Outdoor Endurance Training with Air Pollutant Exposure Versus Sedentary Lifestyle: A Comparison of Airway Immune Responses
Abstract
1. Introduction
2. Material and Methods
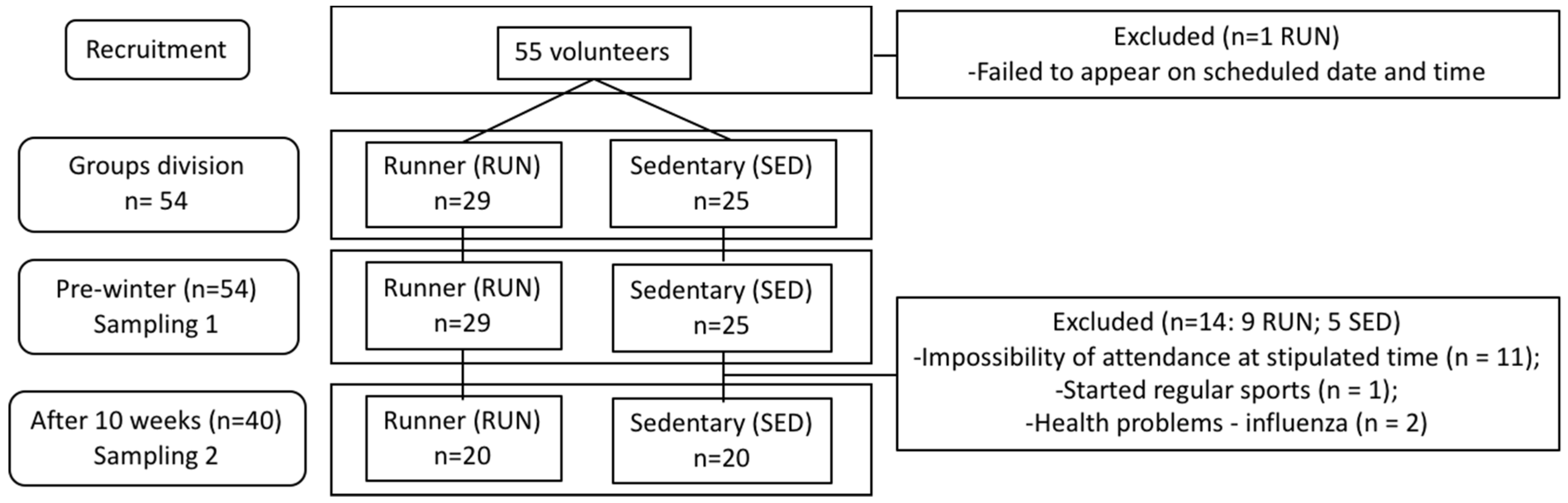
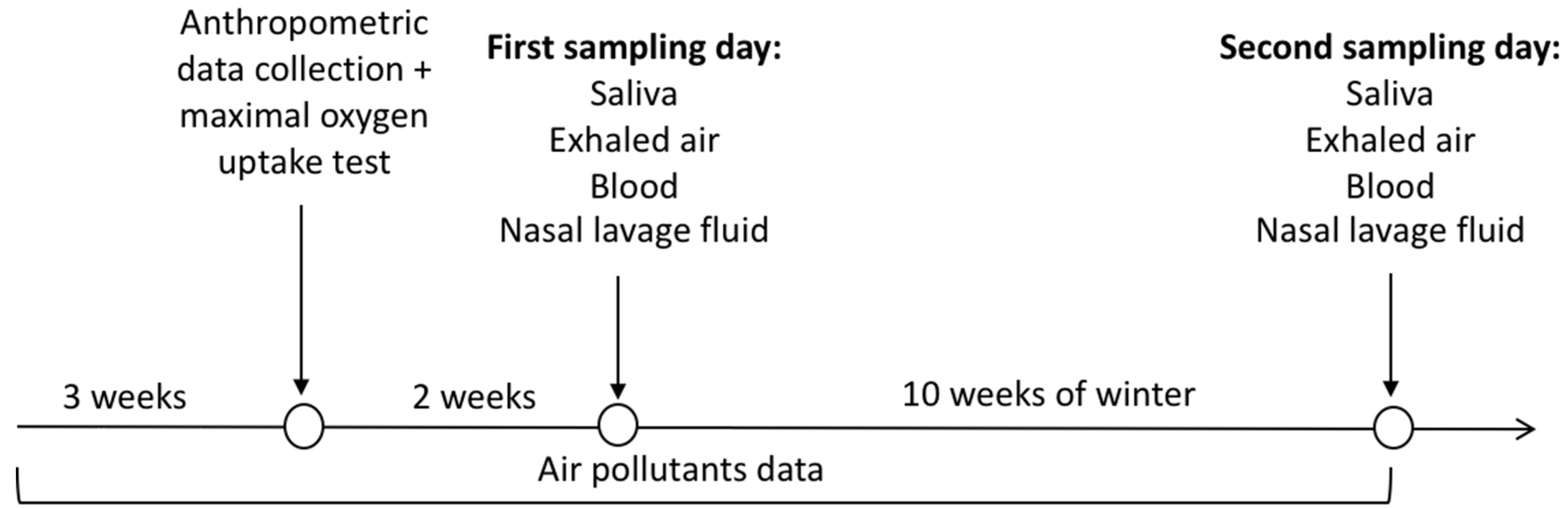
2.1. Experimental Design
2.2. Samples Collection
2.3. Determination of Club Cell Protein (CC16)
2.4. Determination of Cytokines
2.5. Determination of Salivary Concentration
2.6. Determination of Fractional Exhaled Nitric Oxide (FeNO)
2.7. Statistical Analysis
3. Results
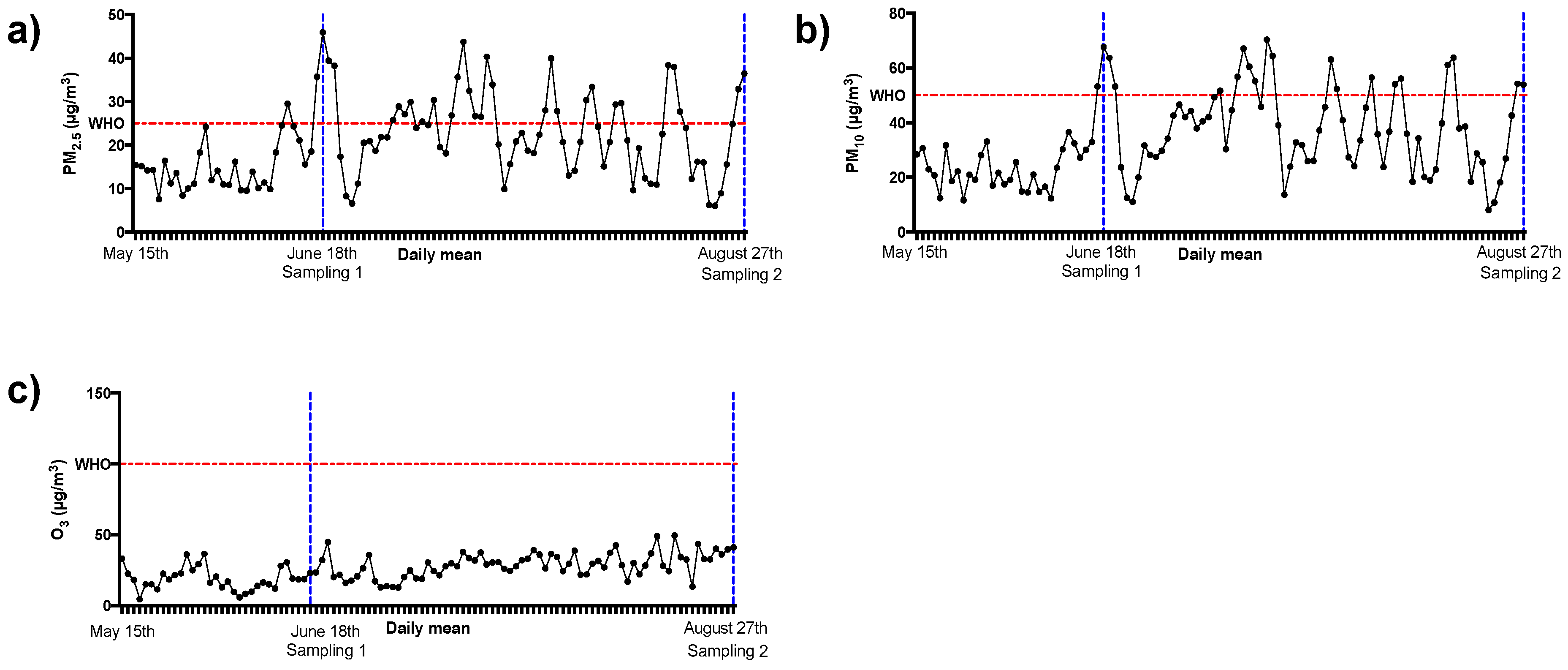
3.1. Air Pollutants Levels
3.2. Fractional Exhaled Nitric Oxide is Reduced in Runners but not CC16
