Effects of Heavy Metal Exposure on Shipyard Welders: A Cautionary Note for 8-Hydroxy-2′-Deoxyguanosine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Exposure to Metals in Workplace Air
2.3. Urinary Biomarker Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization; International Agency for Research on Cancer. Chromium, Nickel and Welding. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1990; Volume 49, pp. 1–648. [Google Scholar]
- Ward, E.M.; Schulte, P.A.; Straif, K.; Hopf, N.B.; Caldwell, J.C.; Carreon, T.; DeMarini, D.M.; Fowler, B.A.; Goldstein, B.D.; Hemminki, K.; et al. Research recommendations for selected IARC-classified agents. Environ. Health Perspect. 2010, 118, 1355–1362. [Google Scholar] [CrossRef]
- Guha, N.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Vilahur, N.; Muller, K.; Straif, K.; et al. Carcinogenicity of welding, molybdenum trioxide, and indium tin oxide. Lancet Oncol. 2017, 18, 581–582. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer, Welding, Molybdenum Trioxide, and Indium Tin Oxide. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer Monographs Working Group, Ed.; International Agency for Research on Cancer: Lyon, France, 2018; Volume 118. [Google Scholar]
- Vallieres, E.; Pintos, J.; Lavoue, J.; Parent, M.E.; Rachet, B.; Siemiatycki, J. Exposure to welding fumes increases lung cancer risk among light smokers but not among heavy smokers: Evidence from two case-control studies in Montreal. Cancer Med. 2012, 1, 47–58. [Google Scholar] [CrossRef]
- Gustavsson, P.; Jakobsson, R.; Johansson, H.; Lewin, F.; Norell, S.; Rutkvist, L.E. Occupational exposures and squamous cell carcinoma of the oral cavity, pharynx, larynx, and oesophagus: A case-control study in Sweden. Occup. Environ. Med. 1998, 55, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Awan, K.H.; Hegde, R.; Cheever, V.J.; Carroll, W.; Khan, S.; Patil, S.; Warnakulasuriya, S. Oral and pharyngeal cancer risk associated with occupational carcinogenic substances: Systematic review. Head Neck 2018, 40, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Puntoni, R.; Merlo, F.; Borsa, L.; Reggiardo, G.; Garrone, E.; Ceppi, M. A historical cohort mortality study among shipyard workers in Genoa, Italy. Am. J. Ind. Med. 2001, 40, 363–370. [Google Scholar] [CrossRef]
- Antonini, J.M. Health effects of welding. Crit. Rev. Toxicol. 2003, 33, 61–103. [Google Scholar] [CrossRef]
- United States Occupational Safety and Health Administration Shipbuilding and Ship Repair. Available online: https://www.osha.gov/SLTC/shipbuildingrepair/ (accessed on 23 May 2019).
- Ennan, A.A.; Kiro, S.A.; Oprya, M.V.; Vishnyakov, V.I. Particle size distribution of welding fume and its dependency on conditions of shielded metal arc welding. J. Aerosol Sci. 2013, 64, 103–110. [Google Scholar] [CrossRef]
