Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-κB, NLRP3, and MAPKs Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animals and Treatments
2.3. Analysis of Serum ALT/AST Activities
2.4. ELISA Assay
2.5. Liver Histological Analysis
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
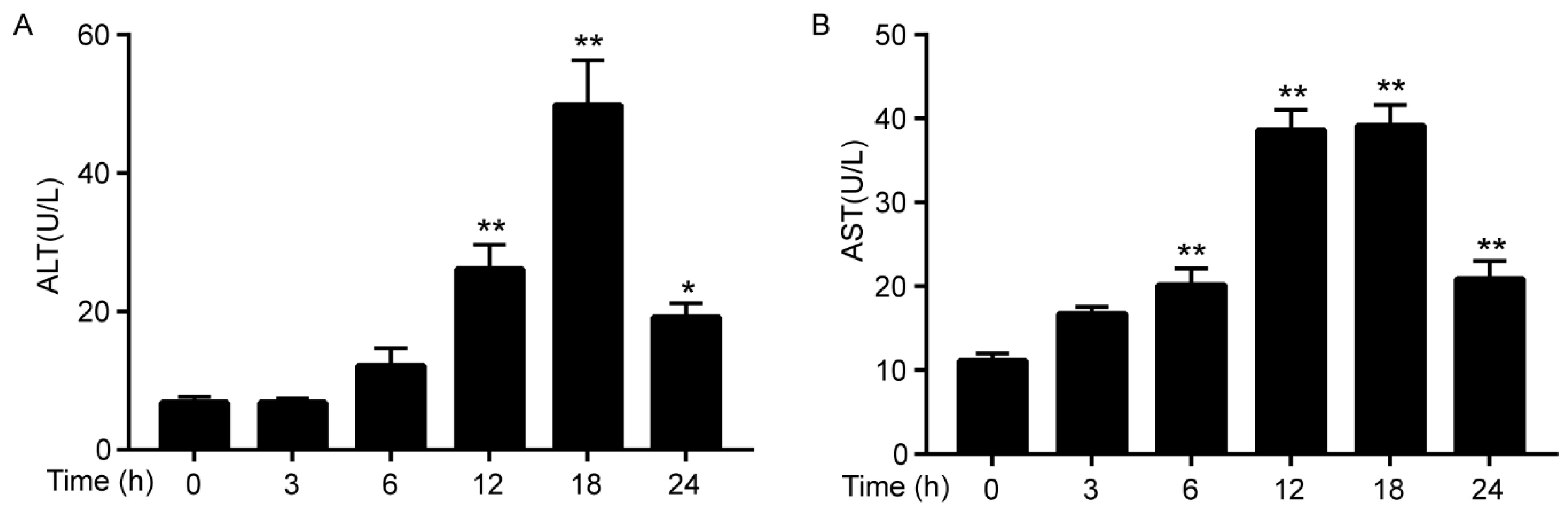
3.1. Cadmium Increases the Activities of ALT/AST
3.2. Cadmium Induces Pro-Inflammatory Cytokines Expression in Serum
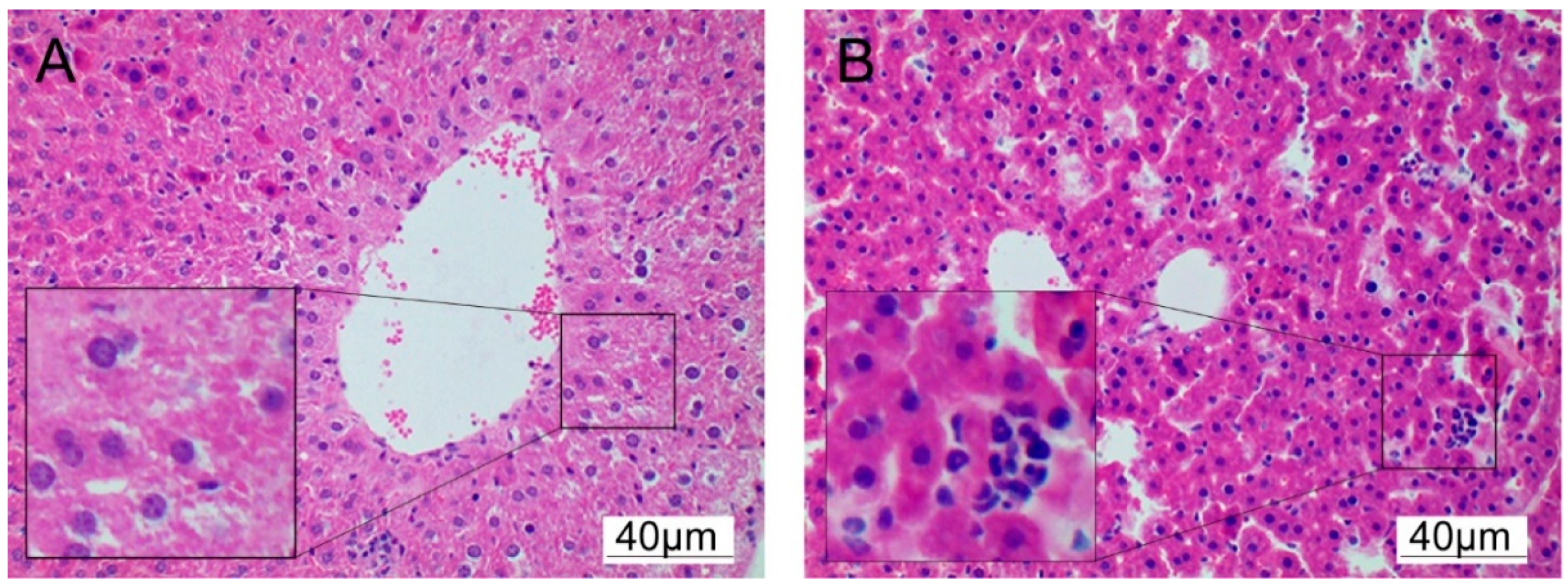
3.3. Cadmium Induces Histopathological Changes
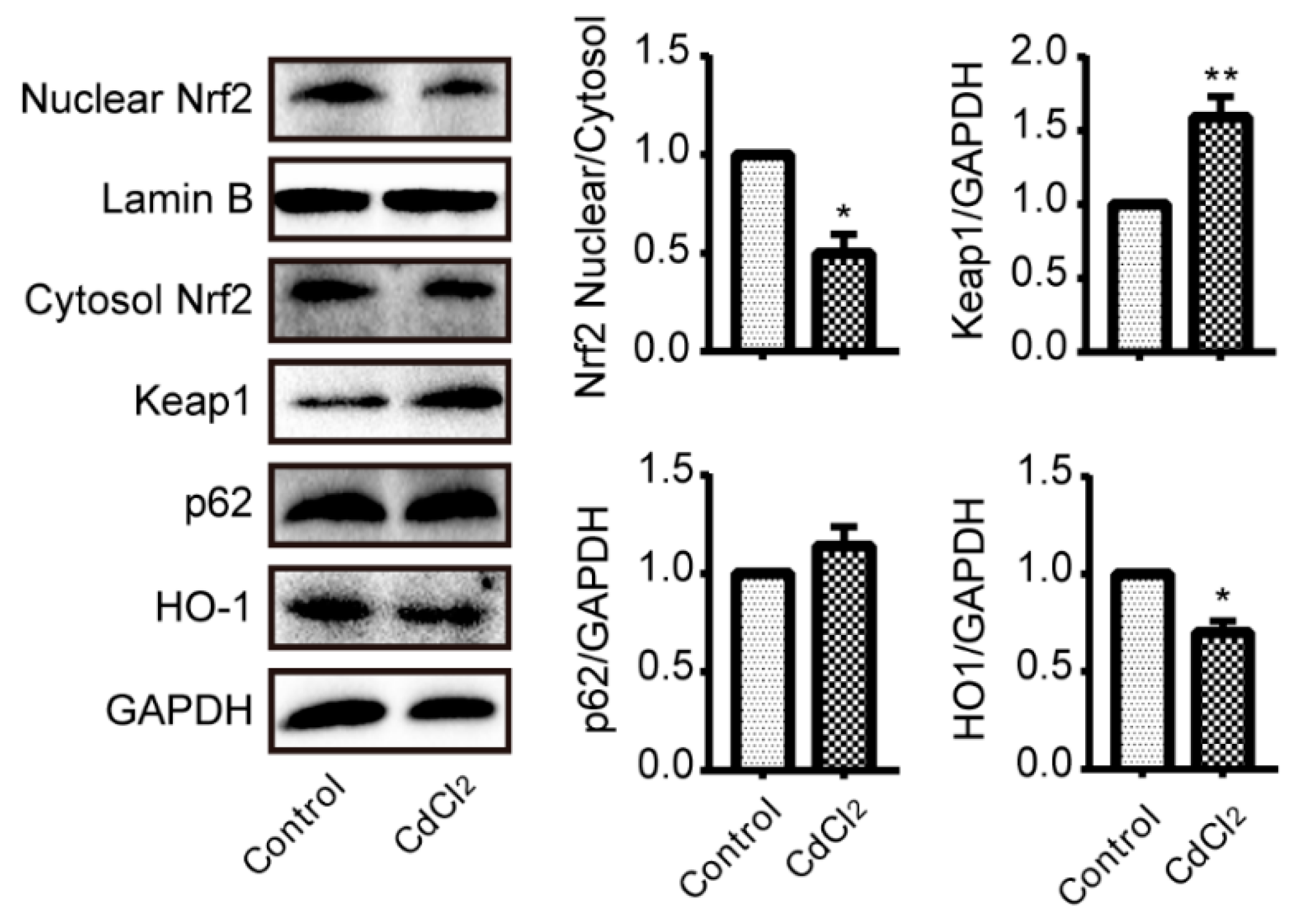
3.4. Cadmium Treatment Results in Nrf2 Inhibition in Liver
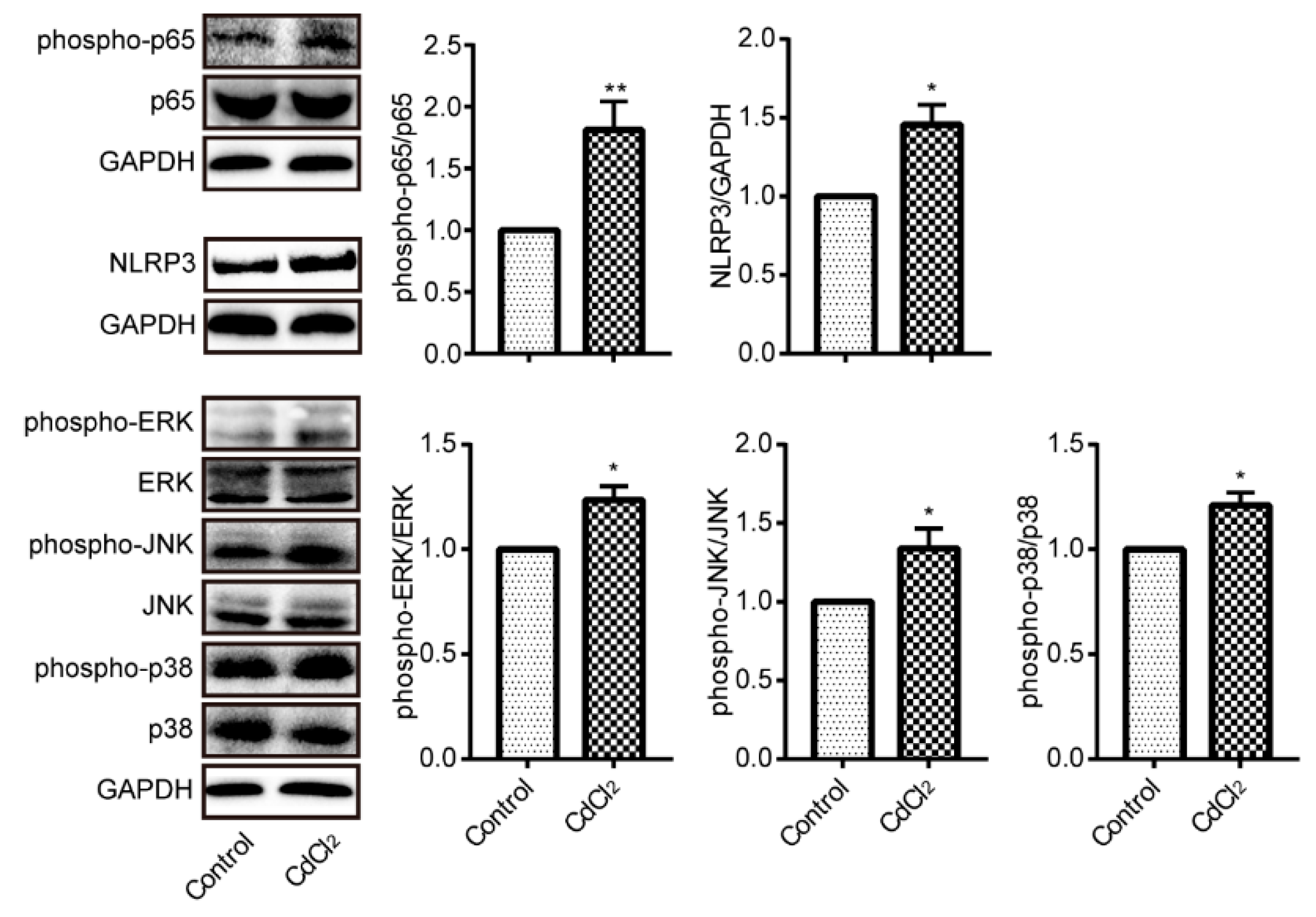
3.5. Cadmium Treatment Results in NF-κB p65, NLRP3, and MAPKs Activation in Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nordberg, G.F.; Kjellstrom, T. Metabolic model for cadmium in man. Environ. Health Perspect 1979, 28, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K.; Mise, N.; Kayama, F.; Iwanaga, K. Augmentation of cadmium-induced oxidative cytotoxicity by pioglitazone in renal tubular epithelial cells. Toxicol. Ind. Health 2019, 35, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Mezynska, M.; Brzoska, M.M. Environmental exposure to cadmium-a risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. Int. 2018, 25, 3211–3232. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Staessen, J.A.; Roels, H.A.; Munters, E.; Cuypers, A.; Richart, T.; Ruttens, A.; Smeets, K.; Clijsters, H.; Vangronsveld, J. Cadmium exposure in the population: From health risks to strategies of prevention. Biometals 2010, 23, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G.F. Toxicological aspects of metallothionein. Cell. Mol. Biol. 2000, 46, 451–463. [Google Scholar]
