Perinatal Exposure to Environmental Endocrine Disruptors in the Emergence of Neurodevelopmental Psychiatric Diseases: A Systematic Review
Abstract
1. Background
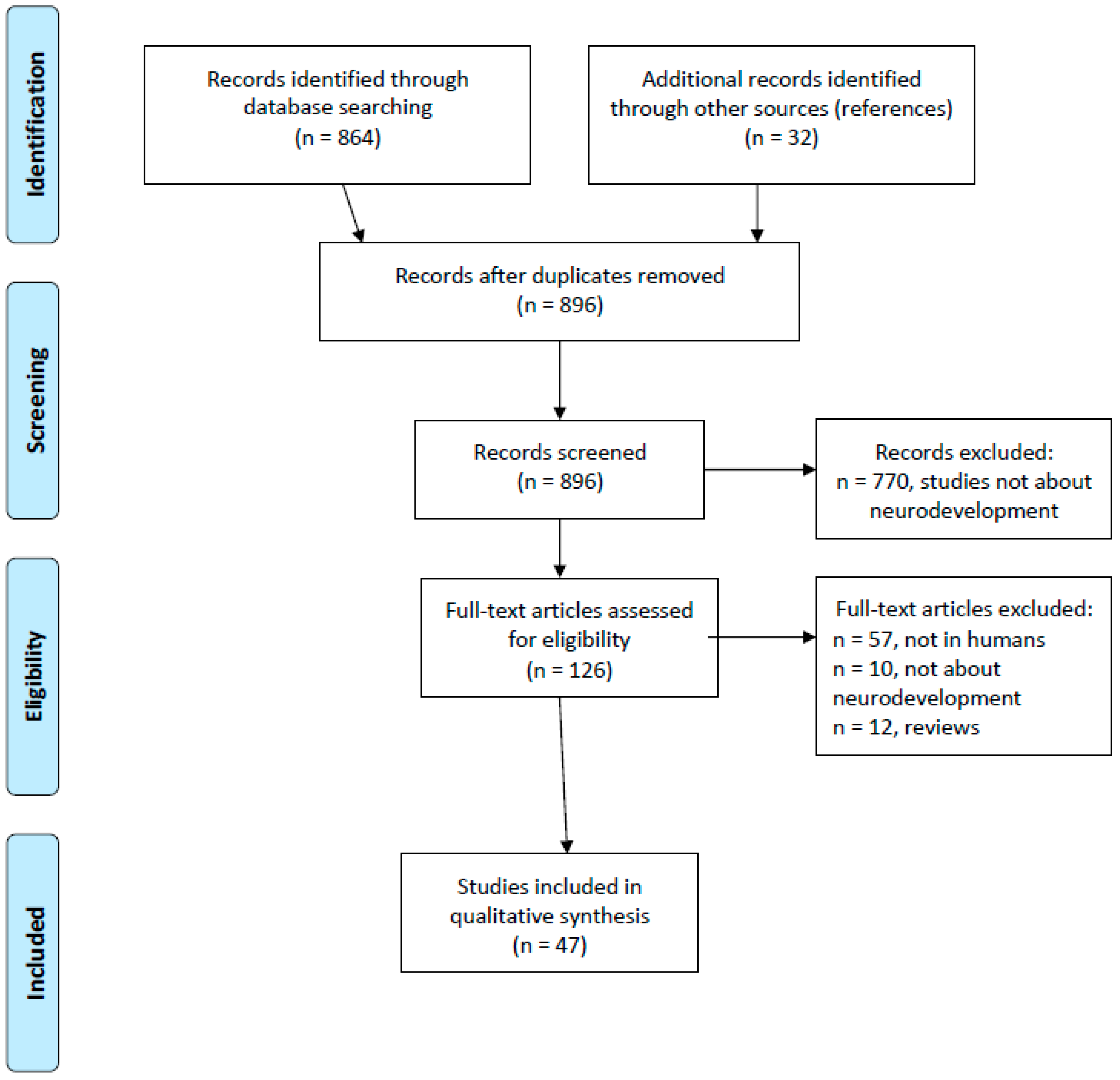
2. Materials and Methods
3. Results
3.1. EDCs Exposure and ASD
3.2. EDCs Exposure and ADHD
3.3. EDCs Exposure and Global Developmental Delay (GDD)
3.4. EDCs Exposure and Intellectual Disability
3.5. Exposure to EDCs and Communication Disorder
3.6. ED Exposure and Behavior/Unspecified Disorders
4. Discussion
- First, the exposure itself can be difficult to define in terms of duration and moment of exposure. The specific measurement of one exposure may be difficult to obtain. As of today, more than 800 products are considered as EDCs, some being commonly found in our environment. We are probably mostly studying mixture effects, and it may be difficult to isolate one specific exposure [74]. Long-lived EDCs are easier to study than many other EDCs (such as BPA and phthalates) that are quickly metabolized in humans and rapidly degraded in the environment. Some papers have tried to overcome this issue in animals, but it has yet to be done in humans [75].In humans, evidence is mostly based on correlations between concentrations of chemicals and outcomes, assessed at one point during an individual’s life, which is not a real-world situation. At some very specific times, even in very low concentrations, any exogenous EDCs may exceed the body’s natural endogenous hormone levels, changing target cells that are sensitive to hormones. Thus, even extremely low dosages of EDCs can alter biological outcomes. Moreover, studies showed that, due to nonmonotonic dose–response curves, the effects of low doses cannot be predicted by the effects observed at high doses [76].
- Second, the pathophysiological processes involved with EDCs have not been clearly elucidated yet. If, by definition, they are all able to alter the physiological endocrine system, their pathophysiology may differ or overlap. EDCs interfere with the endocrine system in multiple ways, making it difficult to link one pathway to one symptom. They can act directly on the behavior and development via sexual hormone perturbations (for example, the anti-androgenic effect of phthalates [77]), but they could interfere directly with the neuronal development as well; for instance, interactions with the vasopressin system [78] and oxytocin [79] have been described, among others.
- Third, the outcome measurement is most often based on neuropsychological assets that may not correlate to a clinical diagnosis, but more to a ‘profile.’ The fact that diagnosis in the developmental disorder categories co-occur frequently makes it difficult to determine the specificity of the cause–effect relationship [80].