- Hewett, P. Estimation of regional pulmonary deposition and exposure for fumes from SMAW and GMAW mild and stainless steel consumables. Am. Ind. Hyg. Assoc. J. 1995, 56, 136–142. [Google Scholar] [CrossRef]
- Lockey, J.E.; Schenker, M.B.; Howden, D.G.; Desmeules, M.J.; Saracci, R.; Sprince, N.L.; Harber, P.I. Current issues in occupational lung disease. Am. Rev. Respir. Dis. 1988, 138, 1047–1050. [Google Scholar]
- Erhola, M.; Toyokuni, S.; Okada, K.; Tanaka, T.; Hiai, H.; Ochi, H.; Uchida, K.; Osawa, T.; Nieminen, M.M.; Alho, H.; et al. Biomarker evidence of DNA oxidation in lung cancer patients: Association of urinary 8-hydroxy-2′-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Lett. 1997, 409, 287–291. [Google Scholar] [CrossRef]
- Toraason, M.; Hayden, C.; Marlow, D.; Rinehart, R.; Mathias, P.; Werren, D.; Olsen, L.D.; Neumeister, C.E.; Mathews, E.S.; Cheever, K.L.; et al. DNA strand breaks, oxidative damage, and 1-OH pyrene in roofers with coal-tar pitch dust and/or asphalt fume exposure. Int. Arch. Occup. Environ. Health 2001, 74, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chiu, A.; Chen, C.T.; Halliwell, B.; Castranova, V.; Vallyathan, V. Reduction of chromium(VI) and its relationship to carcinogenesis. J. Toxicol. Environ. Health B Crit. Rev. 1999, 2, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Kelly, F.J. Oxidative stress: its role in air pollution and adverse health effects. Occup. Environ. Med. 2003, 60, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Chiou, C.C.; Chang, P.Y.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 2004, 339, 1–9. [Google Scholar] [CrossRef]
- Bartsch, H. Studies on biomarkers in cancer etiology and prevention: a summary and challenge of 20 years of interdisciplinary research. Mutat. Res. 2000, 462, 255–279. [Google Scholar] [CrossRef]
- Lai, C.Y.; Lai, C.H.; Chuang, H.C.; Pan, C.H.; Yen, C.C.; Lin, W.Y.; Chen, J.K.; Lin, L.Y.; Chuang, K.J. Physicochemistry and cardiovascular toxicity of metal fume PM2.5: a study of human coronary artery endothelial cells and welding workers. Sci. Rep. 2016, 6, 33515. [Google Scholar] [CrossRef]
- Graczyk, H.; Lewinski, N.; Zhao, J.; Sauvain, J.J.; Suarez, G.; Wild, P.; Danuser, B.; Riediker, M. Increase in oxidative stress levels following welding fume inhalation: A controlled human exposure study. Part. Fibre Toxicol. 2016, 13, 31. [Google Scholar] [CrossRef]
- Nuernberg, A.M.; Boyce, P.D.; Cavallari, J.M.; Fang, S.C.; Eisen, E.A.; Christiani, D.C. Urinary 8-isoprostane and 8-OHdG concentrations in boilermakers with welding exposure. J. Occup. Environ. Med. 2008, 50, 182–189. [Google Scholar] [CrossRef]
- World Health Organization Office of Occupational Health. Biological Monitoring of Chemical Exposure in the Workplace: Guidelines; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.W.; Wang, C.J.; Chang, L.W.; Chao, M.R. Clinical-scale high-throughput analysis of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine by isotope-dilution liquid chromatography-tandem mass spectrometry with on-line solid-phase extraction. Clin. Chem. 2006, 52, 1381–1388. [Google Scholar] [CrossRef]