- Saedi, S.; Jafarzadeh, S.M.; Totonchi, M.; Zamiri, M.J.; Derakhshanfar, A. Effect of Prepubertal Exposure to CdCl2 on the Liver, Hematological, and Biochemical Parameters in Female Rats; an Experimental Study. Biol. Trace Elem. Res. 2019. [Google Scholar] [CrossRef]
- Mezynska, M.; Brzoska, M.M. Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J. Appl. Toxicol. 2019, 39, 117–145. [Google Scholar] [CrossRef]
- Rikans, L.E.; Yamano, T. Mechanisms of cadmium-mediated acute hepatotoxicity. J. Biochem. Mol. Toxicol. 2000, 14, 110–117. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Buha, D.A.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic Effect of Acute Cadmium and Lead Exposure in Rat Blood, Liver, and Kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef]
- Ledda, C.; Loreto, C.; Zammit, C.; Marconi, A.; Fago, L.; Matera, S.; Costanzo, V.; Fuccio, S.G.; Palmucci, S.; Ferrante, M.; et al. Noninfective occupational risk factors for hepatocellular carcinoma: A review (Review). Mol. Med. Rep. 2017, 15, 511–533. [Google Scholar] [CrossRef]
- Salama, S.A.; Arab, H.H.; Hassan, M.H.; Al, R.M.; Maghrabi, I.A. Cadmium-induced hepatocellular injury: Modulatory effects of gamma-glutamyl cysteine on the biomarkers of inflammation, DNA damage, and apoptotic cell death. J. Trace Elem. Med. Biol. 2019, 52, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, E.A.; Stohs, S.J. Cadmium-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J774A.1 cell cultures. Toxicology 1996, 112, 219–226. [Google Scholar] [CrossRef]