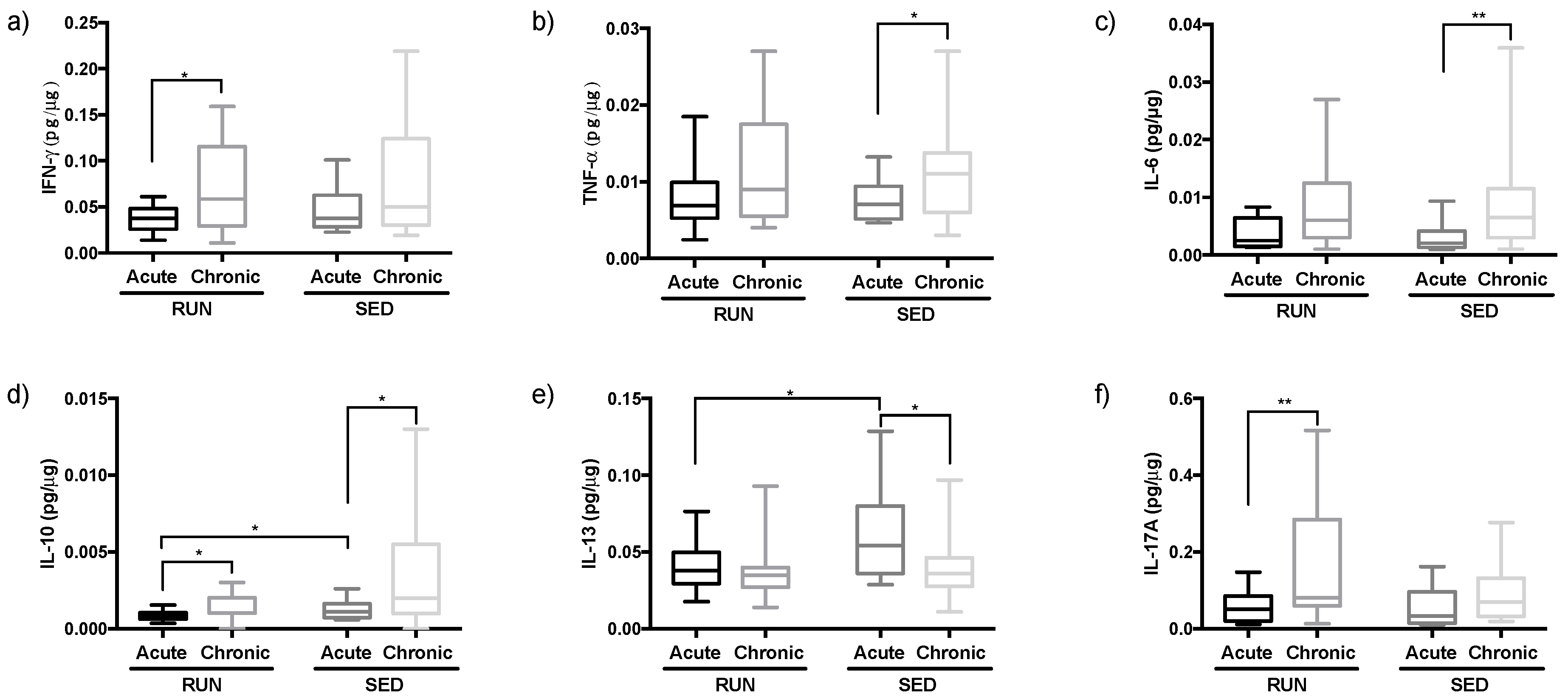
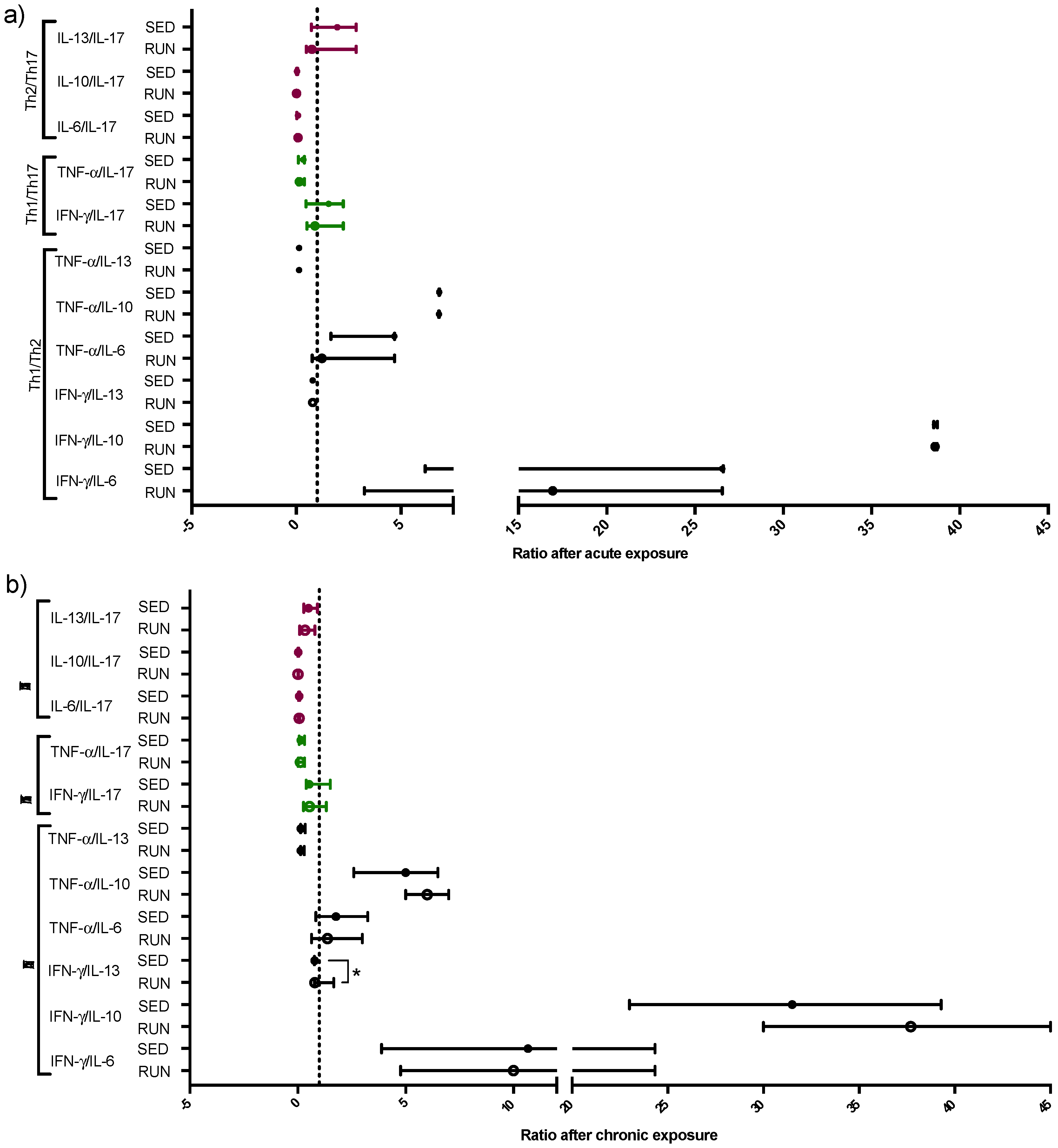
3.3. Different Pattern of Th Immune Response in Upper Airway in the RUN and SED Groups
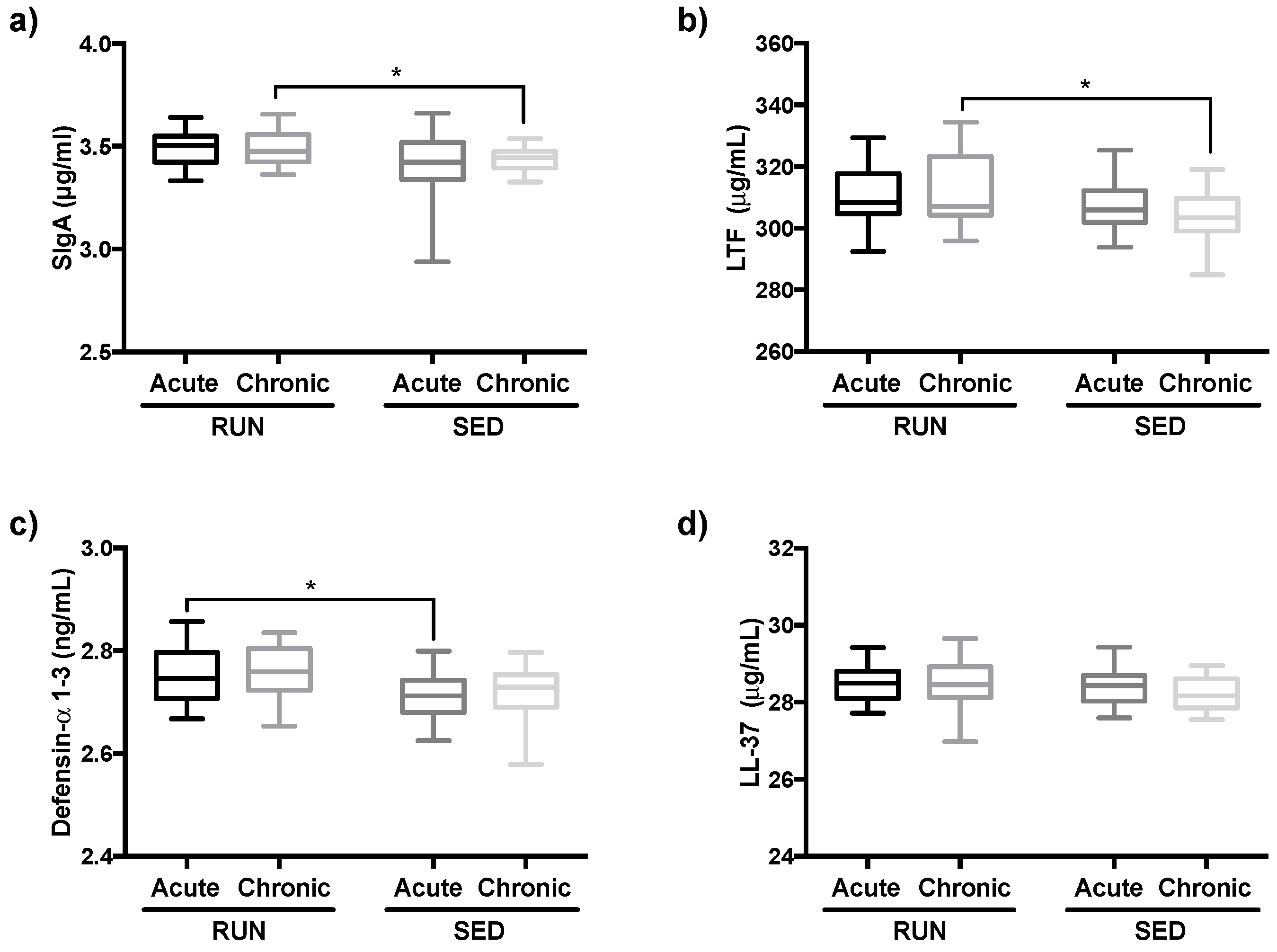
3.4. Salivary Immunological Markers are Increased in Runners
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thurston, G.D.; Kipen, H.; Annesi-Maesano, I.; Balmes, J.; Brook, R.D.; Cromar, K.; de Matteis, S.; Forastiere, F.; Forsberg, B.; Mark, W.F.; et al. A joint ERS/ATS policy statement: What constitutes an adverse health effect of air pollution? An analytical framework. Eur. Respir. J. 2017, 49, 10600419. [Google Scholar] [CrossRef] [PubMed]
- Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016.
- World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Collaborators GRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Sancini, A.; Tomei, F.; Tomei, G.; Caciari, T.; Di Giorgio, V.; André, J.C.; Palermo, P.; Andreozzi, G.; Nardone, N.; Schifano, M.P.; et al. Urban pollution. G. Ital. Med. Lav. Ergon. 2012, 34, 187–196. [Google Scholar] [PubMed]
- Berend, N. Contribution of air pollution to COPD and small airway dysfunction. Respirology 2016, 21, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Cachon, B.F.; Firmin, S.; Verdin, A.; Ayi-Fanou, L.; Billet, S.; Cazier, F.; Martin, P.J.; Aissi, F.; Courcot, D.; Sanni, A.; et al. Proinflammatory effects and oxidative stress within human bronchial epithelial cells exposed to atmospheric particulate matter (PM(2.5) and PM(>2.5)) collected from Cotonou, Benin. Environ. Pollut. 2014, 185, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, Composition, and Lung Diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency US. Ozone Pollution and Your Patients’ Health United States. 2018. Available online: https://www.epa.gov/ozone-pollution-and-your-patients-health/what-ozone (accessed on 21 August 2019).
