Bone-Loading Physical Activity and Alcohol Intake but not BMI Affect Areal Bone Mineral Density in Young College-Aged Korean Women: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Anthropometry and Body Composition
2.3. Assessment of Alcohol Consumption
2.4. Areal Bone Mineral Density
2.5. Bone-Loading Physical Activity
2.6. Calcium Intake
2.7. Statistical Analyses
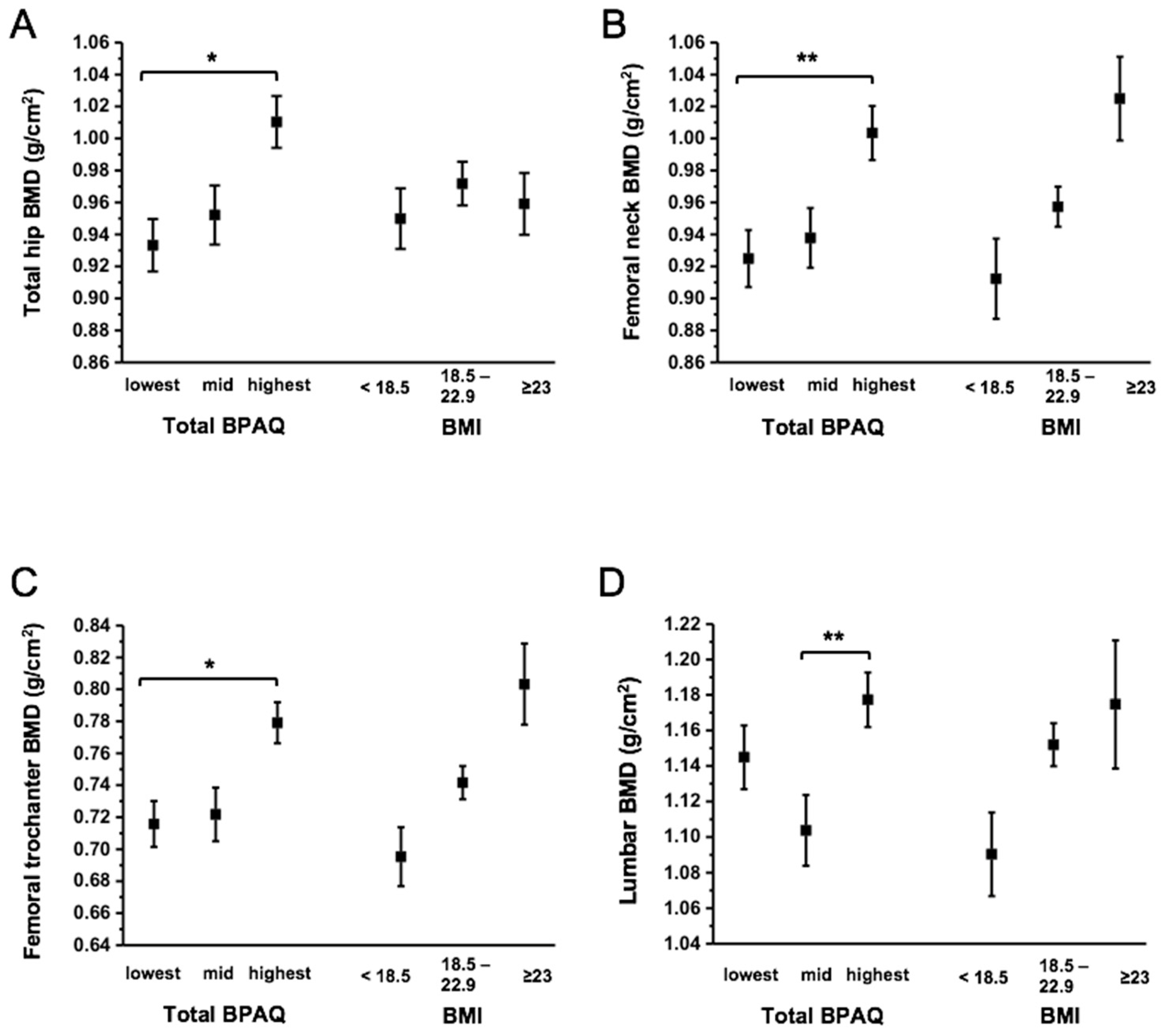
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General; Office of the Surgeon General: Rockville, MD, USA, 2004; p. 16.