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Colborn, T. Statement from the work session on chemically-induced alterations in the developing immune system: The wildlife/human connection. Environ. Health Perspect. 1996, 104, 807–808. [Google Scholar]
- Hugla, J.L.; Thomé, J.P. Effects of polychlorinated biphenyls on liver ultrastructure, hepatic monooxygenases, and reproductive success in the barbel. Ecotoxicol. Environ. Saf. 1999, 42, 265–273. [Google Scholar] [CrossRef]
- Roncati, L.; Termopoli, V.; Pusiol, T. Negative Role of the Environmental Endocrine Disruptors in the Human Neurodevelopment. Front. Neurol. 2016, 7, 143. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, L.; Cao, Y.; Chen, B.; Zheng, L.; Ge, R.-S. Phthalate levels and low birth weight: A nested case-control study of Chinese newborns. J. Pediatr. 2009, 155, 500–504. [Google Scholar] [CrossRef]
- Miao, M.; Yuan, W.; Zhu, G.; He, X.; Li, D.-K. In utero exposure to bisphenol-A and its effect on birth weight of offspring. Reprod. Toxicol. Elmsford NY 2011, 32, 64–68. [Google Scholar] [CrossRef]
- Harley, K.G.; Chevrier, J.; Aguilar Schall, R.; Sjödin, A.; Bradman, A.; Eskenazi, B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am. J. Epidemiol. 2011, 174, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Main, K.M.; Skakkebaek, N.E.; Virtanen, H.E.; Toppari, J. Genital anomalies in boys and the environment. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Paris, F.; Jandel, C.; Kalfa, N.; Orsini, M.; Daurès, J.P.; Sultan, C. Prenatal environmental risk factors for genital malformations in a population of 1442 French male newborns: A nested case-control study. Hum. Reprod. Oxf. Engl. 2011, 26, 3155–3162. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Gray, L.E., Jr.; Marcus, M.; Ojeda, S.R.; Pescovitz, O.H.; Witchel, S.F.; Sippell, W.; Abbott, D.H.; Soto, A.; Tyl, R.W.; et al. Environmental factors and puberty timing: Expert panel research needs. Pediatrics 2008, 121, S192–S207. [Google Scholar] [CrossRef]
- Verhulst, S.L.; Nelen, V.; Hond, E.D.; Koppen, G.; Beunckens, C.; Vael, C.; Schoeters, G.; Desager, K. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ. Health Perspect. 2009, 117, 122–126. [Google Scholar] [CrossRef]
- Moral, R.; Wang, R.; Russo, I.H.; Lamartiniere, C.A.; Pereira, J.; Russo, J. Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J. Endocrinol. 2008, 196, 101–112. [Google Scholar] [CrossRef]
- Anway, M.D.; Skinner, M.K. Epigenetic programming of the germ line: Effects of endocrine disruptors on the development of transgenerational disease. Reprod. Biomed. Online 2008, 16, 23–25. [Google Scholar] [CrossRef]
- Diotel, N.; Charlier, T.D.; Lefebvre d’Hellencourt, C.; Couret, D.; Trudeau, V.L.; Nicolau, J.C.; Meilhac, O.; Kah, O.; Pellegrini, E. Steroid Transport, Local Synthesis, and Signaling within the Brain: Roles in Neurogenesis, Neuroprotection, and Sexual Behaviors. Front. Neurosci. 2018, 12, 84. [Google Scholar] [CrossRef]
- Bernal, J. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 249–259. [Google Scholar] [CrossRef]
- Dickerson, S.M.; Cunningham, S.L.; Patisaul, H.B.; Woller, M.J.; Gore, A.C. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology 2011, 152, 581–594. [Google Scholar] [CrossRef]
- Pellegrini, E.; Diotel, N.; Vaillant-Capitaine, C.; Pérez Maria, R.; Gueguen, M.-M.; Nasri, A.; Cano Nicolau, J.; Kah, O. Steroid modulation of neurogenesis: Focus on radial glial cells in zebrafish. J. Steroid Biochem. Mol. Biol. 2015, 160, 27–36. [Google Scholar] [CrossRef]
- Geens, T.; Neels, H.; Covaci, A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012, 87, 796–802. [Google Scholar] [CrossRef]
- Venerosi, A.; Cutuli, D.; Colonnello, V.; Cardona, D.; Ricceri, L.; Calamandrei, G. Neonatal exposure to chlorpyrifos affects maternal responses and maternal aggression of female mice in adulthood. Neurotoxicol. Teratol. 2008, 30, 468–474. [Google Scholar] [CrossRef]
- Schantz, S.L.; Widholm, J.J. Cognitive effects of endocrine-disrupting chemicals in animals. Environ. Health Perspect. 2001, 109, 1197–1206. [Google Scholar] [CrossRef]
- Ishido, M.; Masuo, Y.; Oka, S.; Niki, E.; Morita, M. p-Nitrotoluene causes hyperactivity in the rat. Neurosci. Lett. 2004, 366, 1–5. [Google Scholar] [CrossRef]
- Xu, X.; Tian, D.; Hong, X.; Chen, L.; Xie, L. Sex-specific influence of exposure to bisphenol-A between adolescence and young adulthood on mouse behaviors. Neuropharmacology 2011, 61, 565–573. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tezlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care intervention: Explanation and elaboration. Plos Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Weintraub, K. The prevalence puzzle: Autism counts. Nature 2011, 479, 22–24. [Google Scholar] [CrossRef]
- Windham, G.C.; Zhang, L.; Gunier, R.; Croen, L.A.; Grether, J.K. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the san francisco bay area. Environ. Health Perspect. 2006, 114, 1438–1444. [Google Scholar] [CrossRef]
- Nishijo, M.; Pham, T.T.; Nguyen, A.T.N.; Tran, N.N.; Nakagawa, H.; Hoang, L.V.; Tran, A.H.; Morikawa, Y.; Ho, M.D.; Kido, T.; et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin in breast milk increases autistic traits of 3-year-old children in Vietnam. Mol. Psychiatry 2014, 19, 1220–1226. [Google Scholar] [CrossRef]
- Ribas-Fitó, N.; Torrent, M.; Carrizo, D.; Júlvez, J.; Grimalt, J.O.; Sunyer, J. Exposure to hexachlorobenzene during pregnancy and children’s social behavior at 4 years of age. Environ. Health Perspect. 2007, 115, 447–450. [Google Scholar] [CrossRef]
