Two Drinking Water Outbreaks Caused by Wastewater Intrusion Including Sapovirus in Finland
Abstract
1. Introduction
2. Materials and Methods
2.1. Outbreak Descriptions and Samples
2.1.1. Outbreak I
2.1.2. Outbreak II
2.2. Environmental Investigation
2.2.1. Sample Collection and Concentration
2.2.2. Detection of Enteric Virus Genomes
2.2.3. Enumeration of Indicator Bacteria
2.2.4. Detection of Microbial Source Tracking (MST) Markers
2.2.5. Detection of Bacterial Pathogens
2.2.6. Detection of Protozoans
3. Results
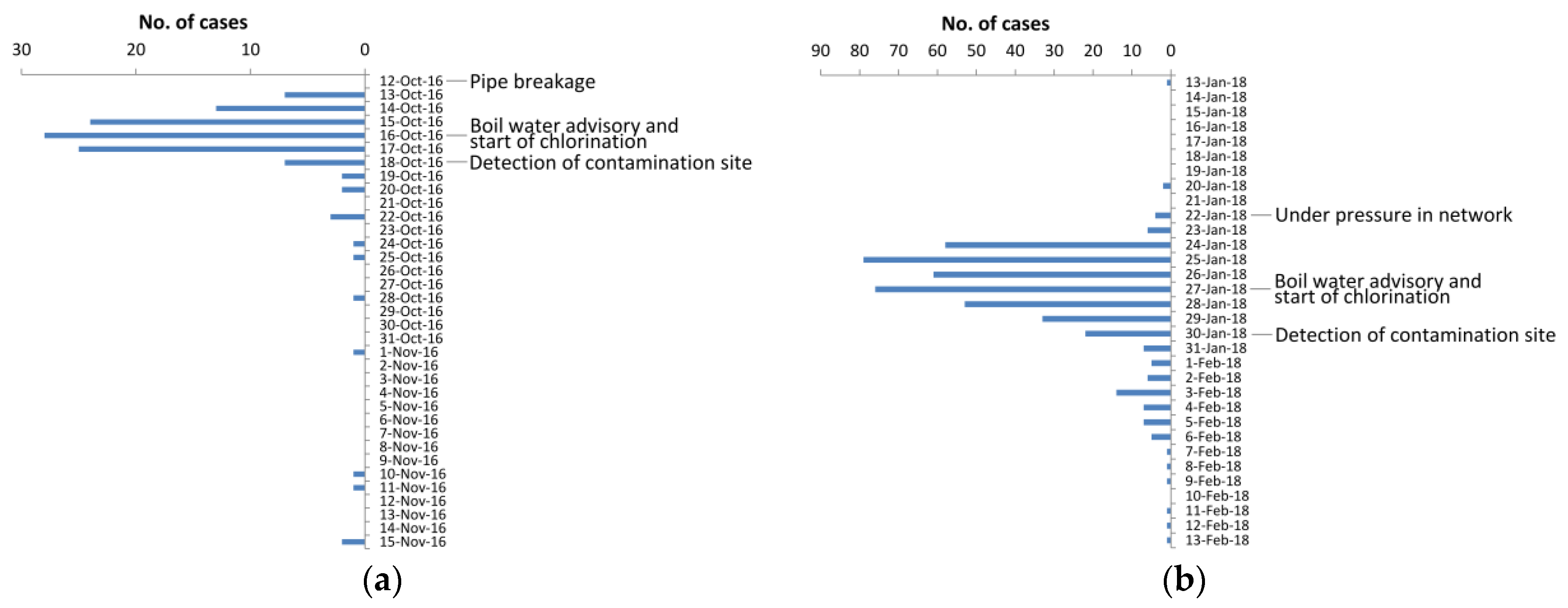
3.1. Clinical Findings
3.2. Environmental Investigations
4. Discussion
5. Conclusions
- This study highlights the importance of sapovirus as a waterborne pathogen, and warrants the need for testing of multiple pathogens during outbreak investigation
- The MST markers proved useful in the detection of contamination and especially HF183 findings were in concordance with the pathogen results, supporting its use in drinking water outbreak investigations
- Boil water advisory, alternative drinking water source and chlorination were effective mitigation actions during the outbreaks
- The role of D. fragilis as human pathogen and its drinking waterborne transmission potential requires further studies
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moreira, N.A.; Bondelind, M. Safe drinking water and waterborne outbreaks. J. Water Health 2017, 15, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Curriero, F.C.; Patz, J.A.; Rose, J.B.; Lele, S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am. J. Public Health 2001, 91, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Vantarakis, A.; Mellou, K.; Spala, G.; Kokkinos, P.; Alamanos, Y. A gastroenteritis outbreak caused by noroviruses in Greece. Int. J. Environ. Res. Public Health 2011, 8, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Wallender, E.K.; Ailes, E.C.; Yoder, J.S.; Roberts, V.A.; Brunkard, J.M. Contributing factors to disease outbreaks associated with untreated groundwater. Groundwater 2014, 52, 886–897. [Google Scholar] [CrossRef]
- Zacheus, O.; Miettinen, I.T. Increased information on waterborne outbreaks through efficient notification system enforces actions towards safe drinking water. J. Water Health 2011, 9, 763–772. [Google Scholar] [CrossRef]
- Guzman-Herrador, B.; Carlander, A.; Ethelberg, S.; de Blasio, B.F.; Kuusi, M.; Lund, V.; Löfdahl, M.; MacDonald, E.; Nichols, G.; Schönning, C.; et al. Waterborne outbreaks in the Nordic countries, 1998 to 2012. Eurosurveillance 2015, 20, 21160. [Google Scholar] [CrossRef]
- Kukkula, M.; Arstila, P.; Klossner, M.L.; Maunula, L.; Bonsdorff, C.H.; Jaatinen, P. Waterborne outbreak of viral gastroenteritis. Scand. J. Infect. Dis. 1997, 29, 415–418. [Google Scholar] [CrossRef]
- Maunula, L.; Klemola, P.; Kauppinen, A.; Söderberg, K.; Nguyen, T.; Pitkänen, T.; Kaijalainen, S.; Simonen, M.L.; Miettinen, I.T.; Lappalainen, M.; et al. Enteric viruses in a large waterborne outbreak of acute gastroenteritis in Finland. Food Environ. Virol. 2009, 1, 31–36. [Google Scholar] [CrossRef]
- Räsänen, S.; Lappalainen, S.; Kaikkonen, S.; Hämäläinen, M.; Salminen, M.; Vesikari, T. Mixed viral infections causing acute gastroenteritis in children in a waterborne outbreak. Epidemiol. Infect. 2010, 138, 1227–1234. [Google Scholar] [CrossRef]