- Kosugi, H.; Enomoto, H.; Ishizuka, Y.; Kikugawa, K. Variations in the level of urinary thiobarbituric acid reactant in healthy humans under different physiological conditions. Biol. Pharm. Bull. 1994, 17, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, H.; Higgins, V.; Woroch, A.; Asgari, S.; Adeli, K. Pediatric reference intervals for clinical chemistry assays on Siemens ADVIA XPT/1800 and Dimension EXL in the CALIPER cohort of healthy children and adolescents. Clin. Chim. Acta 2019, 490, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Pesch, B.; Lotz, A.; Koch, H.M.; Marczynski, B.; Casjens, S.; Kafferlein, H.U.; Welge, P.; Lehnert, M.; Heinze, E.; Van Gelder, R.; et al. Oxidatively damaged guanosine in white blood cells and in urine of welders: Associations with exposure to welding fumes and body iron stores. Arch. Toxicol. 2015, 89, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hedmer, M.; Wojdacz, T.; Hossain, M.B.; Lindh, C.H.; Tinnerberg, H.; Albin, M.; Broberg, K. Oxidative stress, telomere shortening, and DNA methylation in relation to low-to-moderate occupational exposure to welding fumes. Environ. Mol. Mutagen. 2015, 56, 684–693. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhang, X.; Wang, X.C.; Jin, L.F.; Yang, Z.P.; Jiang, C.X.; Chen, Q.; Ren, X.B.; Cao, J.Z.; Wang, Q.; et al. Chronic occupational exposure to hexavalent chromium causes DNA damage in electroplating workers. BMC Public Health 2011, 11, 224. [Google Scholar] [CrossRef]
- Kuo, H.W.; Chang, S.F.; Wu, K.Y.; Wu, F.Y. Chromium (VI) induced oxidative damage to DNA: Increase of urinary 8-hydroxydeoxyguanosine concentrations (8-OHdG) among electroplating workers. Occup. Environ. Med. 2003, 60, 590–594. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lin, J.M.; Lin, W.Y.; Chang, C.W. Cancer risk assessment for occupational exposure to chromium and nickel in welding fumes from pipeline construction, pressure container manufacturing, and shipyard building in Taiwan. J. Occup. Health 2018, 60, 515–524. [Google Scholar] [CrossRef]
- Hewett, P. The particle size distribution, density, and specific surface area of welding fumes from SMAW and GMAW mild and stainless steel consumables. Am. Ind. Hyg. Assoc. J. 1995, 56, 128–135. [Google Scholar] [CrossRef]
- Zimmer, A.T.; Biswas, P. Characterization of the aerosols resulting from arc welding processes. J. Aerosol Sci. 2001, 32, 993–1008. [Google Scholar] [CrossRef]
