Early Lifestyle Interventions in People with Impaired Glucose Tolerance in Northern Colombia: The DEMOJUAN Project
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Oversight
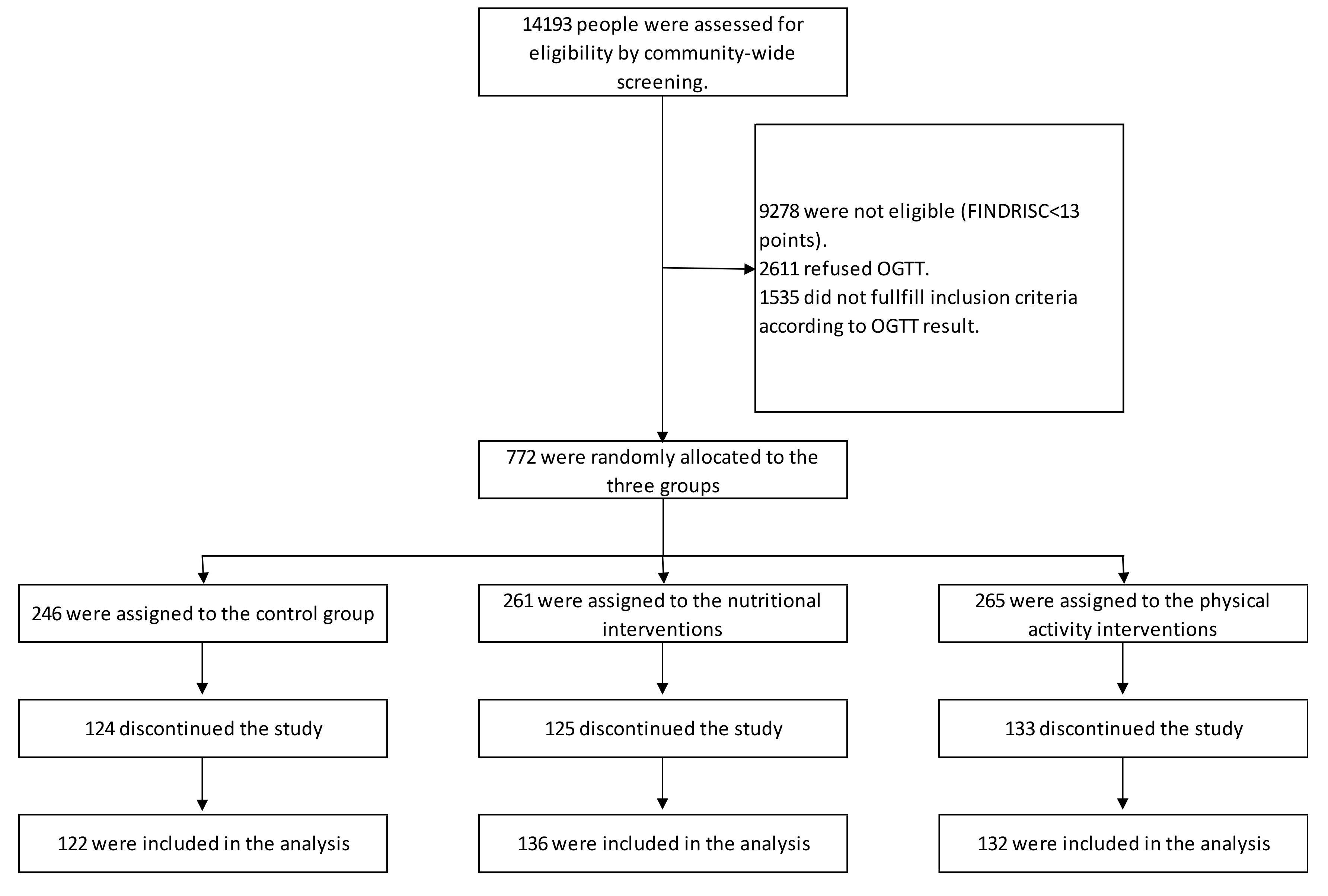
2.2. Study Population
2.3. Randomization and Interventions
2.4. Study Measurements
2.4.1. Non-Invasive Measurements
2.4.2. Biochemical Measurements
2.5. Study Outcomes
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Diabetes Atlas, 7th ed.; International Diabetes Federation (IDF): Brussels, Belgium, 2017.
