Synthesis of Nanoscale Zerovalent Iron (nZVI) Supported on Biochar for Chromium Remediation from Aqueous Solution and Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MSB-nZVI
2.2. Sample Characterizations
2.3. Remediation of Cr(VI) and Total Cr from Aqueous Solution
2.3.1. Effect of pH, Initial Concentration, and Contact Time
2.3.2. Methods to Reveal Cr(VI) Removal Mechanism
2.4. Remediation of Cr(VI)-Contaminated Soil
2.4.1. Analytical Methods for Soil Characterizations
2.4.2. Remediation Experiments of Cr(VI)-Contaminated Soil
3. Results and Discussion
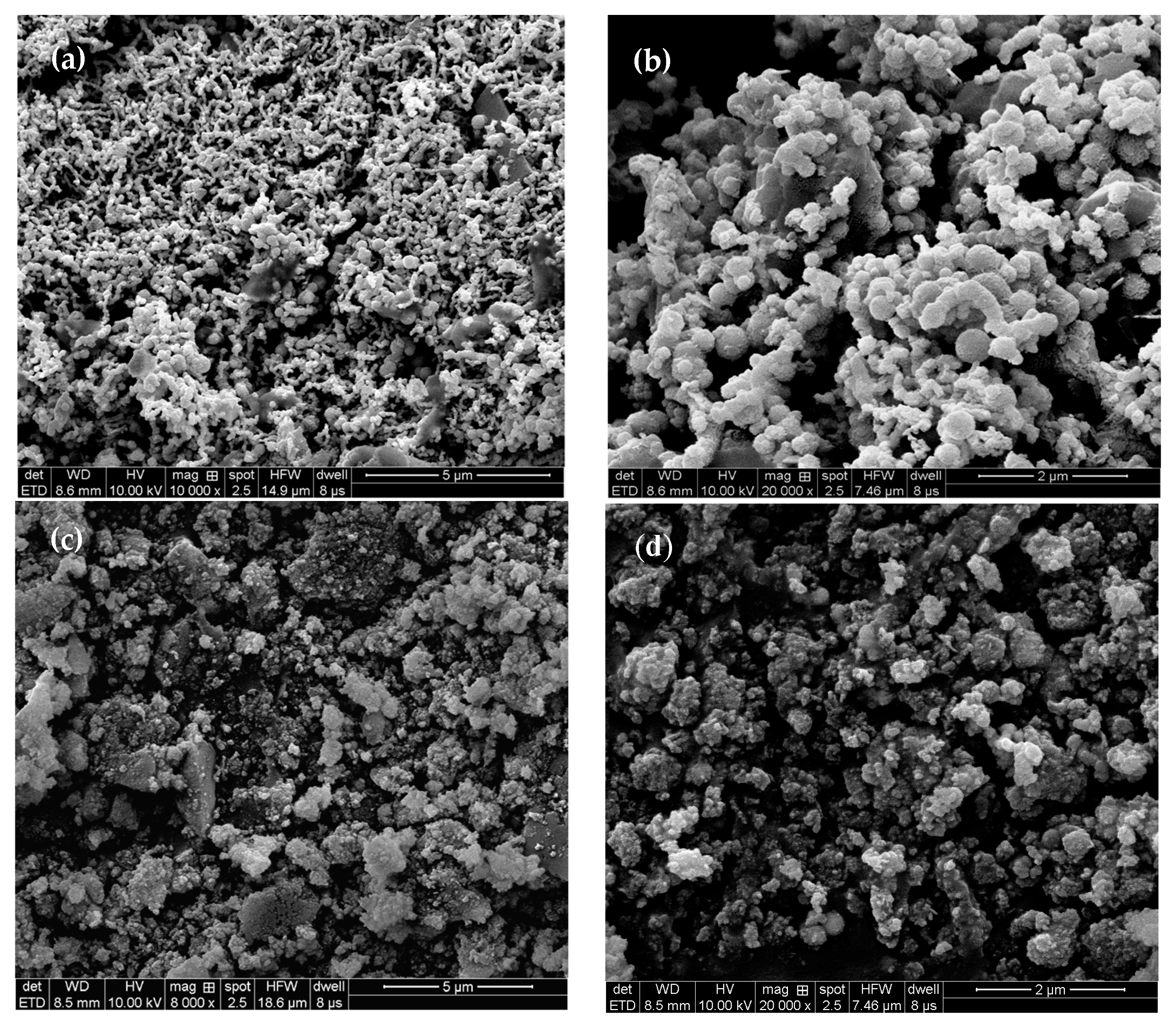
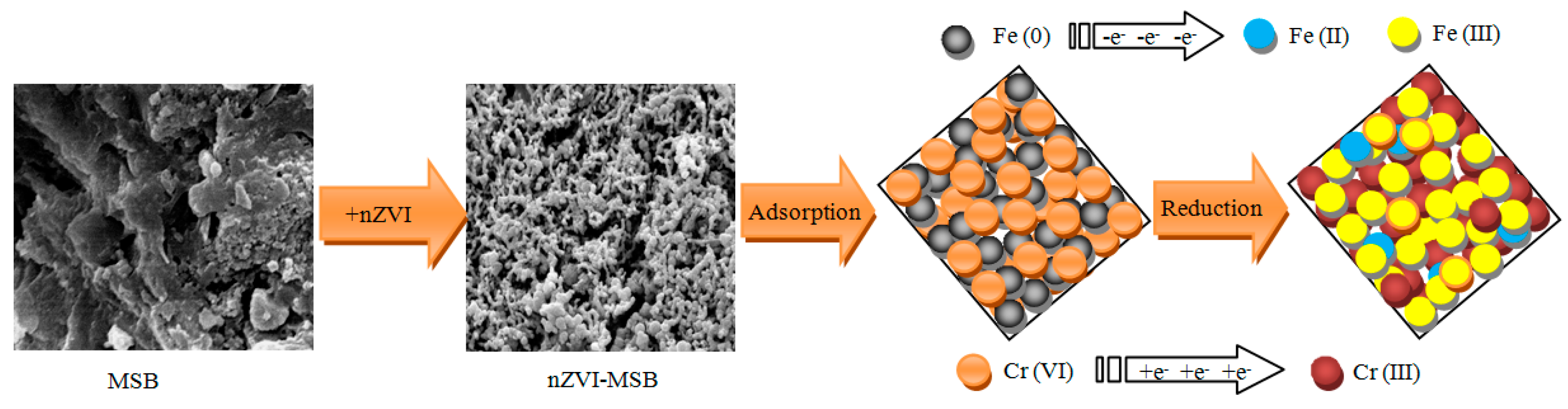
3.1. Characterization of MSB-nZVI
3.2. Cr(VI) Removal from Aqueous Solution