- Jalava, K.; Rintala, H.; Ollgren, J.; Maunula, L.; Gomez-Alvarez, V.; Revez, J.; Palander, M.; Antikainen, J.; Kauppinen, A.; Räsänen, P.; et al. Novel microbiological and spatial statistical methods to improve strength of epidemiological evidence in a community-wide waterborne outbreak. PLoS ONE 2014, 9, e104713. [Google Scholar] [CrossRef]
- Maunula, L.; Kalso, S.; von Bonsdorff, C.H.; Pönkä, A. Wading pool water contaminated with both noroviruses and astroviruses as the source of a gastroenteritis outbreak. Epidemiol. Infect. 2004, 132, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Wang, Q.; Katayama, K.; Saif, L.J. Comprehensive review of human sapoviruses. Clin. Microbiol. Rev. 2015, 28, 32–53. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Kitajima, M.; Katayama, H. Occurrence and reduction of human viruses, F-specific RNA coliphage genogroups and microbial indicators at a full-scale wastewater treatment plant in Japan. J. Appl. Microbiol. 2013, 114, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, J.M.; Rocha, M.S.; Fumian, T.M.; Ginuino, A.; da Silva, T.P.; de Assis, M.R.; Rodrigues, J.D.S.; Carvalho-Costa, F.A.; Miagostovich, M.P. Occurrence of human sapoviruses in wastewater and stool samples in Rio De Janeiro, Brazil. J. Appl. Microbiol. 2016, 121, 855–862. [Google Scholar] [CrossRef]
- Pang, X.L.; Lee, B.E.; Tyrrell, G.J.; Preiksaitis, J.K. Epidemiology and genotype analysis of sapovirus associated with gastroenteritis outbreaks in Alberta, Canada: 2004–2007. J. Infect. Dis. 2009, 199, 547–551. [Google Scholar] [CrossRef]
- Svraka, S.; Vennema, H.; van der Veer, B.; Hedlund, K.; Thorhagen, M.; Siebenga, J.; Duizer, E.; Koopmans, M. Epidemiology and genotype analysis of emerging sapovirus-associated infections across Europe. J. Clin. Microbiol. 2010, 48, 2191–2198. [Google Scholar] [CrossRef]
- Dey, S.K.; Phathammavong, O.; Nguyen, T.D.; Thongprachum, A.; Chan-It, W.; Okitsu, S.; Mizuguchi, M.; Ushijima, H. Seasonal pattern and genotype distribution of sapovirus infection in Japan, 2003–2009. Epidemiol. Infect. 2012, 140, 74–77. [Google Scholar] [CrossRef]
- Lee, L.E.; Cebelinski, E.A.; Fuller, C.; Keene, W.E.; Smith, K.; Vinje, J.; Besser, J.M. Sapovirus outbreaks in long-term care facilities, Oregon and Minnesota, USA, 2002–2009. Emerg. Infect. Dis. 2012, 18, 873–876. [Google Scholar] [CrossRef]
- Hergens, M.P.; Öhd, J.N.; Alm, E.; Askling, H.H.; Helgesson, S.; Insulander, M.; Lagerqvist, N.; Svenungsson, B.; Tihane, M.; Tolfvenstam, T.; et al. Investigation of a food-borne outbreak of gastroenteritis in a school canteen revealed a variant of sapovirus genogroup V not detected by standard PCR, Sollentuna, Sweden, 2016. Eurosurveillance 2017, 22, 30543. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Kong, X.; Li, H.; Zhang, Q.; Jin, M.; Wang, Y.; Duan, Z. Two gastroenteritis outbreaks caused by sapovirus in Shenzhen, China. J. Med. Virol. 2018, 90, 1695–1702. [Google Scholar] [CrossRef]
- Varela, M.F.; Rivadulla, E.; Lema, A.; Romalde, J.L. Human sapovirus among outpatients with acute gastroenteritis in Spain: A one-year study. Viruses 2019, 11, 144. [Google Scholar] [CrossRef]
- Gerba, C.P.; Goyal, S.M.; LaBelle, R.L.; Cech, I.; Bodgan, G.F. Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. Am. J. Public Health 1979, 69, 1116–1119. [Google Scholar] [CrossRef]
- Payment, P.; Trudel, M.; Plante, R. Elimination of viruses and indicator bacteria at each step of treatment during preparation of drinking water at seven water treatment plants. Appl. Environ. Microbiol. 1985, 49, 1418–1428. [Google Scholar] [PubMed]