- Manuel, D.; Schultz, S. Health-related quality of life and health-adjusted life expectancy of people with diabetes mellitus in Ontario, Canada 1996–1997. Diabetes Care 2004, 27, 407–414. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V.; Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 4, 289–297. [Google Scholar] [CrossRef]
- Turner, R.C.; Cull, C.A.; Frighi, V.; Holman, R.R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus. Progressive requirement for multiple therapies (UKPDS 49). JAMA 1999, 281, 2005–2012. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Kosaka, K.; Noda, M.; Kuzuya, T. Prevention of type 2 diabetes by lifestyle intervention: A Japanese trial in IGTmales. Diabetes Res. Clin. Pract. 2005, 67, 152–162. [Google Scholar] [CrossRef]
- Roumen, C.; Corpeleijn, E.; Feskens, E.J.; Mensink, M.; Saris, W.H.; Blaak, E.E. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: The SLIM study. Diabetes Med. 2008, 25, 597–605. [Google Scholar] [CrossRef]
- Ackermann, R.T.; Finch, E.A.; Caffrey, H.M.; Lipscomb, E.R.; Hays, L.M.; Saha, C. Long-term effects of a community-based lifestyle intervention to prevent type 2 diabetes: The DEPLOY extension pilot study. Chronic Illn. 2011, 7, 279–290. [Google Scholar] [CrossRef]
- Ackermann, R.T.; Finch, E.A.; Brizendine, E.; Zhou, H.; Marrero, D.G. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am. J. Prev. Med. 2008, 35, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Echouffo-Tcheugui, J.; Williamson, D.F. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. (Millwood) 2012, 31, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Barrio, F.; Cabré, J.J.; Piñol, J.L.; Cos, X.; Solé, C.; Bolíbar, B.; Basora, J.; Castell, C.; Solà-Morales, O.; et al. Delaying progression to type 2 diabetes among high-risk Spanish individuals is feasible in real-life primary healthcare settings using intensive lifestyle intervention. Diabetologia 2012, 55, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Gilis-Januszewska, A.; Lindström, J.; Tuomilehto, J.; Piwońska-Solska, B.; Topór-Mądry, R.; Szybiński, Z.; Peltonen, M.; Schwarz, P.E.; Windak, A.; Hubalewska-Dydejczyk, A. Sustained diabetes risk reduction after real life and primary health care setting implementation of the diabetes in Europe prevention using lifestyle, physical activity and nutritional intervention (DE-PLAN) project. BMC Public Health 2017, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Noda, M.; Kuzuya, T.; Kilkkinen, A.; Vartiainen, E.; Heistaro, S.; Philpot, B.; Absetz, P.; Bunker, S.; O’Neil, A.; et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) Diabetes Prevention Project. BMC Public Health 2007, 7, 249. [Google Scholar] [CrossRef]
- Nilsen, V.; Bakke, P.S.; Gallefoss, F. Effects of lifestyle intervention in persons at risk for type 2 diabetes mellitus—Results from a randomised, controlled trial. BMC Public Health 2011, 11, 893. [Google Scholar] [CrossRef]