- Kohrt, W.M.; Bloomfield, S.A.; Little, K.D.; Nelson, M.E.; Yingling, V.R. American College of Sports M: American College of Sports Medicine Position Stand: Physical activity and bone health. Med. Sci. Sports Exerc. 2004, 36, 1985–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; So, W.Y.; Kim, J.; Sung, D.J. Relationship between Bone-Specific Physical Activity Scores and Measures for Body Composition and Bone Mineral Density in Healthy Young College Women. PLoS ONE 2016, 11, e0162127. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lombardi, G.; Jiao, W.; Banfi, G. Effects of Exercise on Bone Status in Female Subjects, from Young Girls to Postmenopausal Women: An Overview of Systematic Reviews and Meta-Analyses. Sports Med. 2016, 46, 1165–1182. [Google Scholar] [CrossRef] [PubMed]
- Nikander, R.; Sievanen, H.; Heinonen, A.; Daly, R.M.; Uusi-Rasi, K.; Kannus, P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010, 8, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welten, D.C.; Kemper, H.C.; Post, G.B.; van Staveren, W.A. A meta-analysis of the effect of calcium intake on bone mass in young and middle aged females and males. J. Nutr. 1995, 125, 2802–2813. [Google Scholar] [CrossRef] [PubMed]
- Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health (NSDUH); Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2015.
- Turner, R.T. Skeletal response to alcohol. Alcohol. Clin. Exp. Res. 2000, 24, 1693–1701. [Google Scholar] [CrossRef]
- Laitinen, K.; Kärkkäinen, M.; Lalla, M.; Lamberg-Allardt, C.; Tunninen, R.; Tähtelä, R.; Välimäki, M. Is alcohol an osteoporosis-inducing agent for young and middle-aged women? Metabolism 1993, 42, 875–881. [Google Scholar] [CrossRef]
- Gaddini, G.W.; Turner, R.T.; Grant, K.A.; Iwaniec, U.T. Alcohol: A Simple Nutrient with Complex Actions on Bone in the Adult Skeleton. Alcohol. Clin. Exp. Res. 2016, 40, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Schapira, D. Alcohol abuse and osteoporosis. Semin. Arthr. Rheum. 1990, 19, 371–376. [Google Scholar] [CrossRef]
- Sampson, H.W. Alcohol and other factors affecting osteoporosis risk in women. Alcohol. Res. Health 2002, 26, 292–298. [Google Scholar]
- Felson, D.T.; Zhang, Y.; Hannan, M.T.; Kannel, W.B.; Kiel, D.P. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am. J. Epidemiol. 1995, 142, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wosje, K.S.; Kalkwarf, H.J. Bone density in relation to alcohol intake among men and women in the United States. Osteoporos. Int. 2007, 18, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Sripanyakorn, S.; Jugdaohsingh, R.; Mander, A.; Davidson, S.L.; Thompson, R.P.; Powell, J.J. Moderate ingestion of alcohol is associated with acute ethanol-induced suppression of circulating CTX in a PTH-independent fashion. J. Bone Miner. Res. 2009, 24, 1380–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.; Choi, S.; Kim, K.; Lee, G.; Park, S.M. Association between alcohol consumption and bone mineral density in elderly Korean men and women. Arch. Osteoporos. 