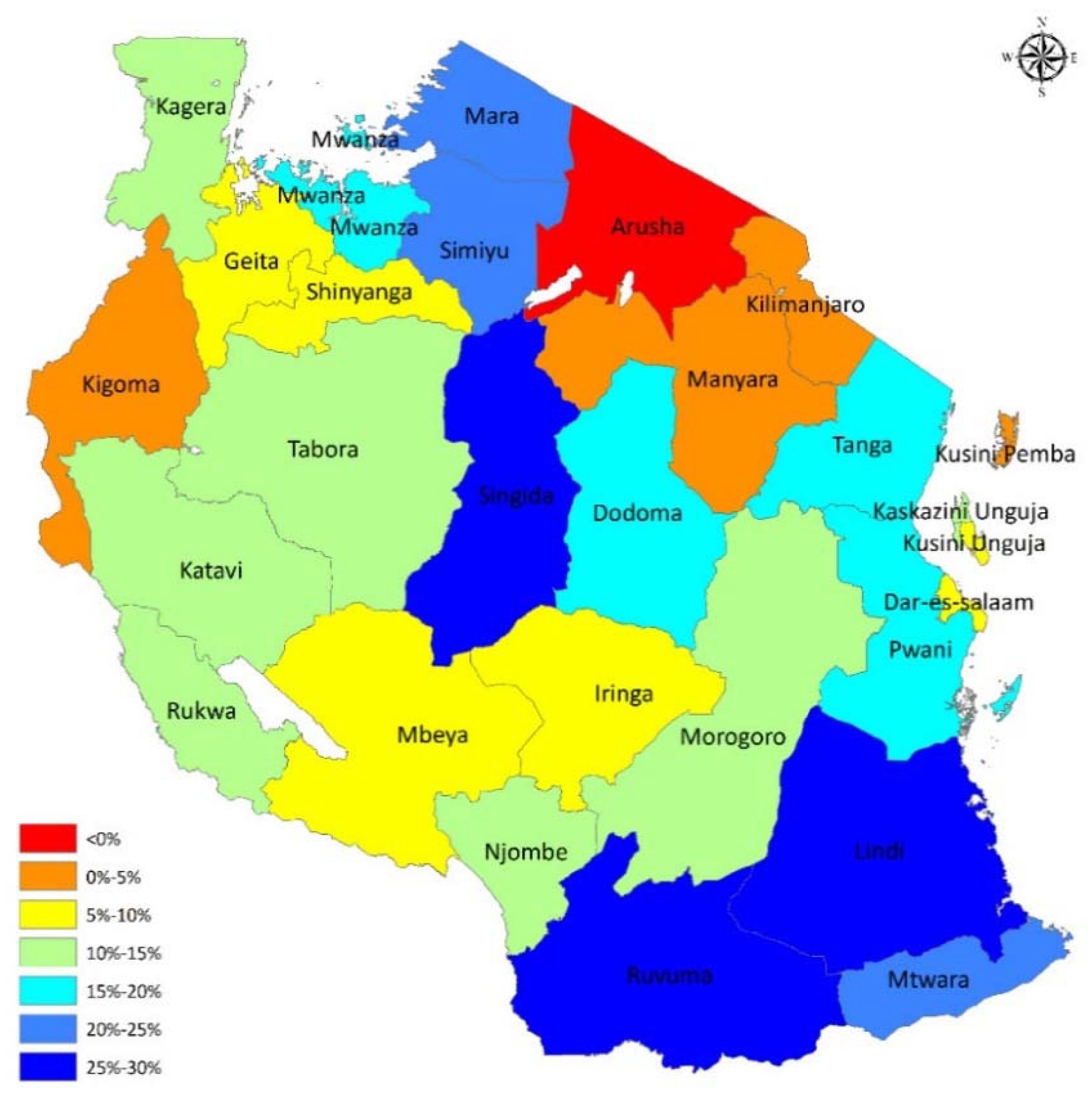
3.1. Changing Landscape in Child Anemia in Tanzania
3.2. Adjusted Differences in the Burden of Anemia between 2005 and 2016
3.3. The Remaining Factors Associated with Child Anemia in Tanzania
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J.; Bertozzi-Villa, A.; Coggeshall, M.S.; Shackelford, K.A.; Steiner, C.; Heuton, K.R.; Gonzalez-Medina, D.; Barber, R.; Huynh, C.; Dicker, D.; et al. Global, regional, and national levels and causes of maternal mortality during 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 980–1004. [Google Scholar] [CrossRef]
- Shah, D.; Sachdev, H.P. Maternal micronutrients and fetal outcome. Indian J. Pediatr. 2004, 71, 985. [Google Scholar] [CrossRef] [PubMed]
- Glazer, Y.; Bilenko, N. Effect of iron deficiency and iron deficiency anemia in the first two years of life on cognitive and mental development during childhood. J. Harefuah 2010, 69, 64–70. [Google Scholar]
- Pivina, L.; Semenova, Y.; Dosa, M.D.; Dauletyarova, M.; Bjorklund, G. Iron Deficiency, Cognitive, Functions and Neurobehavioral Disorders in Children. J. Mol. Neurosci. 2019, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nambiema, A.; Robert, A.; Yaya, I. Prevalence and risk factors of anemia in children aged from 6 to 59 months in Togo: Analysis from Togo demographic and health survey data, 2013–2014. BMC Public Health 2019, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Geletu, A.; Lelisa, A.; Baye, K. Provision of low-iron micronutrient powders on alternate days is associated with lower prevalence of anaemia, stunting, and improved motor milestone acquisition in the first year of life: A retrospective cohort study in rural Ethiopia. Matern. Child Nutr. 2019, 15, e12785. [Google Scholar] [CrossRef]
- Ministry of Health and Social Welfare Tanzania National Nutrition Survey. Available online: https://www.unicef.org/esaro/Tanzania_National_Nutrition_Survey_2014_Final_Report_18012015.pdf (accessed on 13 May 2020).