- Cena, L.G.; Chisholm, W.P.; Keane, M.J.; Cumpston, A.; Chen, B.T. Size Distribution and Estimated Respiratory Deposition of Total Chromium, Hexavalent Chromium, Manganese, and Nickel in Gas Metal Arc Welding Fume Aerosols. Aerosol Sci. Technol. 2014, 48, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Park, J.S.; Kim, P.G. Characterization of Total and Size-Fractionated Manganese Exposure by Work Area in a Shipbuilding Yard. Saf. Health Work 2016, 7, 150–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemmar, A.; Hoet, P.H.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Henkler, F.; Brinkmann, J.; Luch, A. The role of oxidative stress in carcinogenesis induced by metals and xenobiotics. Cancers 2010, 2, 376–396. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kaundal, R.K.; Iyer, S.; Sharma, S.S. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci. 2007, 80, 1236–1244. [Google Scholar] [CrossRef]
- Lupescu, A.; Jilani, K.; Zelenak, C.; Zbidah, M.; Qadri, S.M.; Lang, F. Hexavalent chromium-induced erythrocyte membrane phospholipid asymmetry. Biometals 2012, 25, 309–318. [Google Scholar] [CrossRef]
- Petersen, R.; Thomsen, J.F.; Jorgensen, N.K.; Mikkelsen, S. Half life of chromium in serum and urine in a former plasma cutter of stainless steel. Occup. Environ. Med. 2000, 57, 140–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaki, M.; Suwazono, Y.; Kido, T.; Nishijo, M.; Honda, R.; Kobayashi, E.; Nogawa, K.; Nakagawa, H. Estimation of biological half-life of urinary cadmium in inhabitants after cessation of environmental cadmium pollution using a mixed linear model. Food Addit. Contam. Part A 2015, 32, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Amzal, B.; Julin, B.; Vahter, M.; Wolk, A.; Johanson, G.; Akesson, A. Population toxicokinetic modeling of cadmium for health risk assessment. Environ. Health Perspect. 2009, 117, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Colli, G.; Terzi, R.; Terzi, M.; Catenacci, G. Application of mathematical modelling for assessing the urinary half-times of nickel in stainless steel welders. G. Ital. Med. Lav. Ergon. 2005, 27, 427–430. [Google Scholar] [PubMed]
- Weiss, T.; Pesch, B.; Lotz, A.; Gutwinski, E.; Van Gelder, R.; Punkenburg, E.; Kendzia, B.; Gawrych, K.; Lehnert, M.; Heinze, E.; et al. Levels and predictors of airborne and internal exposure to chromium and nickel among welders-Results of the WELDOX study. Int. J. Hyg. Environ. Health 2013, 216, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, M.; Weiss, T.; Pesch, B.; Lotz, A.; Zilch-Schoneweis, S.; Heinze, E.; Van Gelder, R.; Hahn, J.U.; Bruning, T.; WELDOX Study Group. Reduction in welding fume and metal exposure of stainless steel welders: An example from the WELDOX study. Int. Arch. Occup. Environ. Health 2014, 87, 483–492. [Google Scholar] [CrossRef]
- Gube, M.; Ebel, J.; Brand, P.; Goen, T.; Holzinger, K.; Reisgen, U.; Kraus, T. Biological effect markers in exhaled breath condensate and biomonitoring in welders: Impact of smoking and protection equipment. Int. Arch. Occup. Environ. Health 2010, 83, 803–811. [Google Scholar] [CrossRef]