3.2.1. Effect of pH
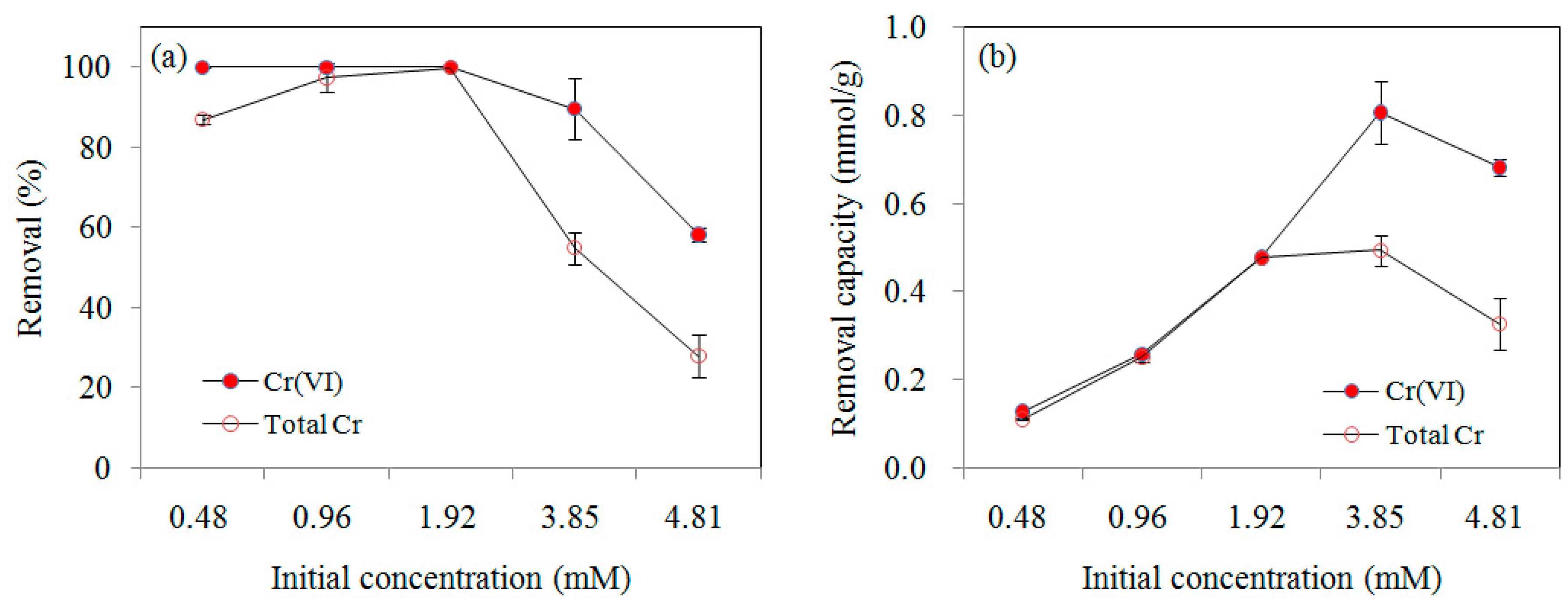
3.2.2. Effect of Initial Concentration
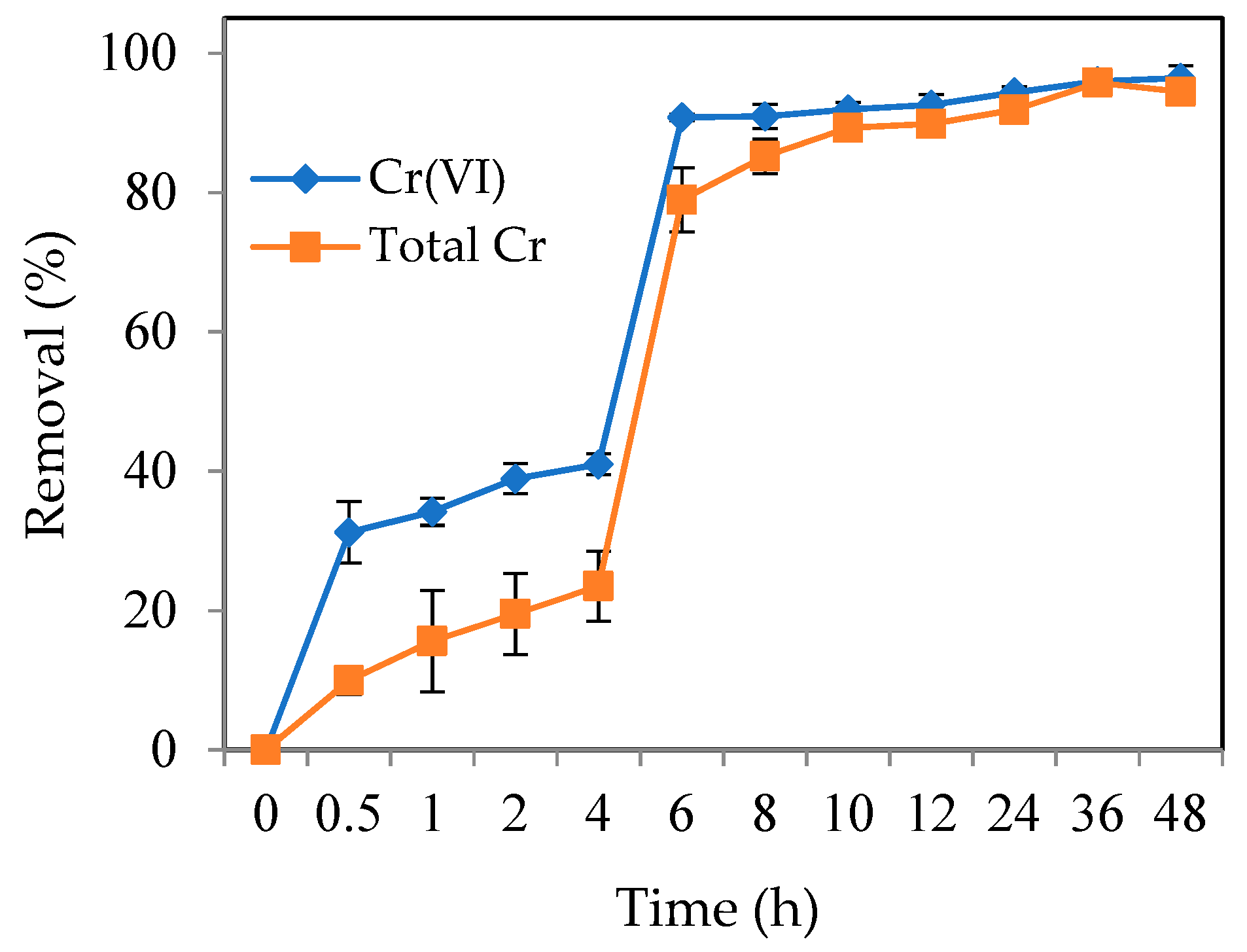
3.2.3. Effect of Contact Time
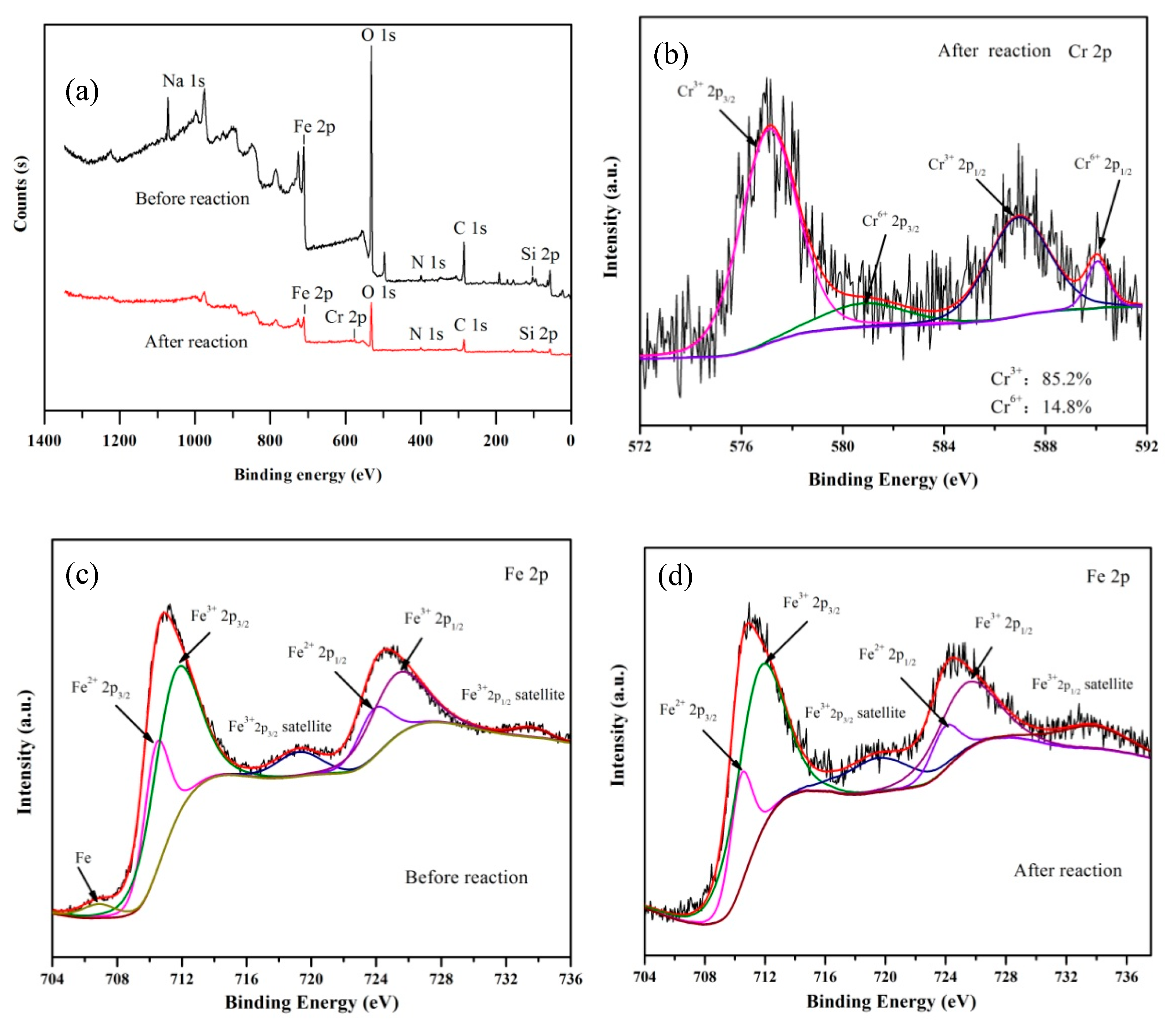
3.2.4. Removal Mechanisms
3.3. Remediation of Cr(VI)-Contaminated Soil
3.3.1. Characterization of Soil Sample
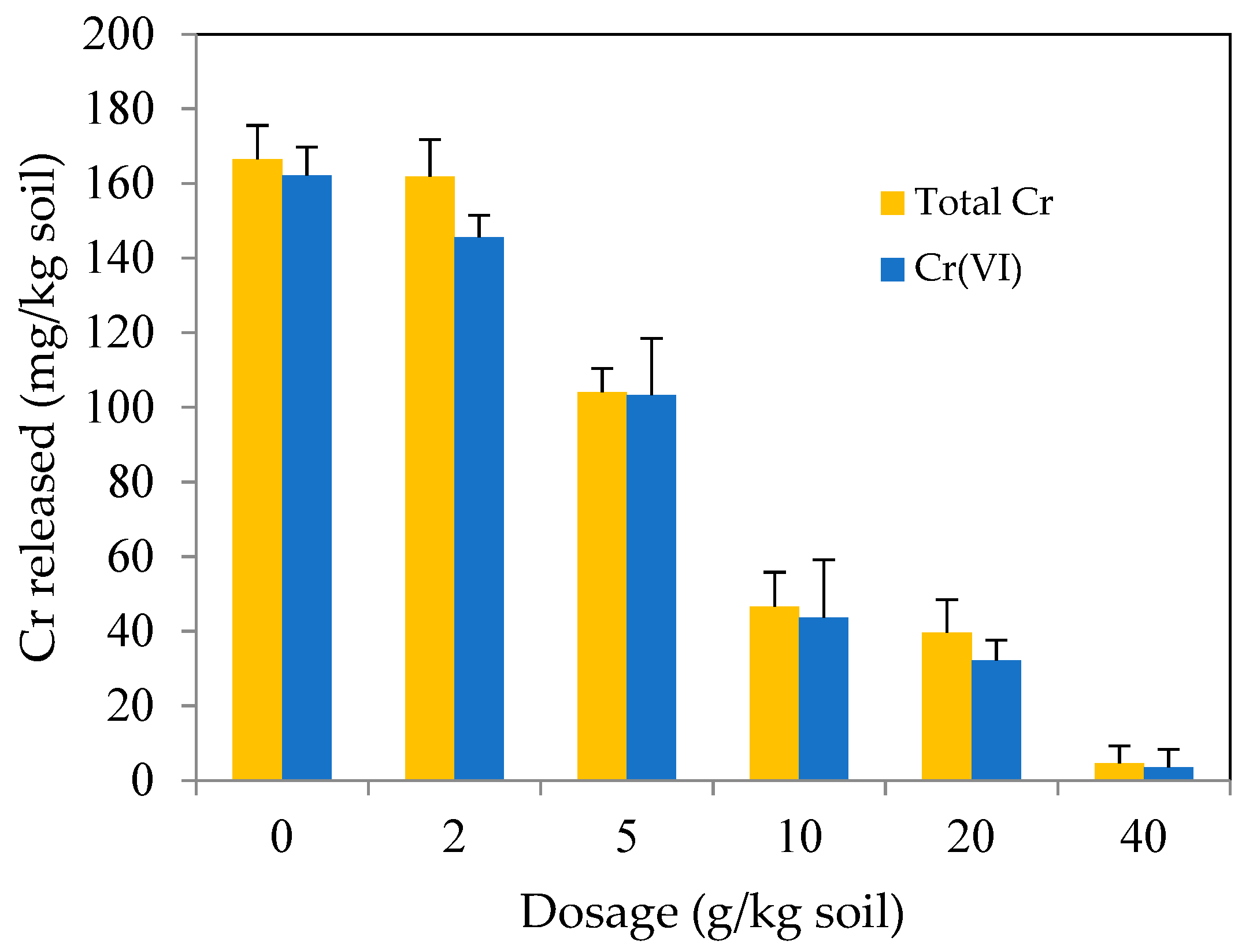
3.3.2. Effect of MSB-nZVI Dosage
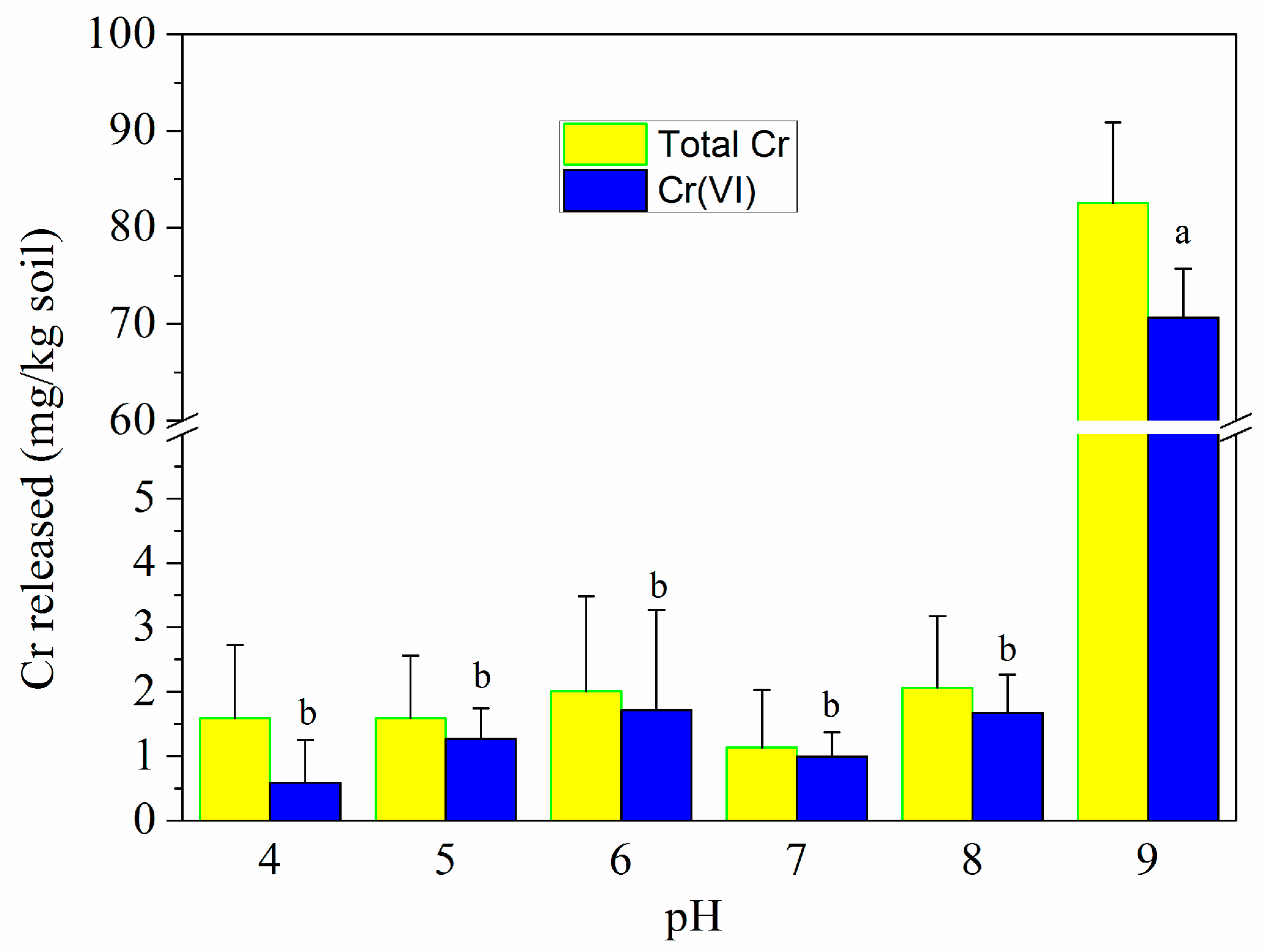
3.3.3. Effect of pH
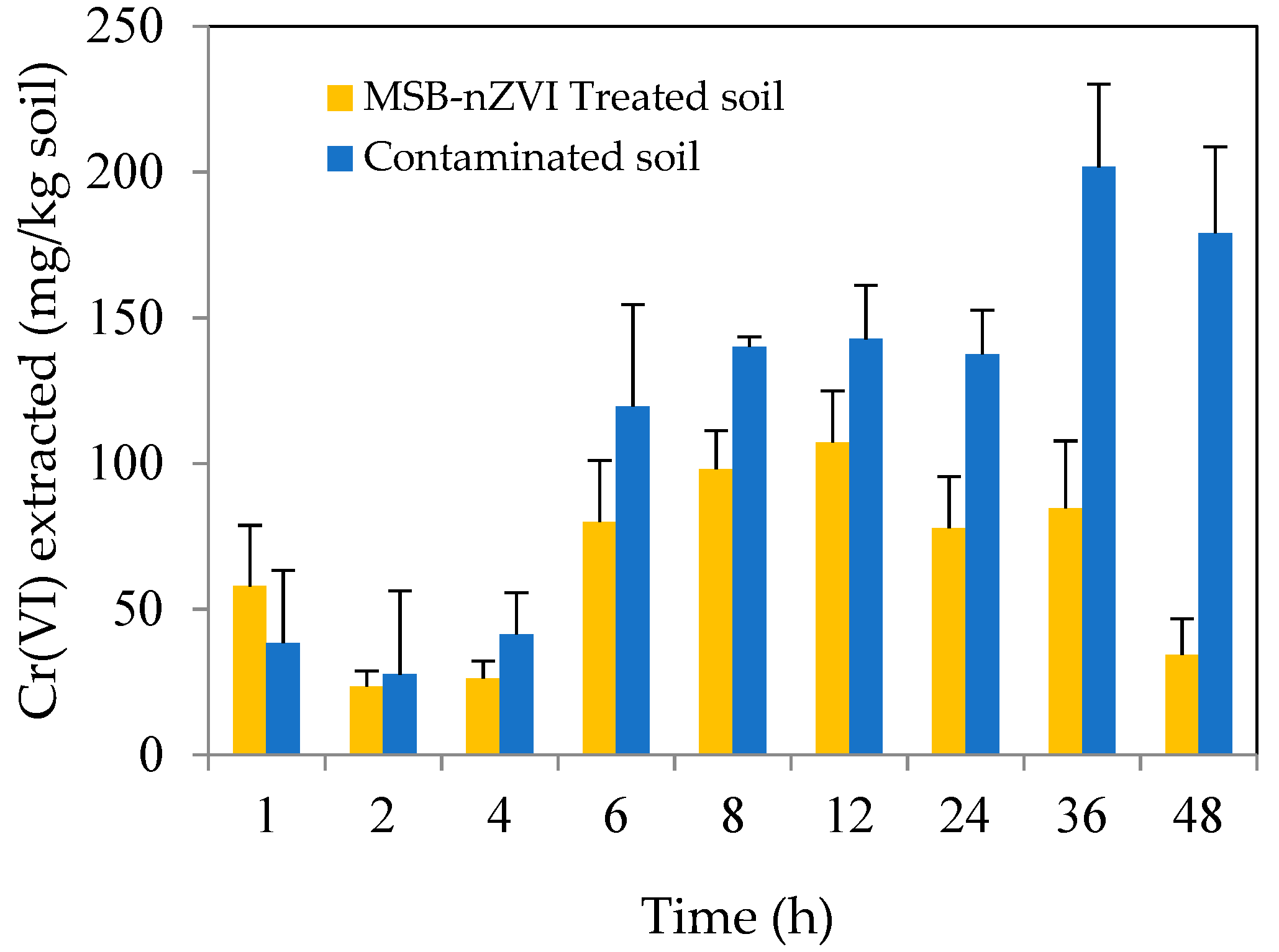
3.3.4. Remediation Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, L.; Liu, Y.G.; Liu, S.B.; Yin, Y.C.; Zeng, G.G.; Tan, X.F.; Hu, X.; Hu, X.G.; Jiang, L.H.; Ding, Y.; et al. Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, W.; Teng, B.; Liu, X.; Zhang, W. Zirconium cross-linked chitosan composite: Preparation, characterization and application in adsorption of Cr(VI). Chem. Eng. J. 2013, 229, 1–8. [Google Scholar] [CrossRef]
- Chinese Standards for Drinking Water Quality (GB5749-2006); The National Standard of the P.R. China; China Standard Press: Beijing, China, 2007; pp. 2–4. (In Chinese)
- Diao, Z.H.; Xu, X.R.; Chen, H.; Jiang, D.; Yang, Y.X.; Kong, L.J.; Sun, Y.X.; Hu, Y.X.; Hao, Q.W.; Liu, L. Simultaneous removal of Cr(VI) and phenol by persulfate activated with bentonite-supported nanoscale zero-valent iron: Reactivity and mechanism. J. Hazard. Mater. 2016, 316, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Kurosu, S.; Kobayashi, M.; Kawase, Y. Zero-valent iron treatment of dark brown colored coffee effluent: Contributions of a core-shell structure to pollutant removals. J. Environ. Manag. 2016, 183, 478–487. [Google Scholar] [CrossRef] [PubMed]
- El-Temsah, Y.S.; Joner, E.J. Effects of nano-sized zero-valent iron (nZVI) on DDT degradation in soil and its toxicity to collembola and ostracods. Chemosphere 2013, 92, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Cheng, C.Y.; Li, C.W.; Chai, P.H.; Chang, Y.M. Reduction of chromate from electroplating wastewater from pH 1–2 using fluidized zero valent iron process. J. Hazard. Mater. 2007, 142, 362–367. [Google Scholar] [PubMed]