- Tanzania Demographic and Health Survey and Malaria Indicator Survey (TDHS-MIS) 2015–2016. Available online: http://www.tzdpg.or.tz/fileadmin/documents/dpg_internal/dpg_working_groups_clusters/cluster_2/health/Key_Sector_Documents/Monitoring___Evaluation/TDHS_2015-16_final_report.pdf (accessed on 13 May 2020).
- Sunguya, B.F.; Zhu, S.; Mpembeni, R.; Huang, J. Trends in prevalence and determinants of stunting in Tanzania: An analysis of Tanzania demographic health surveys (1991–2016). Nutr. J. 2019, 18, 85. [Google Scholar] [CrossRef]
- Al Kibria, G.M.; Swasey, K.; Hasan, M.Z.; Sharmeen, A.; Day, B. Prevalence and factors associated with underweight, overweight and obesity among women of reproductive age in India. Global Health Res. Policy 2019, 4, 24. [Google Scholar] [CrossRef]
- National Multisectoral Nutrition Action Plan (NMNAP)for the period July 2016–June 2021. Available online: https://extranet.who.int/nutrition/gina/sites/default/files/1_TZA%202016%20NMNAP.pdf (accessed on 13 May 2020).
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Ruel, M.T.; Alderman, H. Nutrition-sensitive interventions and programmes: How can they help to accelerate progress in improving maternal and child nutrition? Lancet 2013, 382, 536–551. [Google Scholar] [CrossRef]
- Gebreweld, A.; Ali, N.; Ali, R.; Fisha, T. Prevalence of anemia and its associated factors among children under five years of age attending at Guguftu health center, South Wollo, Northeast Ethiopia. PLoS ONE 2019, 14, e0218961. [Google Scholar] [CrossRef] [PubMed]
- Population and Housing Census. Population Distribution by Administrative Areas. Available online: http://www.tzdpg.or.tz/fileadmin/documents/dpg_internal/dpg_working_groups_clusters/cluster_2/water/WSDP/Background_information/2012_Census_General_Report.pdf (accessed on 13 May 2020).