- Gascon, M.; Vrijheid, M.; Martínez, D.; Forns, J.; Grimalt, J.O.; Torrent, M.; Sunyer, J. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ. Int. 2011, 37, 605–611. [Google Scholar] [CrossRef]
- Miodovnik, A.; Engel, S.M.; Zhu, C.; Ye, X.; Soorya, L.V.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011, 32, 261–267. [Google Scholar] [CrossRef]
- Nowack, N.; Wittsiepe, J.; Kasper-Sonnenberg, M.; Wilhelm, M.; Schölmerich, A. Influence of Low-Level Prenatal Exposure to PCDD/Fs and PCBs on Empathizing, Systemizing and Autistic Traits: Results from the Duisburg Birth Cohort Study. PLoS ONE 2015, 10, e0129906. [Google Scholar] [CrossRef]
- Volk, H.E.; Hertz-Picciotto, I.; Delwiche, L.; Lurmann, F.; McConnell, R. Residential proximity to freeways and autism in the CHARGE study. Environ. Health Perspect. 2011, 119, 873–877. [Google Scholar] [CrossRef]
- Stein, T.P.; Schluter, M.D.; Steer, R.A.; Guo, L.; Ming, X. Bisphenol A Exposure in Children With Autism Spectrum Disorders. Autism Res. Off. J. Int. Soc. Autism Res. 2015, 8, 272–283. [Google Scholar] [CrossRef]
- Testa, C.; Nuti, F.; Hayek, J.; De Felice, C.; Chelli, M.; Rovero, P.; Latini, G.; Papini, A.M. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro 2012, 4, 223–229. [Google Scholar] [CrossRef]
- Rahbar, M.H.; Swingle, H.M.; Christian, M.A.; Hessabi, M.; Lee, M.; Pitcher, M.R.; Campbell, S.; Mitchell, A.; Krone, R.; Loveland, K.A.; et al. Environmental Exposure to Dioxins, Dibenzofurans, Bisphenol A, and Phthalates in Children with and without Autism Spectrum Disorder Living near the Gulf of Mexico. Int. J. Environ. Res. Public Health 2017, 14, 1425. [Google Scholar] [CrossRef]
- Danielson, M.L.; Bitsko, R.H.; Ghandour, R.M.; Holbrook, J.R.; Kogan, M.D.; Blumberg, S.J. Prevalence of Parent-Reported ADHD Diagnosis and Associated Treatment Among U.S. Children and Adolescents, 2016. J. Clin. Child Adolesc. Psychol. Off. J. Soc. Clin. Child Adolesc. Psychol. Am. Psychol. Assoc. Div. 2018, 53, 1–14. [Google Scholar] [CrossRef]
- Jacobson, J.L.; Jacobson, S.W. Prenatal exposure to polychlorinated biphenyls and attention at school age. J. Pediatr. 2003, 143, 780–788. [Google Scholar] [CrossRef]
- Sagiv, S.K.; Thurston, S.W.; Bellinger, D.C.; Tolbert, P.E.; Altshul, L.M.; Korrick, S.A. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am. J. Epidemiol. 2010, 171, 593–601. [Google Scholar] [CrossRef]
- Morales, E.; Julvez, J.; Torrent, M.; de Cid, R.; Guxens, M.; Bustamante, M.; Künzli, N.; Sunyer, J. Association of early-life exposure to household gas appliances and undoor nitrogen diosxide with cognition and attention behavior in preschoolers. Am. J. Epidemiol. 2009, 169, 1327–1336. [Google Scholar] [CrossRef]
- Eskenazi, B.; Chevrier, J.; Rauch, S.A.; Kogut, K.; Harley, K.G.; Johnson, C.; Trujillo, C.; Sjödin, A.; Bradman, A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ. Health Perspect. 2013, 121, 257–262. [Google Scholar] [CrossRef]
- Harley, K.G.; Gunier, R.B.; Kogut, K.; Johnson, C.; Bradman, A.; Calafat, A.M.; Eskenazi, B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ. Res. 2013, 126, 43–50. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Davis, K.; Boylan, K.; Fisher, M.; Fu, J. Bisphenol A, phthalates and lead and learning and behavioral problems in Canadian children 6–11 years of age: CHMS 2007–2009. Neurotoxicology 2016, 54, 89–98. [Google Scholar] [CrossRef]
- Janulewicz, P.; White, R.; Winter, M.; Weinberg, J.; Gallagher, L.; Vieira, V.; Webster, T.; Aschengrau, A. Risk of learning and behavioral disorders following prenatal and early postnatal exposure to tetrachloroethylene (PCE)-contaminated drinking water. Neurotoxicol Teratol. 2008, 30, 175–185. [Google Scholar] [CrossRef]
- Laslo, D.; Barrera, M.; Knittel-Keren, D.; Kozer, E.; Wolpin, J.; Khattak, S.; Hackmann, R.; Rovet, J.; Koren, G. Child Neurodevelopmental Outcome and Maternal Occupational Exposure to Solvents. Arch Pediatr Adolesc Med. 2004, 158, 956–961. [Google Scholar] [CrossRef]
- Chopra, V.; Harley, K.; Lahiff, M.; Eskenazi, B. Association between phthalates and attention deficit disorder and learning disability in U.S. children, 6–15 years. Environ. Res. 2014, 128, 64–69. [Google Scholar] [CrossRef]
- Verner, M.A.; Plusquellec, P.; Muckle, G.; Ayotte, P.; Dewailly, E.; Jacobson, S.W.; Charbonneau, M.; Haddad, S. Alteration of infant attention and activity by polychlorinated biphenyls: Unravelling critical window of susceptibility using physiologically based pharmacokinetic modeling. Neurotoxicology. 2010, 31, 424–431. [Google Scholar] [CrossRef]
- Perera, F.; Wang, S.; Vishnevetsky, J.; Zhang, B.; Cole, K.; Tang, D.; Rauh, V.; Phillips, D. Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behavior scores in New York city children. Environ. Health Perspect. 2011, 119, 1176–1181. [Google Scholar] [CrossRef]
- Hudziak, J.J.; Achenbach, T.M.; Althoff, R.R.; Pine, D.S. A dimensional approach to developmental psychopathology. Int. J. Methods Psychiatr. Res. 2007, 16, S16–S23. [Google Scholar] [CrossRef]
- Martin, J.; Hamshere, M.L.; Stergiakouli, E.; O’Donovan, M.C.; Thapar, A. Genetic risk for attention-deficit/hyperactivity disorder contributes to neurodevelopmental traits in the general population. Biol. Psychiatry 2014, 76, 664–671. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, E.-H.; Kim, E.-J.; Park, H.; Ha, M.; Kim, J.-H.; Hong, Y.-C.; Chang, N.; Kim, B.-N. Prenatal exposure to phthalates and infant development at 6 months: Prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ. Health Perspect. 2011, 119, 1495–1500. [Google Scholar] [CrossRef]
