Investigation of Microenvironmental Exposures to Particle-Bound Polycyclic Aromatic Hydrocarbons for Elementary School Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subject Characteristics
2.2. Instrumental Measurement and Laboratory Analysis
2.3. Data Processing and Statistical Analysis
3. Results and Discussion
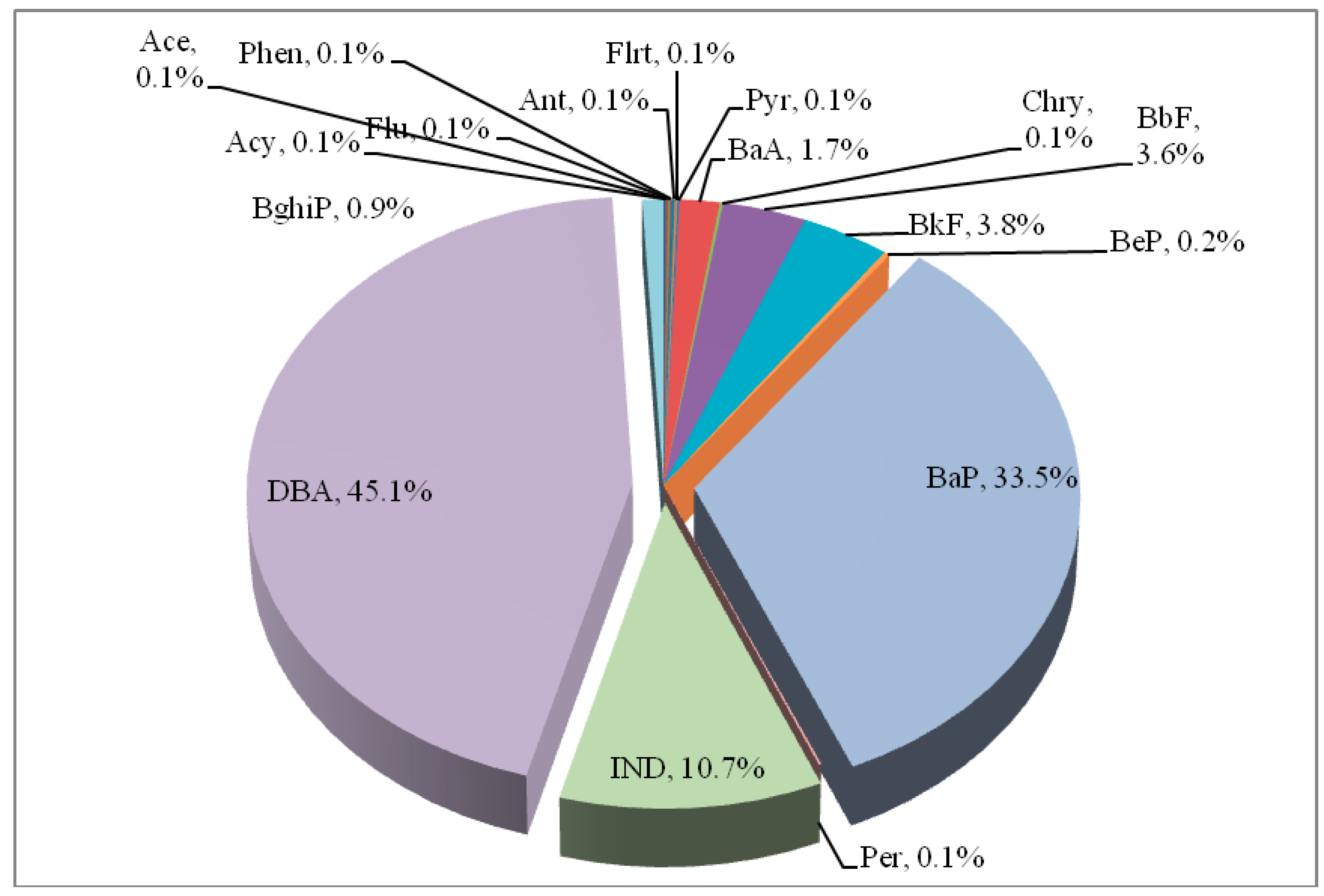
3.1. Descriptive Analyses for p-PAHs Exposures
3.2. Analysis of p-PAHs Exposures Based on Univariate Analysis and Diagnostic Ratios
3.3. Principal Component Analysis for p-PAH Exposure Sources
3.4. BaPeq Concentratons and Inhalation Cancer Risk Assessment
3.5. Strength and Limitations of the Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, N.; Mengersen, K.; Kimlin, M.; Zhou, M.; Tong, S.; Fang, L.; Wang, B.; Hu, W. Lung cancer and particulate pollution: A critical review of spatial and temporal analysis evidence. Environ. Res. 2018, 164, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, R.; Chen, Z.; Li, Q.; Huang, S.; Zhu, Z.; Zhou, L.F. Fine particulate matter (PM2.5): The culprit for chronic lung diseases in China. Chronic Dis. Transl. Med. 2018, 4, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Talbott, E.O.; Rager, J.R.; Benson, S.; Brink, L.A.; Bilonick, R.A.; Wu, C. A case-crossover analysis of the impact of PM2.5 on cardiovascular disease hospitalizations for selected CDC tracking states. Environ. Res. 2014, 134, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Nikasinovic, L.; Just, J.; Sahraoui, F.; Seta, N.; Grimfeld, A.; Momas, I. Nasal inflammation and personal exposure to fine particles PM2.5 in asthmatic children. J. Allergy Clin. Immunol. 2006, 117, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Buteau, S.; Goldberg, M.S. A structured review of panel studies used to investigate associations between ambient air pollution and heart rate variability. Environ. Res. 2016, 148, 207–247. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.R.; Young, L.H.; Hsu, H.T.; Lin, M.Y.; Chen, Y.C.; Hwang, B.F.; Tsai, P.J. PM2.5 components and outpatient visits for asthma: A time-stratified case-crossover study in a suburban area. Environ. Pollut. 2017, 231, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Feretti, D.; Pedrazzani, R.; Ceretti, E.; Dal Grande, M.; Zerbini, I.; Viola, G.C.V.; Gelatti, U.; Donato, F.; Zani, C. “Risk is in the air”: Polycyclic aromatic hydrocarbons, metals and mutagenicity of atmospheric particulate matter in a town of Northern Italy (Respira study). Mutat. Res. 2019, 842, 35–49. [Google Scholar] [CrossRef]