- Faber, M.; Laubscher, R.; Berti, C. Poor dietary diversity and low nutrient density of the complementary diet for 6- to 24-month-old children in urban and rural KwaZulu-Natal, South Africa. Matern. Child Nutr. 2016, 12, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.N.; Angeles-Agdeppa, I.; Lenighan, Y.M.; Capanzana, M.V.; Montoliu, I. Diet Diversity and Micronutrient Adequacy among Filipino School-Age Children. Nutrients 2019, 11, 2197. [Google Scholar] [CrossRef] [PubMed]
- Tanzania Demographic and Health Survey 2004–2005. Available online: https://dhsprogram.com/pubs/pdf/FR173/FR173-TZ04-05.pdf (accessed on 13 May 2020).
- Tanzania Demographic and Health Survey 2010. Available online: https://dhsprogram.com/pubs/pdf/FR243/FR243[24June2011].pdf (accessed on 24 June 2012).
- Aung, T.; Niyeha, D.; Shagihilu, S.; Mpembeni, R.; Kaganda, J.; Sheffel, A.; Heidkamp, R. Optimizing data visualization for reproductive, maternal, newborn, child health, and nutrition (RMNCH&N) policymaking: Data visualization preferences and interpretation capacity among decision-makers in Tanzania. Glob. Health Res. Policy 2019, 4, 4. Available online: https://ghrp.biomedcentral.com/articles/10.1186/s41256-019-0095-1 (accessed on 15 February 2019). [PubMed]
- Petrou, S.; Kupek, E. Poverty and childhood undernutrition in developing countries: A multi-national cohort study. Soc. Sci. Med. 2010, 71, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, S.; Harttgen, K.; Subramanyam, M.A.; Finlay, J.; Klasen, S.; Subramanian, S.V. Association between economic growth and early childhood undernutrition: Evidence from 121 Demographic and Health Surveys from 36 low-income and middle-income countries. Lancet Glob Health 2014, 2, e225–e234. [Google Scholar] [CrossRef]
- Bailey, R.L.; West, K.J.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef]
- McGovern, M.E.; Krishna, A.; Aguayo, V.M.; Subramanian, S.V. A review of the evidence linking child stunting to economic outcomes. Int. J. Epidemiol 2017, 46, 1171–1191. [Google Scholar] [CrossRef]
- Mkonda, M.Y.; He, X. Production Trends of Food Crops: Opportunities, Challenges and Prospects to Improve Tanzanian Rural Livelihoods. Available online: http://www.suaire.suanet.ac.tz:8080/xmlui/handle/123456789/2831 (accessed on 13 May 2020).
- National food security bulletin for December 2018. Available online: https://www.kilimo.go.tz/index.php/en/resources/view/national-food-security-bulletin-for-december-2018 (accessed on 31 December 2018).
- Altare, C.; Delbiso, T.D.; Mutwiri, G.M.; Kopplow, R.; Guha-Sapir, D. Factors Associated with Stunting among Pre-school Children in Southern Highlands of Tanzania. J. Trop. Pediatr. 2016, 62, 390–408. [Google Scholar] [CrossRef]
- Yang, Q.; Yuan, T.; Yang, L.; Zou, J.; Ji, M.; Zhang, Y.; Deng, J.; Lin, Q. Household Food Insecurity, Dietary Diversity, Stunting, and Anaemia among Left-Behind Children in Poor Rural Areas of China. Int. J. Environ. Res. Public Health 2019, 16, 4778. [Google Scholar] [CrossRef]