- Herbstman, J.B.; Sjödin, A.; Kurzon, M.; Lederman, S.A.; Jones, R.S.; Rauh, V.; Needham, L.L.; Tang, D.; Niedzwiecki, M.; Wang, R.Y.; et al. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect. 2010, 118, 712–719. [Google Scholar] [CrossRef]
- Perera, F.P.; Rauh, V.; Whyatt, R.M.; Tsai, W.-Y.; Tang, D.; Diaz, D.; Hoepner, L.; Barr, D.; Tu, Y.-H.; Camann, D.; et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ. Health Perspect. 2006, 114, 1287–1292. [Google Scholar] [CrossRef]
- Whyatt, R.M.; Liu, X.; Rauh, V.A.; Calafat, A.M.; Just, A.C.; Hoepner, L.; Diaz, D.; Quinn, J.; Adibi, J.; Perera, F.P.; et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ. Health Perspect. 2012, 120, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Rauh, V.A.; Garfinkel, R.; Perera, F.P.; Andrews, H.F.; Hoepner, L.; Barr, D.B.; Whitehead, R.; Tang, D.; Whyatt, R.W. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 2006, 118, e1845–e1859. [Google Scholar] [CrossRef]
- Tang, D.; Li, T.; Liu, J.J.; Zhou, Z.; Yuan, T.; Chen, Y.; Rauh, V.A.; Xie, J.; Perera, F. Effects of prenatal exposure to coal-burning pollutants on children’s development in China. Environ. Health Perspect. 2008, 116, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Li, T.; Zhou, Z.; Yuan, T.; Chen, Y.; Qu, L.; Rauh, V.A.; Zhang, Y.; Tang, D. Benefits of reducing prenatal exposure to coal-burning pollutants to children’s neurodevelopment in China. Environ. Health Perspect. 2008, 116, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-J.; Liu, X.; Guo, Y.L.; Guo, N.-W.; Yu, M.-L.; Hsu, C.-C.; Rogan, W.J. A cohort study of behavioral problems and intelligence in children with high prenatal polychlorinated biphenyl exposure. Arch. Gen. Psychiatry 2002, 59, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yolton, K.; Webster, G.M.; Sjödin, A.; Calafat, A.M.; Dietrich, K.N.; Xu, Y.; Xie, C.; Braun, J.M.; Lanphear, B.P.; et al. Prenatal PBDE and PCB Exposures and Reading, Cognition, and Externalizing Behavior in Children. Environ. Health Perspect. 2017, 125, 746–752. [Google Scholar] [CrossRef]
- Edwards, S.C.; Jedrychowski, W.; Butscher, M.; Camann, D.; Kieltyka, A.; Mroz, E.; Flak, E.; Li, Z.; Wang, S.; Rauh, V.; et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ. Health Perspect. 2010, 118, 1326–1331. [Google Scholar] [CrossRef]
- Perera, F.P.; Li, Z.; Whyatt, R.; Hoepner, L.; Wang, S.; Camann, D.; Rauh, V. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics 2009, 124, e195–e202. [Google Scholar] [CrossRef]
- Cho, S.-C.; Bhang, S.-Y.; Hong, Y.-C.; Shin, M.-S.; Kim, B.-N.; Kim, J.-W.; Yoo, H.-J.; Cho, I.H.; Kim, H.-W. Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ. Health Perspect. 2010, 118, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Till, C.; Koren, G.; Rovet, J.F. Prenatal exposure to organic solvents and child neurobehavioral performance. Neurotoxicol. Teratol. 2001, 23, 235–245. [Google Scholar] [CrossRef]
- Yolton, K.; Xu, Y.; Strauss, D.; Altaye, M.; Calafat, A.M.; Khoury, J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol. Teratol. 2011, 33, 558–566. [Google Scholar] [CrossRef]
- Braun, J.M.; Yolton, K.; Dietrich, K.N.; Hornung, R.; Ye, X.; Calafat, A.M.; Lanphear, B.P. Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect. 2009, 117, 1945–1952. [Google Scholar] [CrossRef]
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Yolton, K.; Ye, X.; Dietrich, K.N.; Lanphear, B.P. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011, 128, 873–882. [Google Scholar] [CrossRef]
- Perez-Lobato, R.; Mustieles, V.; Calvente, I.; Jimenez-Diaz, I.; Ramos, R.; Caballero-Casero, N.; López-Jiménez, F.J.; Rubio, S.; Olea, N.; Fernandez, M.F. Exposure to bisphenol A and behavior in school-age children. Neurotoxicology 2016, 53, 12–19. [Google Scholar] [CrossRef]
- Evans, S.F.; Kobrosly, R.W.; Barrett, E.S.; Thurston, S.W.; Calafat, A.M.; Weiss, B.; Stahlhut, R.; Yolton, K.; Swan, S.H. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. Neurotoxicology 2014, 45, 91–99. [Google Scholar] [CrossRef]
- Hong, S.-B.; Hong, Y.-C.; Kim, J.-W.; Park, E.-J.; Shin, M.-S.; Kim, B.-N.; Yoo, H.-J.; Cho, I.-H.; Bhang, S.-Y.; Cho, S.-C. Bisphenol A in relation to behavior and learning of school-age children. J. Child Psychol. Psychiatry 2013, 54, 890–899. [Google Scholar] [CrossRef]
- Engel, S.M.; Zhu, C.; Berkowitz, G.S.; Calafat, A.M.; Silva, M.J.; Miodovnik, A.; Wolff, M.S. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology 2009, 30, 522–528. [Google Scholar] [CrossRef]
- Philippat, C.; Nakiwala, D.; Calafat, A.M.; Botton, J.; De Agostini, M.; Heude, B.; Slama, R.; EDEN Mother–Child Study Group. Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 years. Environ. Health Perspect. 2017, 125, 097014. [Google Scholar] [CrossRef]
- Engel, S.M.; Miodovnik, A.; Canfield, R.L.; Zhu, C.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ. Health Perspect. 2010, 118, 565–571. [Google Scholar] [CrossRef]
- Swan, S.H.; Liu, F.; Hines, M.; Kruse, R.L.; Wang, C.; Redmon, J.B.; Sparks, A.; Weiss, B. Prenatal phthalate exposure and reduced masculine play in boys. Int. J. Androl. 2010, 33, 259–269. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, M.L.; Rogan, W.J.; Gladen, B.C.; Hsu, C.C. A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. Am. J. Public Health 1994, 84, 415–421. [Google Scholar] [CrossRef]