- Chang, K.F.; Fang, G.C.; Chen, J.C.; Wu, Y.S. Atmospheric polycyclic aromatic hydrocarbons (PAHs) in Asia: A review from 1999 to 2004. Environ. Pollut. 2006, 142, 388–396. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, L.; Zheng, X.; Zhang, S.; Song, S.; Li, J.; Hao, J. Characterization and source apportionment of particulate PAHs in the roadside environment in Beijing. Sci. Total Environ. 2014, 470–471, 76–83. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chiang, H.C.; Hsu, C.Y.; Yang, T.T.; Lin, T.Y.; Chen, M.J.; Chen, N.T.; Wu, Y.S. Ambient PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in Changhua County, central Taiwan: Seasonal variation, source apportionment and cancer risk assessment. Environ. Pollut. 2016, 218, 372–382. [Google Scholar] [CrossRef]
- Yang, T.T.; Hsu, C.Y.; Chen, Y.C.; Young, L.H.; Huang, C.H.; Ku, C.H. Characteristics, sources, and health risks of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons in Hsinchu, Taiwan. Aerosol Air Qual. Res. 2017, 17, 563–573. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fan, S.; Meng, Q.; Sun, Y.; Zhang, J.; Zu, F. Polycyclic aromatic hydrocarbons (PAHs) associated with fine particulate matters in Nanjing, China: Distributions, sources and meteorological influences. Atmos. Environ. 2014, 89, 207–215. [Google Scholar] [CrossRef]
- Bootdee, S.; Chantara, S.; Prapamontol, T. Determination of PM2.5 and polycyclic aromatic hydrocarbons from incense burning emission at shrine for health risk assessment. Atmos. Pollut. Res. 2016, 7, 680–689. [Google Scholar] [CrossRef]
- IARC-International Agency for Research on Cancer. Outdoor Air Pollution/IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; IARC-International Agency for Research on Cancer: Lyon, France, 2013; Available online: https://publications.iarc.fr/_publications/media/download/4317/b1f528f1fca20965a2b48a220f47447c1d94e6d1.pdf (accessed on 30 September 2019).