- Qing, H.; Wang, X.; Zhang, N.; Zheng, K.; Du, K.; Zheng, M.; Li, Y.; Chang, Y.; Zhang, L.; Bachert, C. The Effect of PM2.5 on the Inflammatory Responses in Human Upper Airway Mucosa. Am. J. Respir. Crit. Care Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.P.; Toledo, A.C.; Silva, L.B.; Almeida, F.M.; Damaceno-Rodrigues, N.R.; Caldini, E.G.; Santos, A.B.G.; Rivero, D.H.; Hizume, D.C.; Lopes, F.; et al. Anti-inflammatory effects of aerobic exercise in mice exposed to air pollution. Med. Sci. Sports Exerc. 2012, 44, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fujii, T.; Qui, D.; Vincent, R.; Hogg, J.C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am. J. Respir. Crit. Care Med. 2001, 164, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Hage, P. Air Pollution: Adverse Effects on Athletic Performance. Phys. Sportsmed. 1982, 10, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Fitch, K.D. Management of allergic Olympic athletes. J. Allergy Clin. Immunol. 1984, 73 Pt 2, 722–727. [Google Scholar] [CrossRef]
- Rundell, K.W. Effect of air pollution on athlete health and performance. Br. J. Sports Med. 2012, 46, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, T.; Kipen, H.; Wang, G.; Huang, W.; Rich, D.; Zhu, P.; Wang, Y.; Lu, S.E.; Ohman-Strickland, P.; et al. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res. Rep. (Health Eff. Inst.) 2013, 174, 5. [Google Scholar]
- Rich, D.Q.; Liu, K.; Zhang, J.; Thurston, S.W.; Stevens, T.P.; Pan, Y.; Kane, C.; Weinberger, B.; Ohman-Strickland, P.; Woodruff, T.J.; et al. Differences in Birth Weight Associated with the 2008 Beijing Olympics Air Pollution Reduction: Results from a Natural Experiment. Environ. Health Perspect. 2015, 123, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, X.; Ying, Z.; Jiang, L.; Zhong, M.; Wang, A.; Chen, L.; Lu, B.; Sun, Q. Post-Effect of Air Quality Improvement on Biomarkers for Systemic Inflammation and Microparticles in Asthma Patients After the 2008 Beijing Olympic Games: A Pilot Study. Inflammation 2017, 40, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, Y.; Cai, X. A health-based assessment of particulate air pollution in urban areas of Beijing in 2000–2004. Sci. Total Environ. 2007, 376, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, M.; Xing, J.; Wu, Y.; Zhou, Y.; Lei, Y.; He, K.; Fu, L.; Hao, J. Quantifying the air pollutants emission reduction during the 2008 Olympic games in Beijing. Environ. Sci. Technol. 2010, 44, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Pierson, W.E.; Covert, D.S.; Koenig, J.Q.; Namekata, T.; Kim, Y.S. Implications of air pollution effects on athletic performance. Med. Sci. Sports Exerc. 1986, 18, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Bermon, S. Airway inflammation and upper respiratory tract infection in athletes: Is there a link? Exerc. Immunol. Rev. 2007, 13, 6–14. [Google Scholar] [PubMed]
- Daigle, C.C.; Chalupa, D.C.; Gibb, F.R.; Morrow, P.E.; Oberdörster, G.; Utell, M.J.; Frampton, M.W. Ultrafine particle deposition in humans during rest and exercise. Inhal. Toxicol. 2003, 15, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Guidi, G.C.; Maffulli, N. Air pollution and sports performance in Beijing. Int. J. Sports Med. 2008, 29, 696–698. [Google Scholar] [CrossRef] [PubMed][Green Version]
- An, R.; Zhang, S.; Ji, M.; Guan, C. Impact of ambient air pollution on physical activity among adults: A systematic review and meta-analysis. Perspect. Public Health 2018, 138, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Voss, J.D.; Knight, B. The association of ambient air pollution and physical inactivity in the United States. PLoS ONE 2014, 9, e90143. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Tainio, M.; de Nazelle, A.J.; Götschi, T.; Kahlmeier, S.; Rojas-Rueda, D.; Nieuwenhuijsen, M.J.; de Sa Thiago, H.; Kelly, P.; Woodcock, J. Can air pollution negate the health benefits of cycling and walking? Prev. Med. 2016, 87, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Harriss, D.J.; Atkinson, G. Ethical Standards in Sport and Exercise Science Research: 2016 Update. Int. J. Sports Med. 2015, 36, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll Dent. 2014, 81, 14–18. [Google Scholar]
- Ellestad, M.H.; Allen, W.; Wan, M.C.; Kemp, G.L. Maximal treadmill stress testing for cardiovascular evaluation. Circulation 1969, 39, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Meneghelo, R.S.; Araújo, C.; Stein, R.; Mastrocolla, L.; Albuquerque, P.; Serra, S., III. Diretrizes da Sociedade Brasileira de Cardiologia sobre teste ergométrico. Arquivos Brasileiros Cardiologia 2010, 95, 1–26. [Google Scholar]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Camarda, S.R.d.A.; Tebexreni, A.S.; Páfaro, C.N.; Sasai, F.B.; Tambeiro, V.L.; Juliano, Y.; Barros Neto, T.L.D. Comparison of maximal heart rate using the prediction equations proposed by Karvonen and Tanaka. Arquivos Brasileiros Cardiologia 2008, 91, 311–314. [Google Scholar]