- Harwood, V.J.; Levine, A.D.; Scott, T.M.; Chivukula, V.; Lukasik, J.; Farrah, S.R.; Rose, J.B. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl. Environ. Microbiol. 2005, 71, 3163–3170. [Google Scholar] [CrossRef]
- Siefring, S.; Varma, M.; Atikovic, E.; Wymer, L.; Haugland, R.A. Improved real-time PCR assays for the detection of fecal indicator bacteria in surface waters with different instrument and reagent systems. J. Water Health 2008, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Kildare, B.J.; Leutenegger, C.M.; McSwain, B.S.; Bambic, D.G.; Rajal, V.B.; Wuertz, S. 16S rRNA-based assays for quantitative detection of universal, human-, cow-, and dog-specific fecal Bacteroidales: A Bayesian approach. Water Res. 2007, 41, 3701–3715. [Google Scholar] [CrossRef]
- Pitkänen, T.; Ryu, H.; Elk, M.; Hokajärvi, A.-M.; Siponen, S.; Vepsäläinen, A.; Räsänen, P.; Santo Domingo, J.W. Detection of fecal bacteria and source tracking identifiers in environmental waters using rRNA-based RT-qPCR and rDNA-based qPCR assays. Environ. Sci. Technol. 2013, 47, 13611–13620. [Google Scholar] [CrossRef]
- Kapoor, V.; Pitkänen, T.; Ryu, H.; Elk, M.; Wendell, D.; Santo Domingo, J.W. Distribution of human-specific bacteroidales and fecal indicator bacteria in an urban watershed impacted by sewage pollution, determined using RNA-and DNA-based quantitative PCR assays. Appl. Environ. Microbiol. 2015, 81, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Hughes, B.; Harwood, V. Current status of marker genes of Bacteroides and related taxa for identifying sewage pollution in environmental waters. Water 2016, 8, 231. [Google Scholar] [CrossRef]
- Communicable Disease Surveillance Centre (CDSC). Strength of association between human illness and water: Revised definitions for use in outbreak investigations. Commun. Dis. Rep. CDR Wkly. 1996, 6, 65–68. [Google Scholar]
- Tillett, H.E.; de Louvois, J.; Wall, P.G. Surveillance of outbreaks of waterborne infectious disease: Categorizing levels of evidence. Epidemiol. Infect. 1998, 120, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Loisy, F.; Atmar, R.L.; Guillon, P.; Le Cann, P.; Pommepuy, M.; Le Guyader, F.S. Real-time RT-PCR for norovirus screening in shellfish. J. Virol. Methods 2005, 123, 1–7. [Google Scholar] [CrossRef]
- Mull, B.; Hill, V.R. Recovery of diverse microbes in high turbidity surface water samples using dead-end ultrafiltration. J. Microbiol. Methods 2012, 91, 429–433. [Google Scholar] [CrossRef]
- Inkinen, J.; Jayaprakash, B.; Siponen, S.; Hokajärvi, A.M.; Pursiainen, A.; Ikonen, J.; Ryzhikov, I.; Täubel, M.; Kauppinen, A.; Paananen, J.; et al. Active eukaryotes in drinking water distribution systems of ground and surface waterworks. Microbiome 2019, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Pitkänen, T.; Miettinen, I.T. Persistent norovirus contamination of groundwater supplies in two waterborne outbreaks. Food Environ. Virol. 2018, 10, 39–50. [Google Scholar] [CrossRef]
- Maunula, L.; Söderberg, K.; Vahtera, H.; Vuorilehto, V.; von Bonsdorff, C.; Valtari, M.; Laakso, T.; Lahti, K. Presence of human noro-and adenoviruses in river and treated wastewater, a longitudinal study and method comparison. J. Water Health 2012, 10, 87–99. [Google Scholar] [CrossRef]