- Plusquellec, P.; Muckle, G.; Dewailly, E.; Ayotte, P.; Bégin, G.; Desrosiers, C.; Després, C.; Saint-Amour, D.; Poitras, K. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology 2010, 31, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Roze, E.; Meijer, L.; Bakker, A.; Van Braeckel, K.; Sauer, P.; Bos, A. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect. 2009, 117, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Bergman, Å.; Heindel, J.J.; Kasten, T.; Kidd, K.A.; Jobling, S.; Neira, M.; Zoeller, R.T.; Becher, G.; Bjerregaard, P.; Bornman, R.; et al. The Impact of Endocrine Disruption: A Consensus Statement on the State of the Science. Environ. Health Perspect. 2013, 121, a104–a106. [Google Scholar] [CrossRef] [PubMed]
- Fini, J.-B.; Mughal, B.B.; Le Mével, S.; Leemans, M.; Lettmann, M.; Spirhanzlova, P.; Affaticati, P.; Jenett, A.; Demeneix, B.A. Human amniotic fluid contaminants alter thyroid hormone signalling and early brain development in Xenopus embryos. Sci. Rep. 2017, 7, 43786. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Bellinger, D.C. Prenatal Exposures to Environmental Chemicals and Children’s Neurodevelopment: An Update. Saf. Health Work 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Kodavanti, P.R.S.; Curras-Collazo, M.C. Neuroendocrine actions of organohalogens: Thyroid hormones, arginine vasopressin, and neuroplasticity. Front. Neuroendocrinol. 2010, 31, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Fusani, L.; Della Seta, D.; Dessì-Fulgheri, F.; Farabollini, F. Altered reproductive success in rat pairs after environmental-like exposure to xenoestrogen. Proc. Biol. Sci. 2007, 274, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Biederman, J.; Petty, C.; Goldin, R.L.; Furtak, S.L.; Wozniak, J. Examining the comorbidity of bipolar disorder and autism spectrum disorders: A large controlled analysis of phenotypic and familial correlates in a referred population of youth with bipolar I disorder with and without autism spectrum disorders. J. Clin. Psychiatry 2013, 74, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Kebir, O.; Krebs, M.-O. Diethylstilbestrol and risk of psychiatric disorders: A critical review and new insights. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 2012, 13, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Rivollier, F.; Chaumette, B.; Bendjemaa, N.; Chayet, M.; Millet, B.; Jaafari, N.; Barhdadi, A.; Lemieux Perreault, L.-P.; Provost, S.; Dubé, M.-P.; et al. Methylomic changes in individuals with psychosis, prenatally exposed to endocrine disrupting compounds: Lessons from diethylstilbestrol. PLoS ONE 2017, 12, e0174783. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef]
- Crews, D.; Gore, A.C.; Hsu, T.S.; Dangleben, N.L.; Spinetta, M.; Schallert, T.; Anway, M.D.; Skinner, M.K. Transgenerational epigenetic imprints on mate preference. Proc. Natl. Acad. Sci. USA 2007, 104, 5942–5946. [Google Scholar] [CrossRef]
- Wolstenholme, J.T.; Taylor, J.A.; Shetty, S.R.J.; Edwards, M.; Connelly, J.J.; Rissman, E.F. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS ONE 2011, 6, e25448. [Google Scholar] [CrossRef]
- Skinner, M.K. Endocrine disruptors in 2015: Epigenetic transgenerational inheritance. Nat. Rev. Endocrinol. 2016, 12, 68–70. [Google Scholar] [CrossRef]
| Authors | Outcome | Exposure | Dose (Medians) | Population | Results |
|---|---|---|---|---|---|
| Testa et al., 2012 [32] | ASD diagnosis (DSM-IV) | Phthalates (postnatal) | Urinary samples 5-OH-mono-ethylhexin phthalates (MEHP) = 0.18 µg/L 5-oxo-MEHP = 0.096 µg/L | n = 48 ASD (mean 11 years) + 45 controls | Higher rates of 5-OH-MEHP, 5-oxo-MEHP and MEHP in ASD patients (p < 0.05). |
| Windham et al., 2006 [24] | ASD diagnosis (DSM-IV) | Air pollutants (prenatal) | Estimated concentrations by US Environmental Protection Agency (EPA) | n = 284 ASD (mean 12 years) + 657 controls | Ajusted odds-ratio (AORs) elevated by 50% in top quartile of chlorinated solvents and heavy metals (IC 1.1–2.1). |
| Volk et al., 2011 [30] | ASD diagnosis (clinical diagnosis) | Air pollutants (neonatal) | Distance to freeways | n = 304 ASD (5–14 years) + 259 controls | Association of maternal residence at birth near a freeway (≤ 309 m) with ASD odds-ratio (OR) = 1.86 (1.04–3.45). |
| Rahbar et al., 2017 [33] | ASD diagnosis (DSM-5) | BPA, phthalates, dioxins and dibenzofurans (postnatal) | Serum samples (dioxins and dibenzofurans) and urine samples (BPA and phthalates) | n = 30 ASD (2–8 years) + 10 controls | Mean concentrations did not differ between the ASD cases and control group (p ≥ 0.27). |
| Nishijo et al., 2014 [25] | ASD diagnosis (Autism Spectrum Rating Scale (ASRS) and Bailey III) | TCDD (pre and postnatal) | Milk sample | n = 153 (3 years) | The high TCDD groups showed higher ASRS scores than the mild-TCDD groups (p = 0.042). No differences in neurodevelopmental scores. |
| Stein et al., 2015 [31] | ASD diagnosis (DSM-IV-TR) | BPA (postnatal) | Urine sample | n = 46 ASD (mean 10 years) + 52 controls | Association between ASD and total BPA and bound BPA (p < 0.05). |
| Ribas et al., 2007 [26] | Social competence California preschool social competence scale (CP-SCS)) | HCB (prenatal) | Cord serum HCB = 0.73 ng/mL | n = 477 (4 years) | Association of postnatal exposure (HCB > 1.5 ng/mL) with social impairment: Relative risk (RR) 4.04 (1.76–9.58). |