- Belda, J.; Parameswaran, K.; Keith, P.K.; Hargreave, F.E. Repeatability and validity of cell and fluid-phase measurements in nasal fluid: A comparison of two methods of nasal lavage. Clin. Exp. Allergy 2001, 31, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Society, A.T.; Society, E.R. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar]
- Kharitonov, S.; Alving, K.; Barnes, P.J. Exhaled and nasal nitric oxide measurements: Recommendations. The European Respiratory Society Task Force. Eur. Respir. J. 1997, 10, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Leme, A.S.; Kasahara, D.I.; Nunes, M.P.; Martins, M.A.; Vieira, J.E. Exhaled nitric oxide collected with two different mouthpieces: A study in asthmatic patients. Braz. J. Med. Biol. Res. 2002, 35, 1133–1137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vaisberg, M.; Paixão, V.; Almeida, E.B.; Santos, J.M.B.; Foster, R.; Rossi, M.; Pithon-Curi, T.C.; Gorjão, R.; Momesso, C.M.; Andrade, M.S.; et al. Daily Intake of Fermented Milk Containing. Nutrients 2019, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goete, L.; Rogers, C.J.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol Rev. 2011, 17, 6–63. [Google Scholar] [PubMed]
- Shimizu, K.; Hanaoka, Y.; Akama, T.; Kono, I. Ageing and free-living daily physical activity effects on salivary beta-defensin 2 secretion. J. Sports Sci. 2017, 35, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Gillum, T.; Kuennen, M.; McKenna, Z.; Castillo, M.; Jordan-Patterson, A.; Bohnert, C. Exercise increases lactoferrin, but decreases lysozyme in salivary granulocytes. Eur. J. Appl. Physiol. 2017, 117, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Kumai, Y.; Akama, T.; Hayashi, E.; Murakami, H.; Soma, R.; Kuno, S.; Kono, I. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br. J. Sports Med. 2003, 37, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huo, X.; Zhang, Y.; Huang, Y.; Zheng, X.; Xu, X. Ambient fine particulate matter inhibits innate airway antimicrobial activity in preschool children in e-waste areas. Environ. Int. 2019, 123, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, J.; Zhou, J.; Wang, J.; Chen, C.; Song, Y.; Pan, J. Urban particulate matter (PM) suppresses airway antibacterial defence. Respir. Res. 2018, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yamamoto, Y.; Tanaka, M.; Kataoka, K.; Kuboniwa, M.; Nakayama, K.; Morimoto, K.; Shizukuishi, S. Association between involuntary smoking and salivary markers related to periodontitis: A 2-year longitudinal study. J. Periodontol. 2008, 79, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Carter, J.D.; Dailey, L.A.; Devlin, R.B.; Samet, J.M. Respiratory epithelial cells demonstrate lactoferrin receptors that increase after metal exposure. Am. J. Physiol. 1999, 276, L933–L940. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, G.S.; Houthuijs, D.; Steerenberg, P.A.; Fletcher, T.; Armstrong, B.; Antova, T.; Lochman, I.; Lochmanova, A.; Rudnai, P.; Erdei, E.; et al. Immune biomarkers in relation to exposure to particulate matter: A cross-sectional survey in 17 cities of Central Europe. Inhal. Toxicol. 2000, 12 (Suppl. 4), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ewing, P.; Otczyk, D.C.; Occhipinti, S.; Kyd, J.M.; Gleeson, M.; Cripps, A.W. Developmental profiles of mucosal immunity in pre-school children. Clin. Dev. Immunol. 2010, 2010, 196785. [Google Scholar] [CrossRef] [PubMed]
- Marth, E.; Möse, J.R.; Bencko, V.; Tichacek, B.; Sixl, W.; Köck, M.; Sixl-Voigt, B. Air pollution emissions and changes in lung function. Zentralblatt Hygiene Umweltmedizin 1989, 188, 439–448. [Google Scholar]
- Wagner, V.; Wagnerová, M.; Zavázal, V.; Kríz, J. Immunoglobulins and some serum proteins in children with altered resistance coming from areas with variously polluted atmosphere. J. Hyg. Epidemiol. Microbiol. Immunol. 1990, 34, 17–26. [Google Scholar] [PubMed]
- Wagner, V.; Wagnerová, M.; Kríz, J.; Kodl, M.; Wokounová, D. Relationship of blood protein levels to outdoor air pollutant concentrations in a semicohort of school-age children living in urban areas differing by quality of air. J. Hyg. Epidemiol. Microbiol. Immunol. 1988, 32, 121–136. [Google Scholar] [PubMed]
- Olin, A.C.; Rosengren, A.; Thelle, D.S.; Lissner, L.; Torén, K. Increased fraction of exhaled nitric oxide predicts new-onset wheeze in a general population. Am. J. Respir. Crit. Care Med. 2010, 181, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhu, T.; Hu, M.; Wu, Z.; Zhang, J.J. Different metrics (number, surface area, and volume concentration) of urban particles with varying sizes in relation to fractional exhaled nitric oxide (FeNO). J. Thorac. Dis. 2019, 11, 1714–1726. [Google Scholar] [CrossRef] [PubMed]