- Nordang, S.; Shoo, T.; Holmboe-Ottesen, G.; Kinabo, J.; Wandel, M. Women’s work in farming, child feeding practices and nutritional status among under-five children in rural Rukwa, Tanzania. Br. J. Nutr. 2015, 114, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Ntwenya, J.E.; Kinabo, J.; Msuya, J.; Mamiro, P.; Majili, Z.S. Dietary patterns and household food insecurity in rural populations of Kilosa district, Tanzania. PLoS ONE 2015, 10, e0126038. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Aaron, G.J. Predictors of anemia in preschool children: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 402S–415S. [Google Scholar] [PubMed]
- Gosdin, L.; Martorell, R.; Bartolini, R.M.; Mehta, R.; Srikantiah, S.; Young, M.F. The co-occurrence of anaemia and stunting in young children. Matern. Child. Nutr. 2018, 14, e12597. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.W.; Borgerhoff, M.M.; Ghiselli, M.E.; Ngadaya, E.; Ngowi, B.; Mfinanga, S.G.; Hartwig, K.; James, S. Ethnicity and child health in northern Tanzania: Maasai pastoralists are disadvantaged compared to neighbouring ethnic groups. PLoS ONE 2014, 9, e110447. [Google Scholar] [CrossRef]
- Chege, P.; Kimiywe, J.; Ndungu, Z. Influence of culture on dietary practices of children under five years among Maasai pastoralists in Kajiado, Kenya. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 131. [Google Scholar] [CrossRef]
- Houghton, L.; Brown, R.; Beaumont, S.; Jennings, S.; Bailey, K.; Haszard, J.; Erhardt, J.; Daniels, L.; Gibson, R. Micronutrient status differs among Maasai and Kamba preschoolers participating in a supplementary feeding program in Southern Kenya. Matern. Child Nutr. 2019, 15, e12805. [Google Scholar] [CrossRef]
- Mgongo, M.; Hussein, T.H.; Stray-Pedersen, B.; Vangen, S.; Msuya, S.E.; Wandel, M. “We give water or porridge, but we don’t really know what the child wants:” A qualitative study on women’s perceptions and practises regarding exclusive breastfeeding in Kilimanjaro region, Tanzania. BMC Pregnancy Childbirth 2018, 18, 323. [Google Scholar] [CrossRef]
| Anemia Status | 2004/2005 | 2015/2016 | p-Value | ||
|---|---|---|---|---|---|
| N | % | n | % | ||
| Normal | 2141 | 29.1 | 3232 | 41.3 | |
| Anemia status | 5220 | 70.9 | 4596 | 58.7 | <0.001 |
| Mild anemia | 1762 | 23.9 | 2091 | 26.7 | 0.834 |
| Moderate anemia | 3153 | 42.8 | 2369 | 30.3 | <0.001 |
| Severe anemia | 305 | 4.1 | 136 | 1.7 | <0.001 |
| Total | 7361 | 7828 | |||
| Variable | Anemia in 2004/2005 | Anemia in 2015/2016 | p-Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age | |||||
| 0–11 | 1250 | 72.63 | 773 | 79.20 | <0.001 |
| 12–23 | 1320 | 82.55 | 1515 | 73.62 | |
| 24–35 | 1094 | 74.07 | 968 | 57.24 | |
| 36–47 | 836 | 62.25 | 697 | 44.06 | |
| 48–59 | 720 | 58.92 | 642 | 42.24 | |
| Sex | |||||
| Male | 2629 | 71.34 | 2388 | 60.29 | 0.115 |
| Female | 2591 | 70.50 | 2208 | 57.10 | |