- Penn, L.; White, M.; Oldroyd, J.; Walker, M.; Alberti, K.G.; Mathers, J.C. Prevention of type 2 diabetes in adults with impaired glucose tolerance: The European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health 2009, 9, 277–283. [Google Scholar] [CrossRef]
- Saaristo, T.; Moilanen, L.; Korpi-Hyövälti, E.; Vanhala, M.; Saltevo, J.; Niskanen, L.; Jokelainen, J.; Peltonen, M.; Oksa, H.; Tuomilehto, J. Lifestyle intervention for prevention of type 2 diabetes in primary health care: One-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010, 33, 2146–2151. [Google Scholar] [CrossRef]
- Acosta, T.; Barengo, N.C.; Arrieta, A.; Ricaurte, C.; Tuomilehto, J.O. A demonstration area for type 2 diabetes prevention in Barranquilla and Juan Mina (Colombia): Baseline characteristics of the study participants. Medicine 2018, 97, e9285. [Google Scholar] [CrossRef] [PubMed]
- Pandis, N.; Chung, B.; Scherer, R.W.; Elbourne, D.; Altman, D.G. CONSORT 2010 statement: Extension checklist for reporting within person randomised trials. BMJ 2017, 357, 2835. [Google Scholar] [CrossRef]
- Lindström, J.; Tuomilehto, J. The diabetes risk score: A practical tool to predict type 2 diabetes risk. Diabetes Care 2003, 26, 725–731. [Google Scholar] [CrossRef]
- Barengo, N.C.; Acosta, T.; Arrieta, A.; Ricaurte, C.; Mayor, D.; Tuomilehto, J.O.; The DEMOJUAN Study Group. Screening for people with glucose metabolism disorders within the framework of the DEMOJUAN project (DEMOnstration area for primary prevention of type 2 diabetes, JUAN Mina and Barranquilla, Colombia). Diabetes Metab. Res. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Saaristo, T.; Peltonen, M.; Lindström, J.; Saarikoski, L.; Sundvall, J.; Eriksson, J.G.; Tuomilehto, J. Cross-sectional evaluation of the Finnish Diabetes Risk Score: A tool to identify undetected type 2 diabetes, abnormal glucose tolerance and metabolic syndrome. Diabetes Vasc. Dis. Res. 2005, 2, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, M.; De Berardis, G.; Rossi, M.C.; Sacco, M.; Belfiglio, M.; Pellegrini, F.; Tognoni, G.; Valentini, M.; Nicolucci, A. Use of the diabetes risk score for opportunistic screening of undiagnosed diabetes and impaired glucose tolerance: The IGLOO (Impaired Glucose Tolerance and Long-Term Outcomes Observational) study. Diabetes Care 2005, 28, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Makrilakis, K.; Liatis, S.; Grammatikou, S.; Perrea, D.; Stathi, C.; Tsiligros, P.; Katsilambros, N. Validation of the Finnish diabetes risk score (FINDRISC) questionnaire for screening forundiagnosed type 2 diabetes, dysglycaemia and the metabolic syndrome in Greece. Diabetes Metab. 2011, 37, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Kengne, A.P.; Beulens, J.W.; Peelen, L.M.; Moons, K.G.; van der Schouw, Y.T.; Schulze, M.B.; Spijkerman, A.M.; Griffin, S.J.; Grobbee, D.E.; Palla, L.; et al. Non-invasive risk scores for prediction of type 2 diabetes (EPIC-InterAct): A validation of existing models. Lancet Diabetes Endocrinol. 2014, 2, 19–29. [Google Scholar] [CrossRef]
- Barengo, N.C.; Tamayo, D.C.; Tono, T.; Tuomilehto, J.A. Colombian diabetes risk score for detecting undiagnosed diabetes and impaired glucose regulation. Prim Care Diabetes 2017, 11, 86–93. [Google Scholar] [CrossRef]