- Stasinos, S.; Nasopoulou, C.; Tsikrika, C.; Zabetakis, I. The bioaccumulation and physiological effects of heavy metals in carrots, onions, and potatoes and dietary implications for Cr and Ni: A review. J. Food Sci. 2014, 79, R765–R780. [Google Scholar] [CrossRef] [Green Version]
| Welders (n = 121) | Office Workers (n = 53) | p-Value | |
|---|---|---|---|
| Personal characteristics, mean ± SD | |||
| Age (years) a | 51.61 ± 7.67 | 51.25 ± 8.08 | 0.78 |
| Seniority (years) a | 32.98 ± 9.76 | 30.08 ± 11.64 | 0.12 |
| Height (cm) a | 168.43 ± 5.62 | 168.89 ± 7.23 | 0.69 |
| Weight (kg) a | 68.34 ± 7.44 | 69.15 ± 9.41 | 0.58 |
| Body Mass Index (kg/m2) a | 24.09 ± 2.36 | 24.21 ± 2.65 | 0.77 |
| Urinary Creatinine (mg/dL, GM, GSD) c | 124.15, 1.68 | 137.01, 1.55 | 0.23 |
| Gender, n (%) | |||
| Male | 121 (100.00) | 53 (100.00) | |
| Education, n (%) b | <0.001 | ||
| Under High School | 89 (73.55) | 8 (15.09) | |
| Above College | 32 (26.45) | 45 (84.91) | |
| Frequency of Particulate Respirator Usage, n (%) b | <0.001 | ||
| Not Regularly | 67 (55.37) | 53 (100.00) | |
| Regularly | 54 (44.63) | 0 (0.00) |
| GM, GSD | Median (Min–Max) | |
|---|---|---|
| Cr (µg/m3) | 5.27, 3.28 | 6.05 (0.15–116.70) |
| Ni (µg/m3) | 6.47, 5.29 | 4.99 (0.05–68.67) |
| Cd (µg/m3) | 0.40, 2.95 | 0.33 (0.02–32.85) |
| Pb (µg/m3) | 5.39, 4.24 | 6.44 (0.31–56.78) |
| Welders (n = 121) | Office Workers (n = 53) | p-Value a | |
|---|---|---|---|
| GM, GSD | GM, GSD | ||
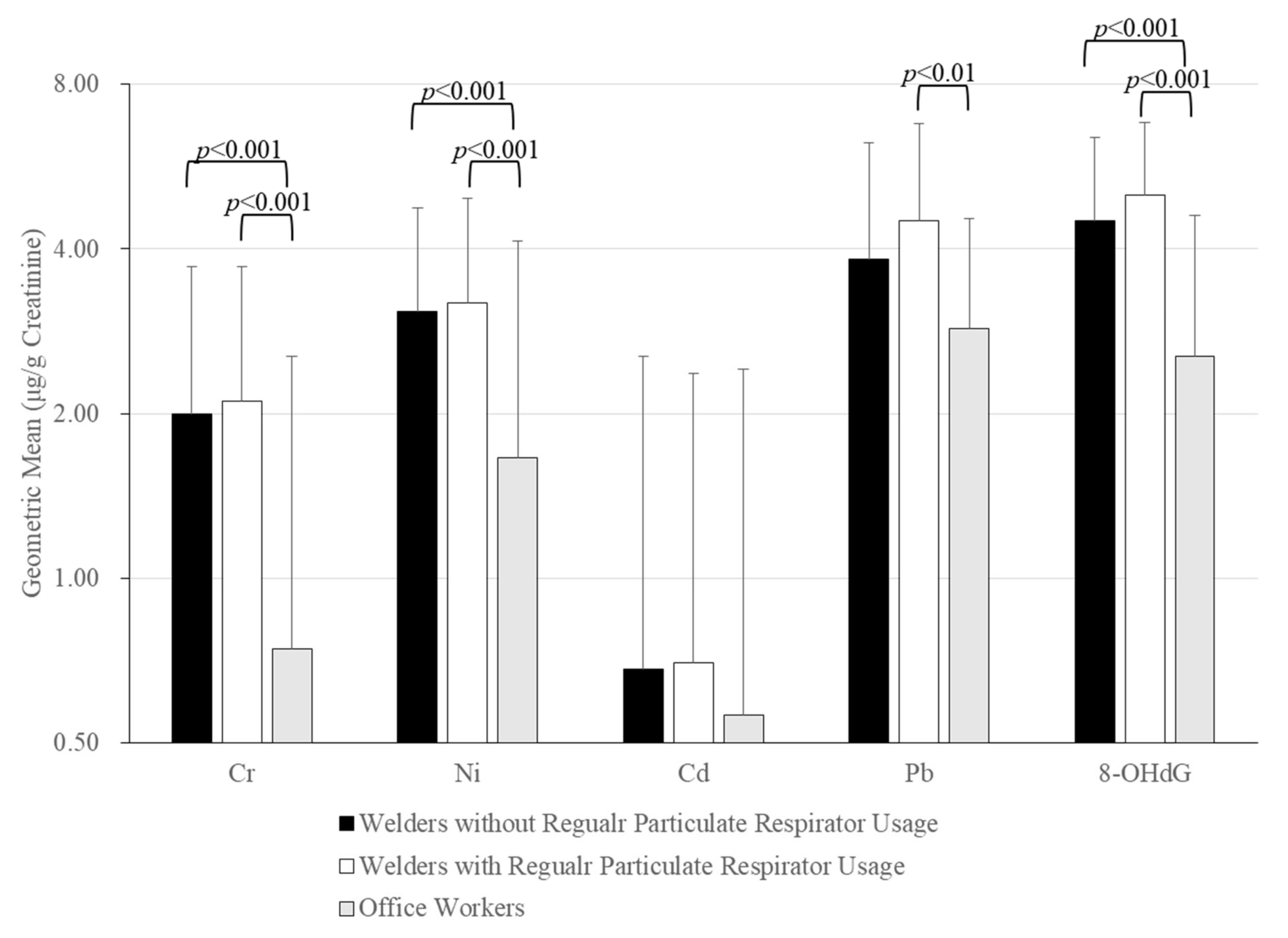
| Urinary Cr (μg/g Creatinine) | 2.06, 1.64 | 0.74, 1.80 | <0.001 |
| Urinary Ni (μg/g Creatinine) | 3.13, 1.72 | 1.66, 2.48 | <0.001 |
| Urinary Cd (μg/g Creatinine) | 0.69, 1.75 | 0.56, 1.85 | <0.05 |
| Urinary Pb (μg/g Creatinine) | 4.18, 2.35 | 2.86, 1.68 | <0.001 |
| Urinary 8-Hydroxy-2′-Deoxyguanosine (μg/g Creatinine) | 4.77, 1.84 | 2.54, 2.07 | <0.001 |
| Urine | Ln Cr (µg/L) | Ln Ni (µg/L) | Ln Cd (µg/L) | Ln Pb (µg/L) | |
|---|---|---|---|---|---|
| Total Dusts | |||||
| Ln Cr (µg/m3) | 0.34 *** | ||||
| Ln Ni (µg/m3) | 0.33 *** | ||||
| Ln Cd (µg/m3) | 0.21 * | ||||
| Ln Pb (µg/m3) | 0.15 | ||||
| Ln U-Ni (μg/L) | Ln U-Cd (μg/L) | Ln U-Pb (μg/L) | Ln U-8-OHdG (μg/L) | Ln U-Creatinine (mg/dL) | |
|---|---|---|---|---|---|
| Ln U-Cr (μg/L) | 0.50 *** | 0.31 *** | 0.31 *** | 0.42 *** | −0.02 |
| Ln U-Ni (μg/L) | 0.52 *** | 0.26 *** | 0.41 *** | 0.07 | |
| Ln U-Cd (μg/L) | 0.43 *** | 0.18 * | 0.19 * | ||
| Ln U-Pb (μg/L) | 0.10 | 0.02 | |||
| Ln U-8-OHdG (μg/L) | 0.04 |
| β (Lower–Upper) | GM % Change | p-Value | |
|---|---|---|---|
| Ln Urinary Cr (μg/L) a,b | |||
| Welders without Regular Particulate Respirator Usage | 0.91 (0.83–1.00) | 148.43 | <0.001 |
| Welders with Regular Particulate Respirator Usage | 0.93 (0.84–1.01) | 153.45 | <0.001 |
| Office Workers | Reference | ||
| Ln Urinary Ni (μg/L) a,b | |||