| Birthweight | |||||
| Low | 343 | 69.86 | 331 | 53.91 | 0.091 |
| Normal or High | 2194 | 69.32 | 2434 | 56.45 | |
| Type of residence | |||||
| Rural | 4322 | 72.13 | 3495 | 60.01 | <0.001 |
| Urban | 898 | 65.60 | 1101 | 54.94 | |
| Family size | |||||
| 1–4 | 1226 | 68.99 | 990 | 55.62 | 0.009 |
| 5–9 | 3033 | 70.32 | 2666 | 57.89 | |
| 10+ | 961 | 75.61 | 940 | 65.14 | |
| Number of children under 5 | |||||
| 1 | 1531 | 69.81 | 1480 | 55.66 | 0.004 |
| 2 | 2087 | 69.89 | 1779 | 57.41 | |
| 3 | 997 | 71.73 | 787 | 62.76 | |
| >3 | 509 | 77.71 | 470 | 69.63 | |
| Mother’s age at first birth | |||||
| <15 | 149 | 63.68 | 136 | 67.66 | 0.045 |
| 15–19 | 3270 | 72.01 | 2754 | 59.38 | |
| 20–24 | 1520 | 69.44 | 1428 | 58.45 | |
| ≥25 | 281 | 70.78 | 278 | 50.92 | |
| Education level (mother) | |||||
| No education | 1423 | 74.78 | 1129 | 66.45 | <0.001 |
| Primary | 3566 | 69.54 | 2873 | 56.87 | |
| Secondary | 182 | 71.65 | 565 | 55.72 | |
| Higher | 49 | 65.33 | 29 | 45.31 | |
| Mother’s Marital Status | |||||
| Married | 4109 | 70.60 | 2911 | 58.94 | <0.001 |
| Living together | 429 | 70.68 | 971 | 59.50 | |
| Widowed/Divorced/Live Apart | 472 | 72.84 | 496 | 57.34 | |
| Never Married | 210 | 73.43 | 218 | 55.61 | |
| Wealth index | |||||
| Poorest | 1293 | 77.06 | 1220 | 63.81 | <0.001 |
| Poorer | 1138 | 73.94 | 1052 | 61.41 | |
| Middle | 1124 | 70.51 | 915 | 59.73 | |
| Richer | 993 | 67.87 | 756 | 53.28 | |
| Richest | 671 | 61.79 | 653 | 52.11 | |
| Variable | N(%) | Model 1 a | Model 2 b | Model 3 c | |||
|---|---|---|---|---|---|---|---|
| AOR(95% CI) | p-Value | AOR(95% CI) | p-Value | AOR(95% CI) | p-Value | ||
| Survey year | |||||||
| 2004–2005 | 7976(46) | 1.00 | |||||
| 2015–2016 | 9520(54) | 0.58(0.55,0.62) | <0.001 | ||||
| Household characteristics | |||||||
| Type of residence | |||||||
| Urban | 4099(23) | 1.00 | |||||
| Rural | 13,397(77) | 0.9(0.81,1.01) | 0.064 | ||||
| Family size | |||||||
| 1–4 | 4230(24) | 1.00 | |||||
| 5–9 | 10,078(58) | 1.02(0.93,1.11) | 0.672 | ||||
| 10+ | 3188(18) | 1.2(1.05,1.37) | 0.007 | ||||
| Number of children under 5 | |||||||
| 1 | 5553(33) | 1.00 | |||||
| 2 | 6790(40) | 0.97(0.89,1.05) | 0.442 | ||||
| 3 | 2993(18) | 1.06(0.94,1.18) | 0.337 | ||||
| >3 | 1518(9) | 1.26(1.07,1.49) | 0.006 | ||||
| Mother’s age at first birth | |||||||
| <15 | 511(3) | 1.00 | |||||
| 15–19 | 10,609(61) | 1.17(0.95,1.44) | 0.139 | ||||
| 20–24 | 5288(30) | 1.13(0.92,1.40) | 0.254 | ||||
| ≥25 | 1089(6) | 1.07(0.84,1.37) | 0.594 | ||||
| Mother’s education level | |||||||
| No education | 4099(23) | 1.00 | |||||
| Primary | 11,668(67) | 0.81(0.74,0.88) | <0.001 | ||||
| Secondary | 1554(9) | 1.01(0.87,1.18) | 0.89 | ||||
| Higher | 175(1) | 0.9(0.62,1.31) | 0.587 | ||||
| Mother’s marital Status | |||||||
| Married | 12,276(70) | 1.00 | |||||
| Living together | 2663(15) | 1.04(0.94,1.15) | 0.456 | ||||
| Widowed/Divorced/Live Apart | 1760(10) | 1(0.90,1.13) | 0.934 | ||||