- Yamano, T.; DeCicco, L.A.; Rikans, L.E. Attenuation of cadmium-induced liver injury in senescent male fischer 344 rats: Role of Kupffer cells and inflammatory cytokines. Toxicol. Appl. Pharmacol. 2000, 162, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; Eguchi, A.; McGeough, M.D.; Pena, C.A.; Johnson, C.D.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014, 59, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Heid, M.E.; Keyel, P.A.; Kamga, C.; Shiva, S.; Watkins, S.C.; Salter, R.D. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J. Immunol. 2013, 191, 5230–5238. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Girardin, S.E. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011, 21, 558–560. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Ding, Y.; Zhou, W.; Tao, L.; Lu, P.; Wang, Y.; Hu, R. Nuclear Factor E2-Related Factor-2 Negatively Regulates NLRP3 Inflammasome Activity by Inhibiting Reactive Oxygen Species-Induced NLRP3 Priming. Antioxid. Redox Signal. 2017, 26, 28–43. [Google Scholar] [CrossRef]
- Rzepecka, J.; Pineda, M.A.; Al-Riyami, L.; Rodgers, D.T.; Huggan, J.K.; Lumb, F.E.; Khalaf, A.I.; Meakin, P.J.; Corbet, M.; Ashford, M.L.; et al. Prophylactic and therapeutic treatment with a synthetic analogue of a parasitic worm product prevents experimental arthritis and inhibits IL-1beta production via NRF2-mediated counter-regulation of the inflammasome. J. Autoimmun. 2015, 60, 59–73. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Ye, X.; Hao, Q.; Zhang, T.; Cui, G.; Yu, M. Nrf2/ARE pathway inhibits ROS-induced NLRP3 inflammasome activation in BV2 cells after cerebral ischemia reperfusion. Inflamm. Res. 2018, 67, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chen, S.; Luo, Y.; Chen, Z.; Liu, L.; Zhou, H.; Chen, W.; Shen, T.; Han, X.; Chen, L.; et al. Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS ONE 2011, 6, e19052. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Liu, J.J.; Klaassen, C.D. Nrf2 activation prevents cadmium-induced acute liver injury. Toxicol. Appl. Pharmacol. 2012, 263, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, Y.; Cao, Z.; Ma, Q.; Pi, H.; Fang, Y.; Yu, Z.; Hu, H.; Zhou, Z. Cadmium induces NLRP3 inflammasome-dependent pyroptosis in vascular endothelial cells. Toxicol. Lett. 2016, 246, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers, G.T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [PubMed]
- Li, W.; Yu, S.; Liu, T.; Kim, J.H.; Blank, V.; Li, H.; Kong, A.N. Heterodimerization with small Maf proteins enhances nuclear retention of Nrf2 via masking the NESzip motif. Biochim. Biophys. Acta 2008, 1783, 1847–1856. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef]
- Almeer, R.S.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel, M.A. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9, 5825. [Google Scholar] [CrossRef]
- Cho, H.Y.; Imani, F.; Miller-DeGraff, L.; Walters, D.; Melendi, G.A.; Yamamoto, M.; Polack, F.P.; Kleeberger, S.R. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am. J. Respir. Crit. Care Med. 2009, 179, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Qu, J.; Shen, X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Fang, Y.; Lu, Y.; Tan, D.; Du, C.; Li, Y.; Ma, Q.; Yu, J.; Chen, M.; Zhou, C.; et al. Melatonin alleviates cadmium-induced liver injury by inhibiting the TXNIP-NLRP3 inflammasome. J. Pineal Res. 2017, 62, e12389. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Joyce, A.R.; Piper, J.T.; McKallip, R.J.; Fariss, M.W. Role of oxidative stress and MAPK signaling in reference moist smokeless tobacco-induced HOK-16B cell death. Toxicol. Lett. 2010, 195, 23–30. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Huang, S. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic. Biol. Med. 2008, 45, 1035–1044. [Google Scholar] [CrossRef]
- Kalariya, N.M.; Wills, N.K.; Ramana, K.V.; Srivastava, S.K.; van Kuijk, F.J. Cadmium-induced apoptotic death of human retinal pigment epithelial cells is mediated by MAPK pathway. Exp. Eye Res. 2009, 89, 494–502. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Nishigaki, Y.; Palaniswami, R.; Nishigaki, I. In vitro studies on mangiferin protection against cadmium-induced human renal endothelial damage and cell death via the MAP kinase and NF-kappaB pathways. J. Recept. Signal. Transduct. Res. 2016, 36, 57–66. [Google Scholar] [CrossRef]
- Dasgupta, P.; Kulkarni, P.; Bhat, N.S.; Gupta, R.; Hashimoto, Y.; Saini, S.; Dar, A.A.; Shahryari, V.; Yamamura, S.; Tanaka, Y.; et al. Abstract 4659: Cadmium induced malignant transformation involves activation of the Erk/MAPK pathway. Cancer Res. 2019, 79, 4659. [Google Scholar]