| Gascon et al., 2011 [27] | Social competence (CP-SCS) | PBDE (pre and postnatal) | Cord blood PBDE 47 at birth = 2.10 ng/g lipid at 4 years = 0.12 ng/g lipid | n = 422 (4 years) | Association of postnatal exposure with social impairment: RR = 2.6 (1.2–5.9). |
| Miodovnik et al., 2011 [28] | Social competence (Social Responsiveness Scale (SRS)) | Phthalates and BPA (prenatal) | Urinary samples BPA 1.25 µg/L LMW phthalates 419 µg/L | n = 137 (7–9 years) | Association of LMWP with social impairment β = 1.53 (p < 0.05). No association with BPA. |
| Nowack et al., 2015 [29] | Autistic Traits (SRS) | Polychlorinated dibenzodioxins (PCDD) and PCB (prenatal) | Maternal blood samples PCDD = 12.91 pg/g PCB = 6.85 mg/g | n = 100 (10 years) | Negative associations between PCDD levels and SRS scores in the whole group (p < 0.05) in girls and in one subscale (social motivation) in boys. For PCB, associations with one subscale for the whole group (autistic mannerisms). |
| Authors | Outcome | Exposure | Dose (Medians) | Population | Results |
|---|---|---|---|---|---|
| Gascon et al., 2011 (2nd part) [27] | ADHD (DSM-IV Criteria, Connors Scale) | PBDE (pre and postnatal) | PBDE 47 Cord blood (birth) = 2.10 ng/g Blood sample (4 years) = 0.12 ng/g | n = 422 (4 years) | Association of postnatal exposure with ADD RR = 1.8 (1.0–3.2). No prenatal association. |
| Ribas et al., 2009 (2nd part) [26] | ADHD (DSM-IV Criteria, Connors Scale) | HCB (prenatal) | Cord serum = 0.73 ng/mL | n = 477 (4 years) | When HCB > 1.5 ng/mL, association of prenatal exposure with ADHD, RR= 2.71 (1.05–6.96). |
| Sagiv et al., 2009 [36] | ADHD (DSM-IV Criteria, Connors Scale) | PCB and p,p′-dichlorodiphenyldichloroethylene (DDE) (prenatal) | Cord serum = 0.19 ng/g lipid | n = 573 (mean 8.2 years) | Association of ADHD Index/DSM-IV criteria with PCB and p,p′-DDE levels, p < 0.05. |
| Eskenazi et al., 2013 [38] | ADHD (DSM-IV Criteria, Connors Scale) | PBDE (pre and postnatal) | Maternal serum PBDE10 = 28.7 ng/g Child serum (7 years) PBDE10 = 90.9 ng/g | n = 310 (5 years) + 323 (7 years) | Association of prenatal exposure with Connors ADHD DSM-IV total scale AOR = 2.6 (0.2–5.0) in 5-year-old children. Association of postnatal exposure with hyperactivity AOR = 4.8 (0.5–9) and inattention AOR = 2.9 (0.4–5.5) on BASC2 teacher report in 7-year-old children. |
| Morales et al., 2009 [37] | ADHD (DSM-IV Criteria Connors Scale) | Gas appliance and nitrogen dioxide (neonatal) | NO2 concentration = 15.8 ppb | n = 482 (4 years) | Association of use of gas appliances with ADHD symptoms OR = 2.72 (1.01–7.28). Association of nitrogen dioxide concentrations with ADHD symptoms, OR = 1.04 (1.00–1.09). |
| Arbuckle et al., 2016 [40] | ADHD & Learning (Strenght and difficulties questionnaire (SDQ)) | Phthalates, BPA and lead (postnatal) | Blood sample (lead) and urine sample (BPA and phthalates) | LD n = 94, ADD/ADHD n = 49, (6–11 years) | Association of lead exposure with ADHD (p = 0.047). |
| Janulewicz et al., 2008 [41] | ADHD diagnosis (clinical) | PCE (pre and postnatal) | Estimation in water by area Prenatal = 7.34 g Postnatal = 20.34 g | n = 1063 exposed vs. n = 1023 unexposed (5 years) | No association with ADHD. |
| Harley et al., 2013 [39] | Behavior ((Behavior assessment system forchildren (BASC2) & Conners Scale) | BPA (pre and postnatal) | Urinary maternal (birth) = 1.1 µg/L Child (5 years) = 2.5 µg/L (geometric mean) | n = 292 (7 years) | No association of prenatal exposure with ADHD. Association of postnatal exposure with ADHD in girls on mothers β = 1.3 (0.2–2.3) and teachers report β = 1.7 (0.3–3.1). Association with inattention in boys in teachers report β = 1.7 (0.3–3). |
| Laslo et al., 2004 [42] | Neuropsychological Profile (with Connors Rating Scale) | Organic solvents (prenatal) | Interrogation | n = 32 (3–7 years) | Association with ADHD β = 0.62 (p < 0.02). No association with IQ. |
| Chopra et al., 2014 [43] | ADHD & LD (diagnosis) | Phthalates (postnatal) | Urine sample | ADD n = 102, LD n = 173, both n = 56 (6–15 years) | Association of ADD with urinary concentration of di–2-ethylhexyl phthalates OR = 2.1 (1.1–3.9) and phthalates OR = 2.7 (1.2–6.1). No association with LD. |
| Verner et al., 2010 [44] | Behavior (Behavior rating scale (BRS) & BSID-II) | PCB (pre and postnatal) | Cord blood PCB-153 = 112.3 ng/g lipid | n = 168 (11 months) | Correlation of prenatal PCB-153 level with inattention r 0.205 (p < 0.05) and of postnatal levels with hyperactivity r 0.181 (p < 0.05). |
| Perera et al., 2011 [45] | Behavior (Child behavior checklist (CBCL)) | PAH (prenatal) | Cord blood 32P = 2.45 adducts/108 nt | n = 215 (followed 8 years) | Association of exposure and attention problems at 4,8 years β = 0.38 (0.06–0.69) and 7 years β = 0.22 (0.06–0.38) |
| Jacobson et al., 2003 [35] | ADHD like neuropsychological profile (continuous performance test (CPT), DigitCancellation, etc.) | PCB (prenatal) | Cord serum PCB = 2.7 ng/mL | n = 144 (11 years) | Correlation between exposure and attention deficit r = 0.17 and working memory r= 0.22 (p < 0.05) |
| Authors | Outcome | Exposure | Dose (Medians) | Population | Results |
|---|---|---|---|---|---|
| Kim et al., 2011 [48] | Development (BSID-II) | Phthalates (prenatal exposure) | Urinary mono (2-ethyl-5-hydroxyhexyl) phthalates (MEHHP) = 8.9 μg/L (mean) | n = 460 (6 months) | Association of mental development index (MDI) with MEHHP β = −0.97 (−1.85 −0.08) and mono (2-ethyl-5-oxohexyl) phthalates MEOHP β = −0.95 (−1.87 to −0.03). Association of PDI with MEHHP β = −1.20 (−2.33 to −0.08). |