- Schultz, A.C.; Perelle, S.; di Pasquale, S.; Kovac, K.; de Medici, D.; Fach, P.; Sommer, H.M.; Hoorfar, J. Collaborative validation of a rapid method for efficient virus concentration in bottled water. Int. J. Food Microbiol. 2011, 145, 5158–5166. [Google Scholar] [CrossRef]
- Kim, M.J.; Ko, G.P. Quantitative characterization of the inhibitory effects of salt, humic acid, and heavy metals on the recovery of waterborne norovirus by electropositive filters. J. Water Health 2013, 11, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Miettinen, I.T. Persistence of norovirus GII genome in drinking water and wastewater at different temperatures. Pathogens 2017, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Rönnqvist, M.; Rättö, M.; Tuominen, P.; Salo, S.; Maunula, L. Swabs as a tool for monitoring the presence of norovirus on environmental surfaces in the food industry. J. Food Protect. 2013, 76, 1421–1428. [Google Scholar] [CrossRef]
- Oka, T.; Katayama, K.; Hansman, G.S.; Kageyama, T.; Ogawa, S.; Wu, F.T.; White, P.A.; Takeda, N. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J. Med. Virol. 2006, 78, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- van Maarseveen, N.M.; Wessels, E.; de Brouwer, C.S.; Vossen, A.C.; Claas, E.C. Diagnosis of viral gastroenteritis by simultaneous detection of Adenovirus group F, Astrovirus, Rotavirus group A, Norovirus genogroups I and II, and Sapovirus in two internally controlled multiplex real-time PCR assays. J. Clin. Virol. 2010, 49, 205–210. [Google Scholar] [CrossRef]
- Jothikumar, N.; Cromeans, T.L.; Hill, V.R.; Lu, X.; Sobsey, M.D.; Erdman, D.D. Quantitative real-time PCR assays for detection of human adenoviruses and identification of serotypes 40 and 41. Appl. Environ. Microbiol. 2005, 71, 3131–3136. [Google Scholar] [CrossRef]
- Kauppinen, A.; Al-Hello, H.; Zacheus, O.; Kilponen, J.; Maunula, L.; Huusko, S.; Lappalainen, M.; Miettinen, I.; Blomqvist, S.; Rimhanen-Finne, R. Increase in outbreaks of gastroenteritis linked to bathing water in Finland in summer 2014. Eurosurveillance 2017, 22, 30470. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, P.W.; Zhong, W.M.; Farkas, T.; Cubitt, D.W.; Matson, D.O. Design and evaluation of a primer pair that detects both Norwalk-and Sapporo-like caliciviruses by RT-PCR. J. Virol. Methods 1999, 83, 145–154. [Google Scholar] [CrossRef]
- Vinjé, J.; Hamidjaja, R.A.; Sobsey, M.D. Development and application of a capsid VP1 (region D) based reverse transcription PCR assay for genotyping of genogroup I and II noroviruses. J. Virol. Methods 2004, 116, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kroneman, A.; Vennema, H.; Deforche, K.; Avoort, H.V.D.; Peñaranda, S.; Oberste, M.S.; Vinjé, J.; Koopmans, M. An automated genotyping tool for enteroviruses and noroviruses. J. Clin. Virol. 2011, 51, 121–125. [Google Scholar] [CrossRef]
- Finnish Standard Association. Water Quality—Membrane Filtration Technique for the Enumeration of Total Coliform Bacteria; SFS 3016; Finnish Standard Association: Helsinki, Finland, 2011. [Google Scholar]
- International Organization for Standardization. Water Quality—Enumeration of Escherichia Coli and Coliform Bacteria—Part 1: Membrane Filtration Method for Waters with Low Bacterial Background Flora; ISO 9308-1; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- International Organization for Standardization. Water Quality—Enumeration of Escherichia coli and Coliform Bacteria—Part 2: Most Probable Number Method; ISO 9308-2; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- International Organization for Standardization. Water Quality—Detection and Enumeration of Intestinal Enterococci—Part 2: Membrane Filtration Method; ISO 7899-2; International Organization for Standardization: Geneva, Switzerland, 2000. [Google Scholar]