- Schwarz, P.E.; Lindström, J.; Kissimova-Scarbeck, K.; Szybinski, Z.; Barengo, N.C.; Peltonen, M.; Tuomilehto, J.; DE-PLAN project. The European Perspective of Type 2 Diabetes Prevention: Diabetes in Europe—Prevention using lifestyle, physical activity and nutritional intervention (DE-PLAN) project. Exp. Clin. Endocrinol. Diabetes 2008, 116, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Standl, E.; Bartnik, M.; Van den Berghe, G.; Betteridge, J.; de Boer, M.J.; Cosentino, F.; Jönsson, B.; Laakso, M.; Malmberg, K.; et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2007, 28, 88–136. [Google Scholar] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Bonita, R.; de Courten, M.; Dwyer, T.; Jamrozik, K.; Winkelmann, R. Surveillance of Risk Factors for Noncommunicable Diseases: The WHO STEP Wise Approach; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Ekelund, U.; Sepp, H.; Brage, S.; Becker, W.; Jakes, R.; Hennings, M.; Wareham, N.J. Criterion-related validity of the last 7-day, short form of the International Physical Activity Questionnaire in Swedish adults. Public Health Nutr. 2006, 9, 258–265. [Google Scholar] [CrossRef]
- Hu, G.; Barengo, N.C.; Tuomilehto, J.; Lakka, T.A.; Nissinen, A.; Jousilahti, P. Relationship of physical activity and body mass index to the risk of hypertension: A prospective study in Finland. Hypertension 2004, 43, 25–30. [Google Scholar] [CrossRef]
- Hu, G.; Qiao, Q.; Silventoinen, K.; Eriksson, J.G.; Jousilahti, P.; Lindström, J.; Valle, T.T.; Nissinen, A.; Tuomilehto, J. Occupational, commuting, and leisure-time physical activity in relation to risk for type 2 diabetes in middle-aged Finnish men and women. Diabetologia 2003, 46, 322–329. [Google Scholar] [CrossRef]
- Albanes, D.; Conway, J.M.; Taylor, P.R.; Moe, P.W.; Judd, J. Validation and comparison of eight physical activity questionnaires. Epidemiology 1990, 1, 65–71. [Google Scholar] [CrossRef]
- Salonen, J.T.; Slater, J.S.; Tuomilehto, J.; Rauramaa, R. Leisure time and occupational physical activity: Risk of death from ischemic heart disease. Am. J. Epidemiol. 1988, 127, 87–94. [Google Scholar] [CrossRef]
- Hemiö, K.; Pölönen, A.; Ahonen, K.; Kosola, M.; Viitasalo, K.; Lindström, J. A simple tool for diet evaluation in primary health care: Validation of a 16-item food intake questionnaire. Int. J. Environ. Res. Public Health 2014, 11, 2683–2697. [Google Scholar] [CrossRef]
- Guía de práctica clínica para el diagnóstico, tratamiento y seguimiento de diabetes tipo 1, diabetes tipo 2 en mayores de 18 años y diabetes gestacional [Clinical Practice Guideline for the Diagnosis, Treatment and Monitoring of Type 1 Diabetes, Type 2 Diabetes and Gestational Diabetes in People Aged 18 and Older]; Alianza Cinets; Ministry of Health and Social Protection: Bogotá, Colombia, 2015. Available online: http://med.javeriana.edu.co/publi/vniversitas/serial/v54n4/Recomendaciones%20Diabetes%20tipo%202.pdf (accessed on 16 April 2019).