| Herbstman et al., 2010 [49] | Development (BSID-II) and Intelligence (Wechsler preschool and primary scale of intelligence (WPPSI-R)) | BPDE (prenatal exposure) | Cord blood PBDE47 = 11.2 ng/g lipid PBDE99 = 3.2 ng/g lipid PBDE100 = 1.4 ng/g lipid | n = 96 (3 years) | Association of 24-month MDI (BDE-47, 99, and 100), 48-month full-scale β = −3.29 (−5.95 −0.63) and performance intellectual quotient (IQ) (Brominated diarylethers (BDE)-100). |
| Perera et al., 2006 [50] | Development (BSID-II/CBCL) | PAH (prenatal exposure) | Air concentrations measures | n = 181 (followed 3 years) | No association of prenatal exposure to PAHs with psychomotor developmental index (PDI) or behavioral problems. Association of high prenatal exposure to PAHs (upper quartile) with lower MDI at age 3 β = −5.69 (−9.05 to −2.33) |
| Whyatt et al., 2012 [51] | Development (BSID-II/CBCL) | Phthalates (prenatal exposure) | Urinary MiBP = 9.3 μg/L MnBP 38.0 μg/L (mean) | n = 319 (3 years) | Association of PDI scores with mono-n-butyl phthalates MnBP β = −2.81 95% (−4.63, −1.0) and monoisubitil phthalates (MiBP) β = −2.28 (−3.90, −0.67). In girls, association of MDI scores with MnBP β = −2.67 (−4.70, −0.65). |
| Rauh et al., 2006 [52] | Development (BSID-II/CBCL) | CPF (prenatal exposure) | Umbilical cord blood | n = 234 (3 years) | Association of highly exposure (>6.17 pg/g plasma) with PDI at 3 years compared with lower levels (p = 0.006). Association of highly exposure with ADHD at 3 years of age compared with those with lower levels of exposure (p = 0.018). |
| Tang et al., 2008 [53] | Development (GDS) | PAH (prenatal exposure) | Cord blood adducts = 0.32 adducts/108 nt (mean) | n = 110 (followed 2 years) | Association of increased adduct levels with decreased average GDS developmental quotient (DQ) β = −14.58 (−28.77 to −0.37). |
| Perera et al., 2008 [54] | Development (GDS) | PAH (prenatal exposure) | Cord blood adducts = 0.20 adducts/108 nt (mean) | n = 107 (followed 2 years) | No association of adduct levels with average GDS DQ. |
| Authors | Outcome | Exposure | Dose (Medians) | Population | Results |
|---|---|---|---|---|---|
| Cho et al., 2010 [59] | Intelligence (K-Wechsler intelligence scale for children (WISC)) | Phthalates (postnatal) | Urinary MEHP = 21.3 μg/L, MEOHP = 18.0 μg/L and MPB = 48.9 μg/L (geometric mean) | n = 667 (9 years) | Association of full-scale IQ and verbal IQ scores with MEHP; β = −1.93 and β = −0.91 (p < 0.05) and MEOHP metabolites β = −0.91 and β = −0.81 (p < 0.01) but not with MBP metabolites. |
| Edwards et al., 2010 [57] | Intelligence (Raven’s colored progressive matrices (RCPM)) | PAH (prenatal) | PAH in air = 39.62 ng/m3 (mean) | n = 214 (5 years) | Association of higher (>17.96 ng/m3) prenatal exposure to airborne PAHs with decreased RCPM scores at 5 years of age β = −1.36 (−2.48 −0.23). |
| Perera et al., 2009 [58] | Intelligence (WPPSI-R) | PAH (prenatal) | PAH in air = 3.48 ng/m3 (mean) | n = 249 (5 years) | Association of high PAH levels (>2.26 ng/m3) with full-scale IQ β = −4.307 (p = 0.007) and verbal IQ β = −4.668 (p = 0.003). |
| Zhang et al., 2017 [56] | Reading (Woodcock-Johnson (WJ)-III and Wide range achievement test (WRAT)-4), intelligence (WISC-IV) and Behavior (BASC-2) | PBDE and PCB (prenatal) | Maternal serum (Sum4PBDEs = 35.65 ng/g) (Sum4PCBs = 31.30 ng/g) | n = 239 (at 5 and 8 years) | Association of Sum4PBDE with reading composite score and FISQ, and externalizing problems at 8 years (not at 5). No association with Sum4PCB |
| Lai et al., 2002 [55] | Behavior (WPPSI-R, CBCL and Rutter’s) | PCBs (prenatal) | Interrogation (contaminated oil) | n = 118 (followed 4 years) + 118 controls | Exposed children scored 3 points lower than controls for IQ (p < 0.05, 3 points higher on the CBCL (p = 0.002) and 6 points higher (p = 0.001) on the Rutter’s scale. |
| Authors | Outcome | Exposure | Dose | Population | Results |
|---|---|---|---|---|---|
| Till et al., 2001 [60] | Behavior (NEPSY and CPT) | Organic solvents (prenatal) | Interrogation/checklist | n = 33 (3–7 years) | Association of high exposure with difficulties in receptive (p = 0.025) and expressive (p = 0.044) language as with graphomotor abilities (p = 0.004) when compared to low exposure. |
| Authors | Outcome | Exposure | Dose (Medians) | Population | Results |
|---|---|---|---|---|---|
| Chen et al., 1994 [71] | Behavior (Rutter’s Child Behavior Scale and Werry Weiss Peter’s Activity Scale) | PCB (prenatal) | Interrogation (contaminated oil) | n = 115 (followed 6 years) + 115 controls | Exposed children found to have scores 7% to 43% (mean = 23%) higher than the control children on the Rutter scale at every time point. |
| Braun et al., 2011 [63] | Behavior (BASC2 and behavior rating inventory of executive function (BRIEF)). | BPA (pre and postnatal) | Urinary BPA = 1.2 ng/mL | n = 239 (followed 3 years) | In girls, association of prenatal exposure to BPA in with BASC-2 and BRIEF-P scores (increased 9 to 12 points). No associations of postnatal BPA exposure with behavior. |
| Braun et al., 2009 [62] | Behavior (BASC2) | BPA (prenatal) | Urinary BPA = 1.3 ng/mL | n = 249 (2 years) | In girls, association of BPA exposure with externalizing scores β = 6.0 (0.1–12.0). |