- Dubowsky, S.D.; Wallace, L.A.; Buckley, T.J. The contribution of traffic to indoor concentration of polycyclic aromatic hydrocarbons. J. Expo. Anal. Environ. Epidemiol. 1999, 9, 312–321. [Google Scholar] [CrossRef][Green Version]
- Fromme, H.; Lahrz, T.; Piloty, M.; Gebhardt, H.; Oddoy, A.; Ru¨den, H. Polycyclic aromatic hydrocarbons inside and outside of apartments in an urban area. Sci. Total Environ. 2004, 326, 143–149. [Google Scholar] [CrossRef]
- Ohura, T.; Amagai, T.; Fusaya, M.; Matsushita, H. Polycyclic aromatic hydrocarbons in indoor and outdoor environments and factors affecting their concentrations. Environ. Sci. Technol. 2004, 38, 77–83. [Google Scholar] [CrossRef]
- Tang, C.S.; Chang, L.T.; Lee, H.C.; Chan, C.C. Effects of personal particulate matter on peak expiratory flow rate of asthmatic children. Sci. Total Environ. 2007, 382, 43–51. [Google Scholar] [CrossRef]
- Hopke, P.K.; Gladney, E.S.; Gordon, G.E.; Zoller, W.H.; Jones, A.G. The use of multivariate analysis to identify sources of selected elements in the Boston urban aerosol. Atmos. Environ. 1976, 10, 1015–1025. [Google Scholar] [CrossRef]
- Tsai, P.J.; Shih, T.S.; Chen, H.L.; Lee, W.J.; Lai, C.H.; Liou, S.H. Assessing and predicting the exposures of polycyclic aromatic hydrocarbons (PAHs) and their carcinogenic potencies from vehicle engine exhausts to highway toll station workers. Atmos. Environ. 2004, 38, 333–343. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality Guidelines for Europe, 2nd ed.; WHO, Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Ohura, T.; Noda, T.; Amagai, T.; Fusaya, M. Prediction of personal exposure to PM2.5 and carcinogenic polycyclic aromatic hydrocarbons by their concentrations in residential microenvironments. Environ. Sci. Technol. 2005, 39, 5592–5599. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.I.; Dumyahn, T.; Spengler, J.D. Particulate matter and polycyclic aromatic hydrocarbon concentrations in indoor and outdoor microenvironments in Boston, Massachusetts. J. Expo. Anal. Environ. Epidemiol. 2002, 12, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Naumova, Y.Y.; Eisenreich, S.J.; Turpin, B.J.; Weisel, C.P.; Morandi, M.T.; Colome, S.D.; Totten, L.A.; Stock, T.H.; Winer, A.M.; Alimokhtari, S.; et al. Polycyclic aromatic hydrocarbons in the indoor and outdoor air of three cities in the U.S. Environ. Sci. Technol. 2002, 36, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.K.; Savolainen, H.; Vu Duc, T.; Guillemin, M.; Iselin, F. Impact of thermal proofing of a church on its indoor air quality: The combustion of candles and incense as a source of pollution. Sci. Total Environ. 1991, 102, 241–251. [Google Scholar] [CrossRef]
- Li, C.S.; Ro, Y.S. Indoor characteristics of polycyclic aromatic hydrocarbons in the urban atmosphere of Taipei. Atmos. Environ. 2000, 34, 611–620. [Google Scholar] [CrossRef]
- Lung, S.C.; Hu, S.C. Generation rates and emission factors of particulate matter and particle-bound polycyclic aromatic hydrocarbons of incense sticks. Chemosphere 2003, 50, 673–679. [Google Scholar] [CrossRef]