- WHO Consultation Definition. Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus; World Health Organisation: Geneva, Switzerland, 1999; Report No. 99.2. [Google Scholar]
- American Diabetes Association Position Statement. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjöström, L. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef]
- Knowler, W.C.; Hamman, R.F.; Edelstein, S.L.; Barrett-Connor, E.; Ehrmann, D.A.; Walker, E.A.; Fowler, S.E.; Nathan, D.M.; Kahn, S.E. Diabetes Prevention Program Research Group. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005, 54, 1150–1156. [Google Scholar]
- Gerstein, H.C.; Yusuf, S.; Bosch, J.; Pogue, J.; Sheridan, P.; Dinccag, N.; Hanefeld, M.; Hoogwerf, B.; Laakso, M.; Mohan, V.; et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006, 368, 1096–1105. [Google Scholar] [PubMed]
- Adams, R.; Hebert, C.J.; Mcvey, L.; Williams, R. Implementation of the YMCA Diabetes Prevention Program throughout an Integrated Health System: A Translational Study. Perm J 2016, 20, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; van der Pols, J.C.; Dobson, A.J. Regression to the mean: What it is and how to deal with it. Int. J. Epidemiol. 2005, 34, 215–220. [Google Scholar] [CrossRef] [PubMed]
| Intervention Groups | ||||
|---|---|---|---|---|
| Control | Nutrition | Physical Activity | p-Values | |
| (n = 246) | (n = 261) | (n = 265) | ||
| mean (SD 1) | mean (SD) | mean (SD) | ||
| Age | 53.8 (9.1) | 52.9 (9.3) | 52.2 (8.1) | 0.105 |
| % (n) | % (n) | % (n) | ||
| BMI1 | 0.218 | |||
| <25 kg/m2 | 15 (37) | 17 (43) | 11 (30) | |
| 25–30 kg/m2 | 36 (88) | 41 (106) | 44 (115) | |
| >30 kg/m2 | 49 (121) | 43 (111) | 45 (119) | |
| Waist circumference | 0.59 | |||
| ≥94 cm (men)/≥90 cm (women) | 89 (219) | 88 (229) | 86 (228) | |
| >30 min physical activity/day | 13 (31) | 13 (35) | 15 (40) | 0.703 |
| Daily consumption of fruits and vegetables | 23 (57) | 20 (51) | 23 (61) | 0.528 |
| Anti-hypertension drug use | 46 (114) | 44 (115) | 46 (121) | 0.868 |
| Family history of diabetes | 0.657 | |||
| No | 29 (71) | 23 (61) | 25 (67) | |
| Yes: Grandparent, uncle, aunt, or cousin | 22 (54) | 26 (67) | 23 (61) | |
| Yes: Biological father, mother, or sibling | 49 (121) | 51 (132) | 52 (136) | |
| Previous increased glucose in blood | 28 (69) | 30 (77) | 35 (93) | 0.187 |
| Glucose metabolism | ||||
| Normoglycemia | 18 (22) | 11 (15) | 13 (17) | 0.069 |
| Isolated IFG | 32 (40) | 28 (57) | 23 (38) | |
| Isolated IGT | 35 (44) | 41 (38) | 29 (30) | |
| IFG and IGT | 15 (19) | 20 (28) | 35 (47) | |
| Effectively Analyzed | Control | Nutrition | Physical Activity | p-Values |
|---|---|---|---|---|
| (n = 122) | (n = 136) | (n = 132) | ||
| mean (SD 1) | mean (SD) | mean (SD) | ||
| Age (years) | 53.7 (8.9) | 52.3 (9.1) | 52.6 (8.2) | 0.420 |
| % (n) | % (n) | % (n) | ||
| BMI 1 | 0.732 | |||
| <25 kg/m2 | 13 (16) | 18 (24) | 13 (17) | |
| 25–30 kg/m2 | 43 (52) | 40 (55) | 46 (61) | |
| >30 kg/m2 | 44 (54) | 42 (57) | 41 (54) | |
| Waist circumference | ||||
| ≥94 cm (men)/≥90 cm (women) | 90 (110) | 88 (120) | 82 (108) | 0.118 |
| >30 min physical activity/day | 12 (15) | 12 (16) | 17 (22) | 0.444 |
| Daily consumption of fruits and vegetables | 28 (34) | 19 (26) | 24 (32) | 0.249 |
| Anti-hypertension drug use | 45 (55) | 42 (57) | 46 (60) | 0.815 |
| Family history of diabetes | 0.618 | |||
| No | 30 (37) | 25 (34) | 26 (35) | |