| Never Married | 797(5) | 0.99(0.84,1.17) | 0.891 | ||||
| Wealth index | |||||||
| Poorest | 4132(24) | 1.00 | |||||
| Poorer | 3678(21) | 0.92(0.83,1.02) | 0.12 | ||||
| Middle | 3525(20) | 0.84(0.75,0.93) | 0.001 | ||||
| Richer | 3334(19) | 0.67(0.60,0.76) | <0.001 | ||||
| Richest | 2827(16) | 0.55(0.47,0.64) | <0.001 | ||||
| Individual characteristics | |||||||
| Age | |||||||
| 0–5 | 1856(11) | 1.00 | |||||
| 6–11 | 1910(12) | 1.85(0.77,4.45) | 0.167 | ||||
| 12–23 | 3718(22) | 2.25(0.98,5.17) | 0.057 | ||||
| 24–35 | 3235(20) | 1.41(0.61,3.23) | 0.419 | ||||
| 36–47 | 3030(18) | 0.76(0.34,1.71) | 0.505 | ||||
| 48–59 | 2828(17) | 0.63(0.28,1.45) | 0.278 | ||||
| Sex | |||||||
| Female | 8707(50) | 1.00 | |||||
| Male | 8789(50) | 1.12(0.98,1.27) | 0.085 | ||||
| Birthweight | |||||||
| Low | 1319(13) | 1.00 | |||||
| Normal or High | 8714(87) | 0.92(0.76,1.13) | 0.431 | ||||
| Dietary diversity score * | |||||||
| Below 3 | 1690(43) | 1.00 | |||||
| 3 and above | 2259(57) | 0.93(0.76,1.19) | 0.568 | ||||
| Month of Breastfeeding | |||||||
| <6 | 1859(16) | 1.00 | |||||
| 6–12 | 2696(23) | 1.8(0.78,4.15) | 0.171 | ||||
| 13–24 | 5914(51) | 1.23(0.55,2.74) | 0.611 | ||||
| >24 | 828(7) | 1.08(0.47,2.45) | 0.857 | ||||
| Never breastfed | 230(2) | 1.51(0.62,3.63) | 0.362 | ||||
| Stunting | |||||||
| Normal | 9931(61) | 1.00 | |||||
| stunted | 6245(39) | 1.23(1.06,1.43) | 0.007 | ||||
| Underweight | |||||||
| Normal | 13,791(85) | 1.00 | |||||
| Underweight | 2413(15) | 1.05(0.84,1.32) | 0.64 | ||||
| Variable | N (%) | Model 1 a | Model 2 b | ||
|---|---|---|---|---|---|
| AOR(95% CI) | p-Value | AOR(95% CI) | p-Value | ||
| Household characteristics | |||||
| Type of residence | |||||
| Urban | 4099 (23) | 1.00 | |||
| Rural | 13,397 (77) | 0.87(0.75,1.01) | 0.059 | ||
| Family size | |||||
| 1–4 | 4230 (24) | 1.00 | |||
| 5–9 | 10,078 (58) | 1.01(0.89,1.14) | 0.910 | ||
| 10+ | 3188 (18) | 1.15(0.96,1.37) | 0.127 | ||
| Number of children under five | |||||
| 1 | 5553 (33) | 1.00 | |||
| 2 | 6790 (40) | 0.99(0.88,1.10) | 0.802 | ||
| 3 | 2993 (18) | 1.16(1.00,1.36) | 0.056 | ||
| >3 | 1518 (9) | 1.41(1.13,1.76) | 0.002 | ||
| Mother’s age at first birth | |||||
| <15 | 511 (3) | 1.00 | |||
| 15–19 | 10,609 (61) | 0.8(0.59,1.09) | 0.152 | ||
| 20–24 | 5288 (30) | 0.82(0.60,1.11) | 0.199 | ||
| ≥25 | 1089 (6) | 0.68(0.48,0.97) | 0.033 | ||
| Mother’s education level | |||||
| No education | 4099 (23) | 1.00 | |||
| Primary | 11,668 (67) | 0.72(0.64,0.82) | <0.001 | ||
| Secondary | 1554 (9) | 0.84(0.70,1.01) | 0.061 | ||
| Higher | 175 (1) | 0.66(0.38,1.12) | 0.120 | ||
| Mother’s marital Status | |||||
| Married | 12,276 (70) | 1.00 | |||
| Living together | 2663 (15) | 1.03(0.92,1.16) | 0.600 | ||
| Widowed/Divorced/Live Apart | 1760 (10) | 0.93(0.81,1.08) | 0.374 | ||
| Never Married | 797 (5) | 0.92(0.74,1.14) | 0.431 | ||
| Wealth index | |||||
| Poorest | 4132 (24) | 1.00 | |||