- Fang, Z.; Qiu, X.; Huang, R.; Qiu, X.; Li, M. Removal of chromium in electroplating wastewater by nanoscale zero-valent metal with synergistic effect of reduction and immobilization. Desalination 2011, 280, 224–231. [Google Scholar] [CrossRef]
- Shang, J.; Zong, M.; Yu, Y.; Kong, X.; Du, Q.; Liao, Q. Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar. J. Environ. Manag. 2017, 197, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, A.A.; Li, J.; Abbas, M.H.H. Feasibility of biochar manufactured from organic wastes on the stabilization of heavy metals in a metal smelter contaminated soil. Chemosphere 2014, 117, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Z.; Gascó, G.; Méndez, A.; Shen, Y.; Paz-Ferreiro, J. Use of magnetic biochars for the immobilization of heavy metals in a multi-contaminated soil. Sci. Total Environ. 2018, 622, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ferreiro, J.; Liang, C.; Fu, S.; Mendez, A.; Gasco, G. The Effect of biochar and its interaction with the earthworm Pontoscolex corethrurus on soil microbial community structure in tropical soils. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Z.; Fu, S.; Méndez, A.; Gascó, G.; Paz-Ferreiro, J. Effect of Biochar in Cadmium Availability and Soil Biological Activity in an Anthrosol Following Acid Rain Deposition and Aging. Water Air Soil Pollut. 2015, 226, 164. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Chen, H.; Zhang, M.; Cao, X. Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour. Technol. 2014, 152, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Fang, Z.; Tsang, E.P.; Fang, J.; Zhao, D. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environ. Pollut. 2016, 214, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, W.; Yan, J.; Han, L.; Chen, Y.; Ouyang, D.; Chen, M. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr(VI)removal. Environ. Pollut. 2017, 223, 153–160. [Google Scholar] [CrossRef] [PubMed]
- NY/T 1121.16-2006 (China). Soil Testing, Part 2: Method for Determination of soil pH; Chinese Ministry of Agriculture: Beijing, China, 2006.