| Welders without Regular Particulate Respirator Usage | 0.54 (0.35–0.73) | 71.60 | <0.001 |
| Welders with Regular Particulate Respirator Usage | 0.57 (0.39–0.76) | 76.83 | <0.001 |
| Office Workers | Reference | ||
| Ln Urinary Cd (μg/L) a,b | |||
| Welders without Regular Particulate Respirator Usage | 0.12 (−0.03–0.27) | 12.75 | 0.12 |
| Welders with Regular Particulate Respirator Usage | 0.13 (−0.01–0.27) | 13.88 | 0.07 |
| Office Workers | Reference | ||
| Ln Urinary Pb (μg/L) a,b | |||
| Welders without Regular Particulate Respirator Usage | 0.22 (−0.01–0.44) | 24.61 | 0.06 |
| Welders with Regular Particulate Respirator Usage | 0.34 (0.13–0.56) | 40.49 | <0.01 |
| Office Workers | Reference | ||
| Ln Urinary 8-Hydroxy-2′-Deoxyguanisine (μg/L) a,b | |||
| Welders without Regular Particulate Respirator Usage | 0.50 (0.33–0.68) | 64.87 | <0.001 |
| Welders with Regular Particulate Respirator Usage | 0.58 (0.41–0.74) | 78.60 | <0.001 |
| Office Workers | Reference | ||
| Ln Urinary 8-Hydroxy-2′-Deoxyguanisine (μg/L) a,c | |||
| Ln Urinary Cr (μg/L) | 0.46 (0.31–0.61) | 37.55 | <0.001 |
| Ln Urinary 8-Hydroxy-2′-Deoxyguanisine (μg/L) a,c | |||
| Ln Urinary Ni (μg/L) | 0.38 (0.25–0.51) | 30.13 | <0.001 |
| Ln Urinary 8-Hydroxy-2′-Deoxyguanisine (μg/L) a,c | |||
| Ln Urinary Cd (μg/L) | 0.23 (0.04–0.43) | 17.28 | <0.05 |
| Ln Urinary 8-Hydroxy-2′-Deoxyguanisine (μg/L) a,c | |||
| Ln Urinary Pb (μg/L) | 0.08 (−0.05–0.21) | 5.70 | 0.23 |
| Ln Urinary 8-Hydroxy-2′-Deoxyguanisine (μg/L) a,c | |||
| Ln Urinary Cr (μg/L) | 0.33 (0.16–0.49) | 25.70 | <0.001 |
| Ln Urinary Ni (μg/L) | 0.27 (0.12–0.43) | 20.58 | <0.001 |
| Ln Urinary Cd (μg/L) | −0.06 (−0.27–0.15) | −4.07 | 0.58 |
| Ln Urinary Pb (μg/L) | −0.05 (−0.17–0.08) | −3.41 | 0.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, T.-Y.; Pan, C.-H.; Hsu, Y.-T.; Lai, C.-H. Effects of Heavy Metal Exposure on Shipyard Welders: A Cautionary Note for 8-Hydroxy-2′-Deoxyguanosine. Int. J. Environ. Res. Public Health 2019, 16, 4813. https://doi.org/10.3390/ijerph16234813
Su T-Y, Pan C-H, Hsu Y-T, Lai C-H. Effects of Heavy Metal Exposure on Shipyard Welders: A Cautionary Note for 8-Hydroxy-2′-Deoxyguanosine. International Journal of Environmental Research and Public Health. 2019; 16(23):4813. https://doi.org/10.3390/ijerph16234813
Chicago/Turabian StyleSu, Ting-Yao, Chih-Hong Pan, Yuan-Ting Hsu, and Ching-Huang Lai. 2019. "Effects of Heavy Metal Exposure on Shipyard Welders: A Cautionary Note for 8-Hydroxy-2′-Deoxyguanosine" International Journal of Environmental Research and Public Health 16, no. 23: 4813. https://doi.org/10.3390/ijerph16234813
APA StyleSu, T.-Y., Pan, C.-H., Hsu, Y.-T., & Lai, C.-H. (2019). Effects of Heavy Metal Exposure on Shipyard Welders: A Cautionary Note for 8-Hydroxy-2′-Deoxyguanosine. International Journal of Environmental Research and Public Health, 16(23), 4813. https://doi.org/10.3390/ijerph16234813