| Poorer | 3678 (21) | 0.97(0.85,1.12) | 0.685 | ||
| Middle | 3525 (20) | 0.94(0.81,1.09) | 0.401 | ||
| Richer | 3334 (19) | 0.71(0.61,0.84) | <0.001 | ||
| Richest | 2827 (16) | 0.66(0.53,0.81) | <0.001 | ||
| Individual characteristics | |||||
| Age | |||||
| 0–5* | 1856 (11) | ||||
| 6–11 | 1910 (12) | 1.00 | |||
| 12–23 | 3718 (22) | 1.44(0.82,2.53) | 0.207 | ||
| 24–35 | 3235 (20) | 0.61(0.19,1.96) | 0.402 | ||
| 36–47 | 3030 (18) | 0.38(0.08,1.76) | 0.218 | ||
| 48–59 | 2828 (17) | 0.18(0.03,1.03) | 0.053 | ||
| Sex | |||||
| Female | 8707 (50) | 1.00 | |||
| Male | 8789 (50) | 1.39(1.10,1.75) | 0.005 | ||
| Birthweight | |||||
| Low | 1319 (13) | 1.00 | |||
| Normal or High | 8714 (87) | 0.61(0.40,0.94) | 0.026 | ||
| Dietary diversity score * | |||||
| Below 3 | 1690 (43) | 1.00 | |||
| 3 and above | 2259 (57) | 0.94(0.78,1.15) | 0.458 | ||
| Month of Breastfeeding | |||||
| <6 | 1859 (16) | ||||
| 6–12 | 2696 (23) | 1.00 | |||
| 13–24 | 5914 (51) | 0.5(0.29,0.87) | 0.014 | ||
| >24 | 828 (7) | 0.91(0.25,3.32) | 0.887 | ||
| Never breastfed | 230 (2) | 1.14(0.30,4.41) | 0.845 | ||
| Stunting | |||||
| Normal | 9931 (61) | 1.00 | |||
| stunted | 6245 (39) | 1.08(0.81,1.44) | 0.612 | ||
| Underweight | |||||
| Normal | 13,791 (85) | 1.00 | |||
| Underweight | 2413 (15) | 0.99(0.65,1.50) | 0.953 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunguya, B.F.; Zhu, S.; Paulo, L.S.; Ntoga, B.; Abdallah, F.; Assey, V.; Mpembeni, R.; Huang, J. Regional Disparities in the Decline of Anemia and Remaining Challenges among Children in Tanzania: Analyses of the Tanzania Demographic and Health Survey 2004–2015. Int. J. Environ. Res. Public Health 2020, 17, 3492. https://doi.org/10.3390/ijerph17103492
Sunguya BF, Zhu S, Paulo LS, Ntoga B, Abdallah F, Assey V, Mpembeni R, Huang J. Regional Disparities in the Decline of Anemia and Remaining Challenges among Children in Tanzania: Analyses of the Tanzania Demographic and Health Survey 2004–2015. International Journal of Environmental Research and Public Health. 2020; 17(10):3492. https://doi.org/10.3390/ijerph17103492
Chicago/Turabian StyleSunguya, Bruno F., Si Zhu, Linda Simon Paulo, Bupe Ntoga, Fatma Abdallah, Vincent Assey, Rose Mpembeni, and Jiayan Huang. 2020. "Regional Disparities in the Decline of Anemia and Remaining Challenges among Children in Tanzania: Analyses of the Tanzania Demographic and Health Survey 2004–2015" International Journal of Environmental Research and Public Health 17, no. 10: 3492. https://doi.org/10.3390/ijerph17103492
APA StyleSunguya, B. F., Zhu, S., Paulo, L. S., Ntoga, B., Abdallah, F., Assey, V., Mpembeni, R., & Huang, J. (2020). Regional Disparities in the Decline of Anemia and Remaining Challenges among Children in Tanzania: Analyses of the Tanzania Demographic and Health Survey 2004–2015. International Journal of Environmental Research and Public Health, 17(10), 3492. https://doi.org/10.3390/ijerph17103492