2018, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Paccou, J.; Edwards, M.H.; Ward, K.; Jameson, K.; Moon, R.; Dennison, E.; Cooper, C. Relationships between bone geometry, volumetric bone mineral density and bone microarchitecture of the distal radius and tibia with alcohol consumption. Bone 2015, 78, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Eleftheriou, K.I.; Rawal, J.S.; James, L.E.; Payne, J.R.; Loosemore, M.; Pennell, D.J.; World, M.; Drenos, F.; Haddad, F.S.; Humphries, S.E.; et al. Bone structure and geometry in young men: The influence of smoking, alcohol intake and physical activity. Bone 2013, 52, 17–26. [Google Scholar] [CrossRef]
- Jang, H.D.; Hong, J.Y.; Han, K.; Lee, J.C.; Shin, B.J.; Choi, S.W.; Suh, S.W.; Yang, J.H.; Park, S.Y.; Bang, C. Relationship between bone mineral density and alcohol intake: A nationwide health survey analysis of postmenopausal women. PLoS ONE 2017, 12, e0180132. [Google Scholar] [CrossRef] [Green Version]
- Hyeon, J.H.; Gwak, J.S.; Hong, S.W.; Kwon, H.; Oh, S.W.; Lee, C.M. Relationship between bone mineral density and alcohol consumption in Korean men: The Fourth Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2009. Asia Pac. J. Clin. Nutr. 2016, 25, 308–315. [Google Scholar]
- Seo, S.; Chun, S.; Newell, M.A.; Yun, M. Association between alcohol consumption and Korean young women’s bone health: A cross sectional study from the 2008 to 2011 Korea National Health and Nutrition Examination Survey. BMJ Open 2015, 5, e007914. [Google Scholar] [CrossRef] [Green Version]
- LaBrie, J.W.; Boyle, S.; Earle, A.; Almstedt, H.C. Heavy episodic drinking is associated with poorer bone health in adolescent and young adult women. J. Stud. Alcohol. Drugs 2018, 79, 391–398. [Google Scholar] [CrossRef]
- Tereszkowski, C.M.; Simpson, J.A.; Whiting, S.J.; Buchholz, A.C. Body mass, vitamin D and alcohol intake, lactose intolerance, and television watching influence bone mineral density of young, healthy Canadian women. J. Am. Coll. Nutr. 2012, 31, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Hervás, G.; Ruiz-Litago, F.; Irazusta, J.; Irazusta, A.; Sanz, B.; Gil-Goikouria, J.; Fraile-Bermudez, A.B.; Pérez-Rodrigo, C.; Zarrazquin, I. Bone Health and Its Relationship with Impact Loading and the Continuity of Physical Activity throughout School Periods. Int. J. Environ. Res. Public Health 2019, 16, 2834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindler, J.M.; Ross, H.L.; Laing, E.M.; Modlesky, C.M.; Pollock, N.K.; Baile, C.A.; Lewis, R.D. Load-specific physical activity scores are related to tibia bone architecture. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Snow-Harter, C.; Bouxsein, M.L.; Lewis, B.T.; Carter, D.R.; Marcus, R. Effects of resistance and endurance exercise on bone mineral status of young women: A randomized exercise intervention trial. J. Bone Miner. Res. 1992, 7, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Rantalainen, T.; Heinonen, A.; Linnamo, V.; Komi, P.V.; Takala, T.E.; Kainulainen, H. Short-term bone biochemical response to a single bout of high-impact exercise. J. Sports Sci. Med. 2009, 8, 553–559. [Google Scholar]
- Mann, V.; Huber, C.; Kogianni, G.; Jones, D.; Noble, B. The influence of mechanical stimulation on osteocyte apoptosis and bone viability in human trabecular bone. J. Musculoskelet. Neuronal Interact. 2006, 6, 408–417. [Google Scholar]