- Thornadtsson, A.; Drca, N.; Ricciardolo, F.; Högman, M. Increased levels of alveolar and airway exhaled nitric oxide in runners. Upsala J. Med. Sci. 2017, 122, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Carbonnelle, S.; Nickmilder, M.; de Burbure, C. Non-invasive biomarkers of pulmonary damage and inflammation: Application to children exposed to ozone and trichloramine. Toxicol. Appl. Pharmacol. 2005, 206, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Seys, S.F.; Hox, V.; Van Gerven, L.; Dilissen, E.; Marijsse, G.; Peeters, E.; Dekimpe, E.; Kasran, A.; Aertgeerts, S.; Troosters, T.; et al. Damage-associated molecular pattern and innate cytokine release in the airways of competitive swimmers. Allergy 2015, 70, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.C.; Stone, V.; Florida-James, G. Impact of heat and pollution on oxidative stress and CC16 secretion after 8 km run. Eur. J. Appl. Physiol. 2011, 111, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.; London, N.R.; Tharakan, A.; Surya, N.; Sussan, T.E.; Rao, X.; Lin, S.Y.; Toskala, E.; Rajagopalan, S.; Biswal, S. Airborne Particulate Matter Induces Nonallergic Eosinophilic Sinonasal Inflammation in Mice. Am. J. Respir. Cell Mol. Biol. 2017, 57, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.L.; Liu, S.Y.; Chou, C.C.; Lee, Y.H.; Cheng, T.J. The effect of size-segregated ambient particulate matter on Th1/Th2-like immune responses in mice. PLoS ONE 2017, 12, e0173158. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Townley, R.G. Role of periostin, FENO, IL-13, lebrikzumab, other IL-13 antagonist and dual IL-4/IL-13 antagonist in asthma. Expert Opin. Biol. Ther. 2014, 14, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, D.; Youroukova, V.; Ivanova-Todorova, E.; Tumangelova-Yuzeir, K.; Velikova, T. Serum levels of IL-5, IL-6, IL-8, IL-13 and IL-17A in pre-defined groups of adult patients with moderate and severe bronchial asthma. Respir. Med. 2019, 154, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Mehrotra, S.; Agarwal, S.S. The paradigm of Th1 and Th2 cytokines: Its relevance to autoimmunity and allergy. Immunol Res. 1999, 20, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhao, L.; Xu, C.; Zhang, L.; Zhao, H. High Molecular Weight Hyaluronan Suppresses Macrophage M1 Polarization and Enhances IL-10 Production in PM. Molecules 2019, 24, 1766. [Google Scholar] [CrossRef] [PubMed]
- Vaisberg, M.; Suguri, V.M.; Gregorio, L.C.; Lopes, J.D.; Bachi, A.L. Cytokine kinetics in nasal mucosa and sera: New insights in understanding upper-airway disease of marathon runners. Exerc. Immunol. Rev. 2013, 19, 49–59. [Google Scholar] [PubMed]
- Mayer, A.K.; Bartz, H.; Fey, F.; Schmidt, L.M.; Dalpke, A.H. Airway epithelial cells modify immune responses by inducing an anti-inflammatory microenvironment. Eur. J. Immunol. 2008, 38, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.H.; Song, S.H.; Guo, M.; Zhou, J.; Liu, F.; Peng, L.; Fu, Z.R. Long-term exposure to PM2.5 lowers influenza virus resistance via down-regulating pulmonary macrophage Kdm6a and mediates histones modification in IL-6 and IFN-β promoter regions. Biochem. Biophys. Res. Commun. 2017, 493, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- De Grove, K.C.; Provoost, S.; Brusselle, G.G.; Joos, G.F.; Maes, T. Insights in particulate matter-induced allergic airway inflammation: Focus on the epithelium. Clin. Exp. Allergy 2018, 48, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Liu, J.; Fei, G.; Han, T.; Zhang, K.; Wang, L.; Shi, H.; Zhang, L.; Ruan, Y.; Niu, J. Impact of probable interaction of low temperature and ambient fine particulate matter on the function of rats alveolar macrophages. Environ. Toxicol. Pharmacol. 2017, 49, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, Y.; James, C.; Bernstein, D.I. The Effects of Air Pollution on the Development of Atopic Disease. Clin. Rev. Allergy Immunol. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Domvri, K.; Porpodis, K.; Tzimagiorgis, G.; Chatzopoulou, F.; Kontakiotis, T.; Kyriazis, G.; Papakosta, D. Th2/Th17 cytokine profile in phenotyped Greek asthmatics and relationship to biomarkers of inflammation. Respir. Med. 2019, 151, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.; Kurowski, M.; Moreira, A.; Bullens, D.M.A.; Carlsen, K.H.; Delgado, L.; Kowalski, M.L.; Seys, S.F. Mechanisms of exercise-induced bronchoconstriction in athletes: Current perspectives and future challenges. Allergy 2018, 73, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Huang, D.Y.; Zhang, H.J.; Wang, S.; Chen, X.F. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int. Immunopharmacol. 2017, 50, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Gallegos, M.A.; Ramírez-Venegas, A.; Falfán-Valencia, R. Th17 profile in COPD exacerbations. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Naji, N.; Smith, S.G.; Gauvreau, G.M.; O’Byrne, P.M. T helper 17 cells and related cytokines after allergen inhalation challenge in allergic asthmatics. Int. Arch. Allergy Immunol. 2014, 165, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Ciobanu, C.; Agache, C.; Anghel, M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir. Med. 2010, 104, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, L.; Liotta, F.; Annunziato, F. Th17 regulating lower airway disease. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Schnepf, D.; Becker, J.; Ebert, K.; Tanriver, Y.; Bernasconi, V.; Gad, H.H.; Hartmann, R.; Lycke, N.; Staeheli, P. Interferon-λ enhances adaptive mucosal immunity by boosting release of thymic stromal lymphopoietin. Nat. Immunol. 2019, 20, 593–601. [Google Scholar] [CrossRef] [PubMed]