| Yes: Grandparent, uncle, aunt, or cousin | 17 (21) | 25 (34) | 23 (30) | |
| Yes: Biological father, mother, or sibling | 53 (64) | 50 (68) | 51 (67) | |
| Previous increased glucose in blood | 27 (33) | 27 (36) | 36 (48) | 0.145 |
| Glucose metabolism | <0.001 | |||
| Normoglycemia | 18 (22) | 11 (15) | 13 (17) | |
| Isolated IFG | 35 (43) | 27 (36) | 23 (30) | |
| Isolated IGT | 31 (38) | 42 (57) | 29 (38) | |
| IFG and IGT | 16 (19) | 21 (28) | 36 (47) |
| Control | Nutrition | Physical Activity | ||
|---|---|---|---|---|
| (n = 122) | (n = 136) | (n = 132) | ||
| RR 1 | (95% CI 2) | RR (95% CI) | RR (95% CI) | |
| Primary outcome | ||||
| Reversion to normoglycemia | 1 | Ref. 3 | 0.88 (0.70–1.12) | 0.95 (0.75–1.20) |
| Secondary outcome | ||||
| Type 2 diabetes | 1 | Ref. | 1.38 (0.67–2.84) | 1.43 (0.70–2.93) |
| Group | Control | Nutrition | Physical Activity |
|---|---|---|---|
| (n = 122) | (n = 136) | (n = 132) | |
| % (n) | % (n) | % (n) | |
| Participated in at least one group and one individual intervention session | 0 (0) | 74 (100) | 76 (100) |
| Participated in at least 50% of all individual sessions | 0 (0) | 32 (43) | 46 (61) |
| Participated in at least 50% of all group sessions | 0 (0) | 9 (12) | 15 (20) |
| Control | Nutrition | Physical Activity | ||||
|---|---|---|---|---|---|---|
| (n = 122) | (n = 136) | (n = 132) | ||||
| Mean | SD 1 | Mean | SD | Mean | SD | |
| Fasting glucose | ||||||
| Baseline | 97 | (10) | 101 | (10) | 102 | (11) |
| After follow-up | 101 | (19) | 102 | (21) | 106 | (32) |
| p-value for difference between baseline and follow-up = 0.644 | ||||||
| 2-h glucose | ||||||
| Baseline | 143 | (25) | 141 | (26) | 146 | (26) |
| After follow-up | 122 | (43) | 127 | (52) | 117 | (49) |
| p-value for difference between baseline and follow-up <0.001 | ||||||
| p-value for differences between intervention groups 0.267 | ||||||
| p-value for interaction (intervention and fasting and 2-h glucose levels between baseline and after follow-up group) 0.201 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barengo, N.C.; Acosta, T.; Arrieta, A.; Ricaurte, C.; Smits, D.; Florez, K.; Tuomilehto, J.O. Early Lifestyle Interventions in People with Impaired Glucose Tolerance in Northern Colombia: The DEMOJUAN Project. Int. J. Environ. Res. Public Health 2019, 16, 1403. https://doi.org/10.3390/ijerph16081403
Barengo NC, Acosta T, Arrieta A, Ricaurte C, Smits D, Florez K, Tuomilehto JO. Early Lifestyle Interventions in People with Impaired Glucose Tolerance in Northern Colombia: The DEMOJUAN Project. International Journal of Environmental Research and Public Health. 2019; 16(8):1403. https://doi.org/10.3390/ijerph16081403
Chicago/Turabian StyleBarengo, Noël C., Tania Acosta, Astrid Arrieta, Carlos Ricaurte, Dins Smits, Karen Florez, and Jaakko O. Tuomilehto. 2019. "Early Lifestyle Interventions in People with Impaired Glucose Tolerance in Northern Colombia: The DEMOJUAN Project" International Journal of Environmental Research and Public Health 16, no. 8: 1403. https://doi.org/10.3390/ijerph16081403
APA StyleBarengo, N. C., Acosta, T., Arrieta, A., Ricaurte, C., Smits, D., Florez, K., & Tuomilehto, J. O. (2019). Early Lifestyle Interventions in People with Impaired Glucose Tolerance in Northern Colombia: The DEMOJUAN Project. International Journal of Environmental Research and Public Health, 16(8), 1403. https://doi.org/10.3390/ijerph16081403