- Weeks, B.K.; Beck, B.R. The BPAQ: A bone-specific physical activity assessment instrument. Osteoporos. Int. 2008, 19, 1567–1577. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Baker, B.S.; Sharma-Ghimire, P.; Bemben, D.A.; Bemben, M.G. Association between bone-specific physical activity scores and pQCT-derived measures of bone strength and geometry in healthy young and middle-aged premenopausal women. Arch. Osteoporos. 2018, 13, 83. [Google Scholar] [CrossRef] [Green Version]
- Rantalainen, T.; Weeks, B.K.; Nogueira, R.C.; Beck, B.R. Effects of bone-specific physical activity, gender and maturity on tibial cross-sectional bone material distribution: A cross-sectional pQCT comparison of children and young adults aged 5–29 years. Bone 2015, 72, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Cho, H.N.; Choi, E.; Seo, D.H.; Kim, S.; Park, Y.R.; Choi, K.S.; Rhee, Y. Self-perceptions of body weight status according to age-groups among Korean women: A nationwide population-based survey. PLoS ONE 2019, 14, e0210486. [Google Scholar] [CrossRef]
- Wu, S.F.; Du, X.J. Body Mass Index May Positively Correlate with Bone Mineral Density of Lumbar Vertebra and Femoral Neck in Postmenopausal Females. Med. Sci. Monit. 2016, 22, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Laet, C.; Kanis, J.A.; Oden, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.A.; Kroger, H.; Fujiwara, S.; Garnero, P.; et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Chia, S.C.; Henry, C.J. Body composition in Asians and Caucasians: Comparative analyses and influences on cardiometabolic outcomes. Adv. Food Nutr. Res. 2015, 75, 97–154. [Google Scholar] [PubMed]
- Castro, J.P.; Joseph, L.A.; Shin, J.J.; Arora, S.K.; Nicasio, J.; Shatzkes, J.; Raklyar, I.; Erlikh, I.; Pantone, V.; Bahtiyar, G.; et al. Differential effect of obesity on bone mineral density in White, Hispanic and African American women: A cross sectional study. Nutr. Metab. 2005, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, W.H.; Yeh, W.T. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: An extension of Asian-Pacific recommendations. Asia Pac. J. Clin. Nutr. 2008, 17, 370–374. [Google Scholar] [PubMed]
- O’Leary, C.M. Fetal alcohol syndrome: Diagnosis, epidemiology, and developmental outcomes. J. Paediatr. Child Health 2004, 40, 2–7. [Google Scholar] [CrossRef]
- Alcohol Intake Guidelines Korea. Edited by (KCDC) KCfDCP. Available online: http://health.cdc.go.kr/health/HealthInfoArea/HealthInfo/View.do?idx=14520 (accessed on 17 December 2018).
- Bone-Specific Physical Activity Questionnaire and Calculator. Available online: http://www.fithdysign.com/BPAQ/ (accessed on 5 April 2018).
- Jugdaohsingh, R.; O’Connell, M.A.; Sripanyakorn, S.; Powell, J.J. Moderate alcohol consumption and increased bone mineral density: Potential ethanol and non-ethanol mechanisms. Proc. Nutr. Soc. 2006, 65, 291–310. [Google Scholar] [CrossRef] [Green Version]
- Rico, H.; Gallego-Lago, J.L.; Hernández, E.R.; Villa, L.F.; Sanchez-Atrio, A.; Seco, C.; Gérvas, J.J. Effect of silicon supplement on osteopenia induced by ovariectomy in rats. Calcif. Tissue Int. 2000, 66, 53–55. [Google Scholar] [CrossRef]