| Runners | Sedentary | p Value | |
|---|---|---|---|
| Number (n) | 20 | 20 | |
| Gender | |||
| Women (n) | 4 | 8 | |
| Men (n) | 16 | 12 | |
| Ratio (W/M) | 1/4 | 1/1.5 | 0.1675 |
| Age (years) | 37.4 ± 8.99 | 31.8 ± 10.27 | 0.0746 |
| Weight (kg) | 73.9 ± 16.21 | 77.6 ± 19.65 | 0.5267 |
| Height (cm) | 172 ± 9.72 | 172.2 ± 10.02 | 0.9527 |
| BMI | 24.7 ± 3.81 | 26.0 ± 5.65 | 0.6964 |
| Total body fat (%) | 24.9 ± 6.69 | 30.5 ± 7.17 | 0.0187 |
| O2max (mL/kg/min) | 41.8 ± 6.54 | 29.3 ± 5.02 | <0.0001 |
| Weeks for Measuring Pollutants | PM2.5 | PM10 | O3 |
|---|---|---|---|
| Five weeks prior to first sampling day | 17.72 ± 9.6 | 27.07 ± 13.76 | 19.75 ± 8.24 |
| First sampling week | 32.24 ± 12.28 * | 50.18 ± 15.56 # | 22.67 ± 5.2 |
| Four weeks prior to the sampling week | 14.71 ± 5.52 | 22.29 ± 6.9 | 19.14 ± 8.68 |
| Ratio between sampling week and the previous 4 weeks | 2.19 | 2.25 | 1.18 |
| Ten weeks between sampling days | 22.49 ± 8.90 | 37.39 ± 15.23 | 29.18 ± 8.77 |
| Nine weeks prior to second sampling day | 21.66 ± 8.52 | 31.29 ± 8.17 | 35.96 ± 14.22 |
| Second sampling week | 20.8 ± 12.6 | 34.47 ± 18.55 | 37.24 ± 3.7 |
| Ratio between sampling week and the previous 9 weeks | 0.96 | 1.1 | 1.03 |
| Runners | Sedentary | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | p | Mean ± SD | p | |||
| Th1/Th2 | IFN-γ/IL-6 | Acute | 15.81 ± 2.90 | 0.702 | 17.25 ± 2.61 | 0.493 |
| Chronic | 12.48 ± 2.18 | 16.27 ± 3.42 | ||||
| IFN-γ/IL-10 | Acute | 38.61 ± 0.04 | 0.791 | 38.61 ± 0.03 | 0.016 | |
| Chronic | 43.35 ± 4.20 | 32.51 ± 3.04 | ||||
| IFN-γ/IL-13 | Acute | 0.78 ± 0.00 | 0.091 | 0.78 ± 0.00 | 0.558 | |
| Chronic | 1.21 ± 0.20 | 0.77 ± 0.07 | ||||
| TNF-α/IL-6 | Acute | 2.88 ± 0.65 | 0.393 | 3.20 ± 0.39 | 0.035 | |
| Chronic | 1.89 ± 0.38 | 2.15 ± 0.35 | ||||
| TNF-α/IL-10 | Acute | 6.81 ± 0.00 | 0.052 | 6.83 ± 0.00 | <0.0001 | |
| Chronic | 6.18 ± 0.50 | 4.36 ± 0.53 | ||||
| TNF-α/IL-13 | Acute | 0.13 ± 0.00 | 0.023 | 0.13 ± 0.00 | 0.397 | |
| Chronic | 0.21 ± 0.02 | 0.20 ± 0.03 | ||||
| Th1/Th17 | IFN-γ/IL-17 | Acute | 1.23 ± 0.18 | 0.126 | 1.40 ± 0.22 | 0.101 |
| Chronic | 0.88 ± 0.18 | 0.95 ± 0.17 | ||||
| TNF-α/IL-17 | Acute | 0.22 ± 0.03 | 0.149 | 0.31 ± 0.05 | 0.179 | |
| Chronic | 0.15 ± 0.032 | 0.17 ± 0.02 | ||||
| Th2/Th17 | IL-6/IL-17 | Acute | 0.08 ± 0.013 | 0.423 | 0.06 ± 0.00 | 0.922 |
| Chronic | 0.06 ± 0.00 | 0.06 ± 0.01 | ||||
| IL-10/IL-17 | Acute | 0.02 ± 0.00 | 0.392 | 0.03 ± 0.00 | 0.134 | |
| Chronic | 0.02 ± 0.00 | 0.02 ± 0.00 | ||||
| IL-13/IL-17 | Acute | 1.34 ± 0.25 | 0.015 | 1.80 ± 0.25 | 0.001 | |
| Chronic | 0.53 ± 0.14 | 0.54 ± 0.10 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.d.M.B.d.; Foster, R.; Jonckheere, A.-C.; Rossi, M.; Luna Junior, L.A.; Katekaru, C.M.; de Sá, M.C.; Pagani, L.G.; Almeida, F.M.d.; Amaral, J.d.B.; et al. Outdoor Endurance Training with Air Pollutant Exposure Versus Sedentary Lifestyle: A Comparison of Airway Immune Responses. Int. J. Environ. Res. Public Health 2019, 16, 4418. https://doi.org/10.3390/ijerph16224418
Santos JdMBd, Foster R, Jonckheere A-C, Rossi M, Luna Junior LA, Katekaru CM, de Sá MC, Pagani LG, Almeida FMd, Amaral JdB, et al. Outdoor Endurance Training with Air Pollutant Exposure Versus Sedentary Lifestyle: A Comparison of Airway Immune Responses. International Journal of Environmental Research and Public Health. 2019; 16(22):4418. https://doi.org/10.3390/ijerph16224418
Chicago/Turabian StyleSantos, Juliana de Melo Batista dos, Roberta Foster, Anne-Charlotte Jonckheere, Marcelo Rossi, Luiz Antonio Luna Junior, Catherine Machado Katekaru, Matheus Cavalcante de Sá, Lucas Guimarães Pagani, Francine Maria de Almeida, Jônatas do Bussador Amaral, and et al. 2019. "Outdoor Endurance Training with Air Pollutant Exposure Versus Sedentary Lifestyle: A Comparison of Airway Immune Responses" International Journal of Environmental Research and Public Health 16, no. 22: 4418. https://doi.org/10.3390/ijerph16224418
APA StyleSantos, J. d. M. B. d., Foster, R., Jonckheere, A.-C., Rossi, M., Luna Junior, L. A., Katekaru, C. M., de Sá, M. C., Pagani, L. G., Almeida, F. M. d., Amaral, J. d. B., Vieira, R. d. P., Bachi, A. L. L., Bullens, D. M. A., & Vaisberg, M. (2019). Outdoor Endurance Training with Air Pollutant Exposure Versus Sedentary Lifestyle: A Comparison of Airway Immune Responses. International Journal of Environmental Research and Public Health, 16(22), 4418. https://doi.org/10.3390/ijerph16224418