- Lung, S.C.; Kao, M.C.; Hu, S.C. Contribution of incense burning to indoor PM10 and particle-bound polycyclic aromatic hydrocarbons under two ventilation conditions. Indoor Air 2003, 13, 194–199. [Google Scholar] [CrossRef]
- Obiszewski, M.; Namiesnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Li, C.K.; Kamens, R.M. The use of polycyclic aromatic hydrocarbons as sources signatures in receptor modeling. Atmos. Environ. 1993, 27, 523–532. [Google Scholar] [CrossRef]
- Masclet, P.; Mouvier, G.; Nikolaou, K. Relative decay index and sources of polycyclic aromatic hydrocarbon. Atmos. Environ. 1986, 20, 439–446. [Google Scholar] [CrossRef]
- Simcik, M.F.; Eisenreich, S.J.; Lioy, P.J. Source apportionment and source/sink relationships of PAHs in the coastal atmosphere of Chicago and Lake Michigan. Atmos. Environ. 1999, 33, 5071–5079. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, Y.J.; Kang, C.H. Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmos. Environ. 2002, 36, 2917–2924. [Google Scholar] [CrossRef]
- Caricchia, A.M.; Chiavarini, S.; Pezza, M. Polycyclic aromatic hydrocarbons in the urban atmospheric particulate matter in the city of Naples (Italy). Atmos. Environ. 1999, 33, 3731–3738. [Google Scholar] [CrossRef]
- Manoli, E.; Kouras, A.; Samara, K. Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece. Chemosphere 2004, 56, 867–878. [Google Scholar] [CrossRef]
- Daisey, J.M.; Cheney, J.L.; Lioy, P.J. Profiles of organic particulate emissions from air pollution source: Status and needs for receptor source apportionment modeling. J. Air Pollut. Control Assoc. 1986, 36, 17–33. [Google Scholar] [CrossRef]
- Khalili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Kavouras, I.G.; Koutrakis, P.; Tsapakis, M.; Lagoudaki, E.; Stephanou, E.G.; Von Baer, D.; Oyola, P. Source apportionment of urban particulate aliphatic and polycyclic aromatic hydrocarbons (PAHs) using multivariate methods. Environ. Sci. Technol. 2001, 35, 2288–2294. [Google Scholar] [CrossRef]
- Harrison, R.M.; Smith, D.J.T.; Luhana, L. Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, U.K. Environ. Sci. Technol. 1996, 30, 825–832. [Google Scholar] [CrossRef]
- Miguel, A.H.; Kirchstetter, T.W.; Harley, R.A. On-road emissions of particulate polycyclic aromatic hydrocarbons and black carbon from gasoline and diesel vehicles. Environ. Sci. Technol. 1998, 32, 450–455. [Google Scholar] [CrossRef]
- Mi, H.H. Emission Characteristics of Polycyclic Aromatic Hydrocarbons from Mobile Sources. Ph.D. Thesis, National Cheng Kung University, Tainan, Taiwan, 1998. [Google Scholar]
- Huynh, C.K.; Vu Duc, T.; Schwab, C.; Rollier, H. In-stack dilution technique for the sampling of polycyclic aromatic compounds. Application to effluents of a domestic waste incineration plant. Atmos. Environ. 1984, 18, 255–259. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, S.C.; Chen, T.H.; Wang, J.Z. Evaluation of inhalation exposure to carcinogenic PM10-bound PAHs of people at night markets of an urban area in a metropolis in eastern China. Aerosol Air Qual. Res. 2015, 15, 1944–1954. [Google Scholar] [CrossRef]