- Modlesky, C.M.; Lewis, R.D. Does exercise during growth have a long-term effect on bone health? Exerc. Sport Sci. Rev. 2002, 30, 171–176. [Google Scholar] [CrossRef]
| Variables | Mean ± SEM | Range |
|---|---|---|
| Age (years) | 21.9 ± 0.2 | 19.0–26.8 |
| Height (cm) | 161.9 ± 0.5 | 148.0–174.0 |
| Body Mass (kg) | 53.4 ± 0.6 | 39.0–68.6 |
| Body mass index (kg/m2) | 20.4 ± 0.2 | 15.8–26.3 |
| Menarche age (years) | 12.5 ± 0.1 | 9.0–16.0 |
| Fat-free mass (kg) | 39.9 ± 0.3 | 31.5–47.7 |
| % body fat (%) | 25.0 ± 0.4 | 15.2–35.6 |
| Calcium (mg/day) | 394.2 ± 21.3 | 24.7–1070.3 |
| Total BPAQ | 16.5 ± 1.0 | 1.3–49.3 |
| aBMD (g/cm2) | ||
| Total hip | 0.966 ± 0.010 | 0.727–1.251 |
| Femoral neck | 0.956 ± 0.011 | 0.693–1.242 |
| Femoral trochanter | 0.739 ± 0.009 | 0.475–1.027 |
| Lumbar spine | 1.141 ± 0.011 | 0.907–1.423 |
| Classifications | n (%) | Total Hip | Femoral Neck | Femoral Trochanter | Lumbar Spine |
|---|---|---|---|---|---|
| Frequency of drink | |||||
| Never | 14 (12.5) | 0.965 ± 0.018 | 0.956 ± 0.019 | 0.747 ± 0.014 | 1.118 ± 0.035 |
| Less than 1 per month | 30 (26.8) | 0.947 ± 0.023 | 0.948 ± 0.024 | 0.715 ± 0.021 | 1.143 ± 0.021 |
| 2–4 times per month | 52 (46.4) | 0.951 ± 0.013 | 0.940 ± 0.014 | 0.731 ± 0.011 | 1.125 ± 0.013 |
| ≥2 times per week | 16 (14.3) | 1.052 ± 0.030 a | 1.024 ± 0.031 b | 0.806 ± 0.025 b,c | 1.213 ± 0.030 |
| p-value | 0.002 | 0.049 | 0.008 | 0.128 | |
| Drinking amount per occasion | |||||
| 0–1 glasses | 17 (15.2) | 0.951 ± 0.018 | 0.940 ± 0.022 | 0.735 ± 0.015 | 1.119 ± 0.029 |
| 2–5 glasses | 23 (20.5) | 0.968 ± 0.025 | 0.967 ± 0.027 | 0.735 ± 0.022 | 1.134 ± 0.020 |
| 6–9 glasses | 40 (35.7) | 0.983 ± 0.018 | 0.964 ± 0.017 | 0.751 ± 0.017 | 1.145 ± 0.018 |
| 10 and more glasses | 32 (28.6) | 0.951 ± 0.019 | 0.947 ± 0.021 | 0.731 ± 0.015 | 1.156 ± 0.020 |
| p-value | 0.419 | 0.703 | 0.944 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, D.J.; Singh, H.; Oh, S.-B.; Kim, S. Bone-Loading Physical Activity and Alcohol Intake but not BMI Affect Areal Bone Mineral Density in Young College-Aged Korean Women: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 5063. https://doi.org/10.3390/ijerph16245063
Sung DJ, Singh H, Oh S-B, Kim S. Bone-Loading Physical Activity and Alcohol Intake but not BMI Affect Areal Bone Mineral Density in Young College-Aged Korean Women: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2019; 16(24):5063. https://doi.org/10.3390/ijerph16245063
Chicago/Turabian StyleSung, Dong Jun, Harshvardhan Singh, Seung-Bum Oh, and SoJung Kim. 2019. "Bone-Loading Physical Activity and Alcohol Intake but not BMI Affect Areal Bone Mineral Density in Young College-Aged Korean Women: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 16, no. 24: 5063. https://doi.org/10.3390/ijerph16245063
APA StyleSung, D. J., Singh, H., Oh, S.-B., & Kim, S. (2019). Bone-Loading Physical Activity and Alcohol Intake but not BMI Affect Areal Bone Mineral Density in Young College-Aged Korean Women: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 16(24), 5063. https://doi.org/10.3390/ijerph16245063