| Plusquellec et al., 2010 [72] | Behavior (BSID-II) | PCBs, Hg and Pb (pre and postnatal) | Cord blood: PCB-153 = 120.6 µg/kl Pb = 5µg/dL, Hg = 22.2 µg/L (mean). 5 years old blood: PCB-153 =159 µg/dL Pb = 5.4 µg/dL Hg = 9.6 µg/L (mean) | n = 110 (5 years) | Association of postnatal exposure to Pb with impulsivity and irritability β = 0.20 (p < 0.05). Association of prenatal exposure with PCB 153 with anxiety β = 0.26 (p < 0.01). Association of postnatal exposure with global activity latency β = −0.25 (p < 0.05). |
| Perez et al., 2015 [64] | Behavior (CBCL) | BPA (postnatal) | Urine sample = 18.48 microg/L | n = 300 (9–11 years) | Higher BPA concentrations associated with worse behavioral scores on several syndrome scores such as somatic complaints (p = 0.015), social problems (p = 0.043) and thought problems (p = 0.017). |
| Evans et al., 2014 [65] | Behavior (CBCL) | BPA (prenatal) | Urine sample at 27 weeks of pregnancy 1.10 microg/L | n = 153 (6–10 years) | We observed a significant interaction between maternal urinary BPA and sex for several behaviors (externalizing, aggression, anxiety disorder, oppositional/defiant disorder and conduct disorder traits), but no significant associations between BPA and scores on any CBCL scales. |
| Hong et al., 2013 [66] | Behavior (CBCL/Learning Disability Evaluation Scale (LDES)) | BPA (postnatal) | Urinary BPA = 1.32 lg/g Cr (geometric mean) | n = 1089 (8–11 years) | Significant (p < 0.05) association between exposure and internalizing problems β = 1.07, attention problems β = 1.22, social problems β = 0.93 and anxiety/depression β = 0.66. On the LEDS, significant associations were found for thinking β = −0.36, writing β = −0.31, calculations β = −0.43 and learning quotient β = −1.90. |
| Yolton et al., 2011 [61] | Behavior (NICU Network Neurobehavioral Scale (NNNS)) | BPA (prenatal) | Urinary BPA = 1.7 ng/mL (mean at 26 weeks) | n = 350 (5 weeks) | No association of prenatal exposure to BPA was found with neurobehavioral traits. |
| Yolton et al., 2011 (2nd part) [61] | Behavior (NNNS) | Phthalates (prenatal) | Urinary DBP sum = 113 μM/L DHEP sum = 245 μM/L (mean) | n = 350 (5 weeks) | Association of higher DBP metabolites with decreased regulation (p = 0.04), decreased handling (p = 0.02). Association of higher total (DEHP) metabolites with more nonoptimal reflexes (p = 0.03). |
| Swan et al., 2010 [70] | Behavior (Pediatric Attachment Style Indicator (PASI)) | Phthalates (prenatal) | Urinary DBP sum in boys = 35.6 μM/L in girls = 42.5 μM/L DHEP sum in boys = 23.4 μM/L in girls = 27.0 μM/L (mean) | n = 143 (mean 60 months for boy and 59 months for girls) | Associations of MiBP and DHEP with a decreased (less masculine) composite score in boys r = −4.53 and −4.20 (p = 0.01 and 0.04). Associations of MEHHP and MEOHP and the DEHP sum with decreased masculine score r = −3.29, −2.94 and −3.18 (p = 0.02, 0.04 and 0.04), respectively. |
| Philippat et al., 2017 [68] | Behavior (SDQ) | Phthalates and phenols (among them is BPA) (prenatal) | Urine sample during pregnancy | n = 529 (3.1 years old) and n = 464 (5.6 years) | BPA was associated with relationship problems at 3 years and hyperactivity/inattention at 5. MnBP was associated with internalizing behavior, relationship problem and emotional symptoms at 3. MPzP was associated with internalizing problems and relationship problems at 3. |
| Engel et al., 2010 [69] | Behavior and Cognitive functioning (BASC and BRIEF) | Phthalates (prenatal) | Urinary low-molecular-weight phthalates LMWP = 1.88 μM/L | n = 188 children (4–9 years) | Association of LMWP exposure with conduct problems β = 2.40 (1.34–3.46) |
| Engel et al., 2009 [67] | Neonatal Behavior (Brazelton Neonatal Behavioral Assessment Scale BNBAS) | Phthalates (prenatal) | Urinary Sum LMW = 2.23 μM/L Sum high molecular weight (HMW) = 0.46 μM/L | n = 295 (5 days) | No associations of exposure to phthalates and BNBAS scores. |
| Roze et al., 2009 [73] | Neuropsychological Profile (WPSSI-R and NEPSY-II) | Organohalogens (prenatal) | Maternal serum BDE-47a = 0.9 ng/g lipid (mean) | n = 62 (5–6 years) | For specified metabolites, association of exposure to brominated flame retardants with worse attention, better coordination, better behavior and better total intelligence. Association of exposure to chlorinated OHCs with less choreiform dyskinesia, worse fine manipulative abilities, worse inhibition and worst behavior. Association of PCP with worse coordination and worse performance intelligence. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivollier, F.; Krebs, M.-O.; Kebir, O. Perinatal Exposure to Environmental Endocrine Disruptors in the Emergence of Neurodevelopmental Psychiatric Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1318. https://doi.org/10.3390/ijerph16081318
Rivollier F, Krebs M-O, Kebir O. Perinatal Exposure to Environmental Endocrine Disruptors in the Emergence of Neurodevelopmental Psychiatric Diseases: A Systematic Review. International Journal of Environmental Research and Public Health. 2019; 16(8):1318. https://doi.org/10.3390/ijerph16081318
Chicago/Turabian StyleRivollier, Fabrice, Marie-Odile Krebs, and Oussama Kebir. 2019. "Perinatal Exposure to Environmental Endocrine Disruptors in the Emergence of Neurodevelopmental Psychiatric Diseases: A Systematic Review" International Journal of Environmental Research and Public Health 16, no. 8: 1318. https://doi.org/10.3390/ijerph16081318
APA StyleRivollier, F., Krebs, M.-O., & Kebir, O. (2019). Perinatal Exposure to Environmental Endocrine Disruptors in the Emergence of Neurodevelopmental Psychiatric Diseases: A Systematic Review. International Journal of Environmental Research and Public Health, 16(8), 1318. https://doi.org/10.3390/ijerph16081318