- Wu, M.T.; Lin, P.C.; Pan, C.H.; Peng, C.Y. Risk assessment of personal exposure to polycyclic aromatic hydrocarbons and aldehydes in three commercial cooking workplaces. Sci. Rep. 2019, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, I.C.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Petry, T.; Schmid, P.; Schlatter, C. The use of toxic equivalency factors in assessing occupational and environmental health risk associated with exposure to airborne mixtures of polycyclic aromatic hydrocarbons (PAHs). Chemosphere 1996, 32, 639–648. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, S.J.; Huang, K.L.; Lee, W.J.; Lin, W.Y.; Tsai, J.H.; Chaung, H.C. PAHs, PAH-induced carcinogenic potency, and particle-extract-induced cytotoxicity of traffic-related nano/ultrafine particles. Environ. Sci. Technol. 2008, 42, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.C.; Wu, Y.S.; Chen, J.C.; Fu, P.P.C.; Chang, C.N.; Ho, T.T.; Chen, M.H. Characteristic study of polycyclic aromatic hydrocarbons for fine and coarse particulates at pastureland near industrial park sampling site of central Taiwan. Chemosphere 2005, 60, 427–433. [Google Scholar] [CrossRef]
- Mao, I.F.; Chen, C.N.; Lin, Y.C.; Chen, M.L. Airborne particle PM2.5/PM10 mass distribution and particle-bound PAH concentrations near a medical waste incinerator. Atmos. Environ. 2007, 41, 2467–2475. [Google Scholar] [CrossRef]
- Chiang, K.C.; Chio, C.P.; Chiang, Y.H.; Liao, C.M. Assessing hazardous risks of human exposure to temple airborne polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2009, 166, 676–685. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Hsu, Y.W.; Lee, H.S. Study of human exposure to particulate PAHs using personal air samplers. Arch. Environ. Contam. Toxicol. 2003, 44, 454–459. [Google Scholar] [CrossRef]
- Lin, Y.C.; Pan, C.H.; Chen, C.J.; Wu, K.Y.; Chang-Chien, G.P.; Ho, C.K.; Wu, T.N.; Chuang, H.Y.; Kuo, H.W.; Wu, M.T. Associations between exposure to polycyclic aromatic hydrocarbons and temporal change of urinary 1-hydroxypyrene levels in Taiwanese coke-oven workers. J. Occup. Environ. Med. 2006, 48, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Slezakova, K.; Delerue-Matos, C.; Pereira, M.C.; Morais, S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int. 2019, 124, 180–204. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Armstrong, B.; Hutchinson, E.; Unwin, J.; Fletcher, T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: A review and meta-analysis. Environ. Health Perspect. 2004, 112, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, N.; Cuadras, A.; Rovira, E.; Marcé, R.M.; Borrull, F. Risk assessment related to atmospheric polycyclic aromatic hydrocarbons in gas and particle phases near industrial sites. Environ. Health Perspect. 2011, 119, 1110–1116. [Google Scholar] [CrossRef]
| Compound | Abbreviation | Number of Rings | Molecular Weight (g/mol) | Recovery (%) (Mean ± SD 1) | MDL 2 (pg/μL) |