- NY/T 1121.16-2006 (China). Soil Testing, Part 16: Method for Determination of Total Water-Soluble Salt; Chinese Ministry of Agriculture: Beijing, China, 2006.
- Dong, X.L.; Ma, L.Q.; Li, Y.C. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) inaquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Kou, L.; Wang, T.; Liang, D.; Hu, S. Evaluation of activated carbon fiber supported nanoscale zero-valent iron for chromium (VI) removal from groundwater in a permeable reactive column. J. Environ. Manag. 2017, 201, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Ponder, S.M.; Darab, J.G.; Mallouk, T.E. Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ. Sci. Technol. 2000, 34, 2564–2569. [Google Scholar] [CrossRef]
- Qiu, X.Q.; Fang, Z.Q.; Yan, X.M.; Cheng, W.; Lin, K.R. Chemical stability and toxicity of nanoscale zero-valent iron in the remediation of chromium contaminated watershed. Chem. Eng. J. 2013, 220, 61–66. [Google Scholar] [CrossRef]
- Montesinos, V.N.; Quici, N.; Halac, E.B.; Leyva, A.G.; Custo, G.; Bengio, S.; Zampieri, G.; Litter, M.I. Highly efficient removal of Cr(VI) from water with nanoparticulated zero valent iron: Understanding the Fe(III)-Cr(III) passive outer layer structure. Chem. Eng. J. 2014, 244, 569–575. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2012, 64, 1782–1806. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wu, M.F.; Tang, T.T.; Xing, Q.J.; Peng, C.Q.; Li, F.; Liu, H.; Luo, X.B.; Zou, J.P.; Min, X.B.; et al. Mechanism investigation of anoxic Cr(VI) removal by nano zero-valent iron based on XPS analysis in time scale. Chem. Eng. J. 2018, 335, 945–953. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, L.; Ouyang, D.; Chen, Y.; Han, L.; Chen, M. Effective removal of Cr(VI) by attapulgite-supported nanoscale zero-valent iron from aqueous solution: Enhanced adsorption and crystallization. Chemosphere 2019, 221, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Fang, Z.; Tsang, P.E.; Zheng, L.; Cheng, W.; Fang, J.; Zhao, D. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, L.; Ma, S.; Shang, Z. Effect factors, kinetics and thermodynamics of remediation in the chromium contaminated soils by nanoscale zero valent Fe/Cu bimetallic particles. Chem. Eng. J. 2016, 302, 663–669. [Google Scholar]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, M.; Li, H. Synthesis of Nanoscale Zerovalent Iron (nZVI) Supported on Biochar for Chromium Remediation from Aqueous Solution and Soil. Int. J. Environ. Res. Public Health 2019, 16, 4430. https://doi.org/10.3390/ijerph16224430
Wang H, Zhang M, Li H. Synthesis of Nanoscale Zerovalent Iron (nZVI) Supported on Biochar for Chromium Remediation from Aqueous Solution and Soil. International Journal of Environmental Research and Public Health. 2019; 16(22):4430. https://doi.org/10.3390/ijerph16224430
Chicago/Turabian StyleWang, Haixia, Mingliang Zhang, and Hongyi Li. 2019. "Synthesis of Nanoscale Zerovalent Iron (nZVI) Supported on Biochar for Chromium Remediation from Aqueous Solution and Soil" International Journal of Environmental Research and Public Health 16, no. 22: 4430. https://doi.org/10.3390/ijerph16224430
APA StyleWang, H., Zhang, M., & Li, H. (2019). Synthesis of Nanoscale Zerovalent Iron (nZVI) Supported on Biochar for Chromium Remediation from Aqueous Solution and Soil. International Journal of Environmental Research and Public Health, 16(22), 4430. https://doi.org/10.3390/ijerph16224430