- Zou, H.; Liu, X.; Han, T.; Hu, D.; Wang, Y.; Yuan, Y.; Gu, J.; Bian, J.; Zhu, J.; Liu, Z.P. Salidroside Protects against Cadmium-Induced Hepatotoxicity in Rats via GJIC and MAPK Pathways. PLoS ONE 2015, 10, e129788. [Google Scholar] [CrossRef] [PubMed]
- Nemmiche, S. Oxidative Signaling Response to Cadmium Exposure. Toxicol. Sci. 2017, 156, 4–10. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhu, Y.; Lu, Z.; Guo, W.; Tumen, B.; He, Y.; Chen, C.; Hu, S.; Xu, K.; Wang, Y.; et al. Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-κB, NLRP3, and MAPKs Signaling Pathway. Int. J. Environ. Res. Public Health 2020, 17, 138. https://doi.org/10.3390/ijerph17010138
Liu C, Zhu Y, Lu Z, Guo W, Tumen B, He Y, Chen C, Hu S, Xu K, Wang Y, et al. Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-κB, NLRP3, and MAPKs Signaling Pathway. International Journal of Environmental Research and Public Health. 2020; 17(1):138. https://doi.org/10.3390/ijerph17010138
Chicago/Turabian StyleLiu, Chang, Yaohui Zhu, Zhenxiang Lu, Weina Guo, Bayaer Tumen, Yalan He, Chao Chen, Shanshan Hu, Kangzhi Xu, Yan Wang, and et al. 2020. "Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-κB, NLRP3, and MAPKs Signaling Pathway" International Journal of Environmental Research and Public Health 17, no. 1: 138. https://doi.org/10.3390/ijerph17010138
APA StyleLiu, C., Zhu, Y., Lu, Z., Guo, W., Tumen, B., He, Y., Chen, C., Hu, S., Xu, K., Wang, Y., Li, L., & Li, S. (2020). Cadmium Induces Acute Liver Injury by Inhibiting Nrf2 and the Role of NF-κB, NLRP3, and MAPKs Signaling Pathway. International Journal of Environmental Research and Public Health, 17(1), 138. https://doi.org/10.3390/ijerph17010138