|---|---|---|---|---|---|
| Acenaphthylene | Acy | 3 | 152 | 77.4 ± 12.5% | 5 |
| Acenaphthene | Ace | 3 | 154 | 74.4 ± 14.4% | 5 |
| Fluorene | Flu | 3 | 166 | 78.5 ± 8.4% | 5 |
| Phenanthrene | Phen | 3 | 178 | 82.6 ± 14.8% | 5 |
| Anthracene | Ant | 3 | 178 | 74.0 ± 7.3% | 5 |
| Fluoranthene | Flrt | 4 | 202 | 78.5 ± 8.4% | 5 |
| Pyrene | Pyr | 4 | 202 | 90.3 ± 15.6% | 5 |
| Benz[a]anthracene | BaA | 4 | 228 | 98.6 ± 21.1% | 10 |
| Chrysene | Chry | 4 | 228 | 91.2 ± 22.6% | 10 |
| Benzo[b]fluoranthene | BbF | 5 | 252 | 94.2 ± 9.3% | 20 |
| Benzo[k]fluoranthene | BkF | 5 | 252 | 93.3 ± 24.2% | 20 |
| Benz[e]pyrene | BeP | 5 | 252 | 95.1 ± 16.8% | 20 |
| Benzo[a]pyrene | BaP | 5 | 252 | 92.0 ± 19.5% | 20 |
| Perylene | Per | 5 | 252 | 91.8 ± 14.5% | 20 |
| Indeno[1,2,3-cd]pyrene | IND | 6 | 276 | 94.1 ± 23.6% | 10 |
| Dibenz[a,h]anthracene | DBA | 5 | 278 | 87.3 ± 18.8% | 10 |
| Benzo[ghi]perylene | BghiP | 6 | 276 | 93.4±17.6% | 10 |
| Compound | GM 1 | GSD 2 | Minimum | Median | Maximum |
|---|---|---|---|---|---|
| Acy | 0.055 | 0.056 | 0.021 | 0.042 | 0.257 |
| Ace | 0.043 | 0.050 | 0.029 | 0.039 | 0.388 |
| Flu | 0.059 | 0.140 | 0.021 | 0.040 | 0.544 |
| Phen | 0.062 | 0.169 | 0.029 | 0.040 | 0.752 |
| Ant | 0.054 | 0.191 | 0.029 | 0.040 | 0.947 |
| Flrt | 0.096 | 0.250 | 0.029 | 0.052 | 0.944 |
| Pyr | 0.077 | 0.263 | 0.021 | 0.042 | 1.020 |
| BaA | 0.055 | 0.646 | 0.029 | 0.040 | 4.602 |
| Chry | 0.055 | 0.553 | 0.029 | 0.040 | 3.896 |
| BbF | 0.243 | 0.474 | 0.041 | 0.246 | 2.965 |
| BkF | 0.164 | 0.876 | 0.058 | 0.103 | 4.817 |
| BeP | 0.170 | 0.290 | 0.058 | 0.134 | 1.443 |
| BaP | 0.233 | 0.400 | 0.041 | 0.266 | 2.478 |
| Per | 0.103 | 0.180 | 0.058 | 0.081 | 0.843 |
| IND | 0.894 | 0.772 | 0.146 | 0.961 | 4.170 |
| DBA | 0.298 | 0.557 | 0.115 | 0.185 | 2.563 |
| BghiP | 0.786 | 0.587 | 0.082 | 0.821 | 2.285 |
| Total p-PAHs 3 | 4.443 | 3.395 | 1.292 | 4.127 | 17.468 |
| Compound | ETS 2 | Incense burning | ||||
|---|---|---|---|---|---|---|
| Non-Exposed 3 (n = 39) | Exposed 3 (n = 13) | p-Value | Non-Exposed 3 (n = 37) | Exposed 3 (n = 15) | p-Value | |
| Acy | 0.043 | 0.041 | 0.59 | 0.040 | 0.046 | 0.44 |
| Ace | 0.039 | 0.037 | 0.22 | 0.038 | 0.040 | 0.55 |
| Flu | 0.040 | 0.037 | 0.37 | 0.038 | 0.046 | 0.038* |
| Phen | 0.041 | 0.039 | 0.63 | 0.039 | 0.054 | 0.023* |
| Ant | 0.040 | 0.037 | 0.27 | 0.038 | 0.041 | 0.12 |
| Flrt | 0.050 | 0.104 | 0.73 | 0.045 | 0.175 | 0.06 |
| Pyr | 0.045 | 0.039 | 0.45 | 0.040 | 0.078 | 0.038* |
| BaA | 0.043 | 0.037 | 0.37 | 0.038 | 0.058 | 0.013* |
| Chry | 0.041 | 0.037 | 0.36 | 0.038 | 0.051 | 0.009* |
| BbF | 0.182 | 0.305 | 0.26 | 0.218 | 0.437 | 0.37 |
| BkF | 0.099 | 0.159 | 0.89 | 0.098 | 0.159 | 0.10 |
| BeP | 0.108 | 0.237 | 0.05 | 0.111 | 0.292 | 0.16 |
| BaP | 0.237 | 0.367 | 0.27 | 0.237 | 0.407 | 0.18 |
| Per | 0.081 | 0.081 | 0.98 | 0.079 | 0.092 | 0.22 |
| IND | 0.864 | 1.672 | 0.003* | 0.901 | 1.415 | 0.12 |
| DBA | 0.185 | 0.161 | 0.71 | 0.162 | 0.313 | 0.10 |
| BghiP | 0.738 | 1.516 | 0.002* | 0.766 | 1.156 | 0.26 |
| Total p-PAHs 4 | 3.312 | 6.241 | 0.06 | 3.312 | 7.163 | 0.009 * |
| Diagnostic Ratio | Value | Pollution Source | Literature |
|---|---|---|---|
| BaA/BaP | 0.5 | Gasoline exhaust | 32 |
| 1.0 | Diesel exhaust | ||
| BaA/Chry | 0.28–1.2 | Gasoline exhaust | |
| 0.17–0.36 | Diesel exhaust | ||
| BaP/BghiP | 1.25 1.7 | Motor vehicle emissions Home heating | 33 |
| BaP/BghiP | 0.30–0.78 | Affected by the emission of motor vehicles, the greater the ratio, the greater the impact | 34 |
| BaP/BghiP | 0.3–0.4 0.46–0.81 | Gasoline exhaust Diesel exhaust | 34 |
| BaP/BghiP | >0.6 | Motor vehicle exhaust, indicating a significant traffic flow | 35 |
| IND/BghiP | 0.4 1.0 | Gasoline exhaust Diesel exhaust | 36 |
| BghiP/BaP | 1.16 | Diesel exhaust | 37 |
| BghiP/IND | 3.5 1.1 | Gasoline exhaust Diesel exhaust | 32 |
| Flrt/Pyr | 1 | Motor vehicle | 38 |
| Flrt/Pyr | 0.15 0.5 1.7 3 65 | Urban incinerator Petroleum refinery Fertilizer plant Coal-fired power plant Steel mill | 33 |
| BaP/(BaP+CHR) Flrt/(Flrt+Pyr) IND/(IND+BghiP) | 0.49 <0.5 0.18 | Gasoline exhaust | 39, 40 |
| BaP/(BaP+CHR) Flrt/(Flrt+Pyr) IND/(IND+BghiP) | 0.73 >0.5 0.35–0.70 | Diesel exhaust | 39, 40 |
| PC * | Factor Loading | Eigenvalue | Explained Variance |
|---|---|---|---|
| PC 1 | 4.431 | 25.54% | |
| BaP | 0.902 | ||
| BbF | 0.895 | ||
| BghiP | 0.860 | ||
| BeP | 0.849 | ||
| IND | 0.808 | ||
| PC 2 | 4.064 | 23.90% | |
| Pyr | 0.933 | ||
| Flu | 0.926 | ||
| DBA | 0.861 | ||
| Flrt | 0.667 | ||
| Acy | 0.147 | ||
| PC 3 | 1.993 | 11.72% | |
| Chy | 0.964 | ||
| BaA | 0.948 | ||
| BkF | 0.560 | ||
| PC 4 | 1.595 | 9.38% | |
| Ant | 0.855 | ||
| Pnen | 0.834 | ||
| PC 5 | 1.283 | 7.55% | |
| Ace | 0.835 | ||
| Per | 0.800 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.-S.; Lung, S.-C.C.; Chang, T.-Y.; Tu, H.-H.; Chang, L.-T. Investigation of Microenvironmental Exposures to Particle-Bound Polycyclic Aromatic Hydrocarbons for Elementary School Children. Int. J. Environ. Res. Public Health 2019, 16, 4390. https://doi.org/10.3390/ijerph16224390
Tang C-S, Lung S-CC, Chang T-Y, Tu H-H, Chang L-T. Investigation of Microenvironmental Exposures to Particle-Bound Polycyclic Aromatic Hydrocarbons for Elementary School Children. International Journal of Environmental Research and Public Health. 2019; 16(22):4390. https://doi.org/10.3390/ijerph16224390
Chicago/Turabian StyleTang, Chin-Sheng, Shih-Chun Candice Lung, Ta-Yuan Chang, Han-Hsiang Tu, and Li-Te Chang. 2019. "Investigation of Microenvironmental Exposures to Particle-Bound Polycyclic Aromatic Hydrocarbons for Elementary School Children" International Journal of Environmental Research and Public Health 16, no. 22: 4390. https://doi.org/10.3390/ijerph16224390
APA StyleTang, C.-S., Lung, S.-C. C., Chang, T.-Y., Tu, H.-H., & Chang, L.-T. (2019). Investigation of Microenvironmental Exposures to Particle-Bound Polycyclic Aromatic Hydrocarbons for Elementary School Children. International Journal of Environmental Research and Public Health, 16(22), 4390. https://doi.org/10.3390/ijerph16224390