- International Organization for Standardization. Water Quality—Enumeration of Clostridium Perfringens—Method Using Membrane Filtration; ISO 14189; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- International Organization for Standardization. Water Quality—Detection and Enumeration of Thermotolerant Campylobacter Species; ISO 17995; International Organization for Standardization: Geneva, Switzerland, 2005. [Google Scholar]
- Antikainen, J.; Kantele, A.; Pakkanen, S.H.; Lääveri, T.; Riutta, J.; Vaara, M.; Kirveskari, J. A quantitative polymerase chain reaction assay for rapid detection of 9 pathogens directly from stools of travelers with diarrhea. Clin. Gastroenterol. Hepatol. 2013, 11, 1300–1307. [Google Scholar] [CrossRef]
- Hill, V.; Narayanan, J.; Gallen, R.; Ferdinand, K.; Cromeans, T.; Vinjé, J. Development of a nucleic acid extraction procedure for simultaneous recovery of DNA and RNA from diverse microbes in water. Pathogens 2015, 4, 335–354. [Google Scholar] [CrossRef] [PubMed]
- Jothikumar, N.; da Silva, A.J.; Moura, I.; Qvarnstrom, Y.; Hill, V.R. Detection and differentiation of Cryptosporidium hominis and Cryptosporidium parvum by dual TaqMan assays. J. Med. Microbiol. 2008, 57, 1099–1105. [Google Scholar] [CrossRef]
- International Organization for Standardization. Water Quality—Isolation and Identification of Cryptosporidium oocysts and Giardia cysts from Water; ISO 15553; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Bosch, A.; Lucena, F.; Diez, J.M.; Gajardo, R.; Blasi, M.; Jofre, J. Waterborne viruses associated with hepatitis outbreak. J. Am. Water Work. Assoc. 1991, 83, 80–83. [Google Scholar] [CrossRef]
- Maunula, L.; Miettinen, I.T.; von Bonsdorff, C. Norovirus outbreaks from drinking water. Emerg. Infect. Dis. 2005, 11, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.; Prato, R.; Chironna, M.; Sallustio, A.; Caputi, G.; Conversano, M.; Atti, M.C.D.; D’Ancona, F.P.; Germinario, C.A.; Quarto, M. Large outbreak of viral gastroenteritis caused by contaminated drinking water in Apulia, Italy, May–October 2006. Eurosurveillance 2007, 12, 3176. [Google Scholar] [CrossRef]
- Blanco, A.; Guix, S.; Fuster, N.; Fuentes, C.; Bartolomé, R.; Cornejo, T.; Pintó, R.M.; Bosch, A. Norovirus in bottled water associated with gastroenteritis outbreak, Spain, 2016. Emerg. Infect. Dis. 2017, 23, 1531–1534. [Google Scholar] [CrossRef]
- Diston, D.; Sinreich, M.; Zimmermann, S.; Baumgartner, A.; Felleisen, R. Evaluation of molecular-and culture-dependent MST markers to detect fecal contamination and indicate viral presence in good quality groundwater. Environ. Sci. Technol. 2015, 49, 7142–7151. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, M.J.; Torvinen, E.; Kusnetsov, J.; Pitkänen, T.; Maunula, L.; von Bonsdorff, C.; Martikainen, P.J.; Wilks, S.A.; Keevil, C.W.; Miettinen, I.T. Survival of Mycobacterium avium, Legionella pneumophila, Escherichia coli, and caliciviruses in drinking water-associated biofilms grown under high-shear turbulent flow. Appl. Environ. Microbiol. 2007, 73, 2854–2859. [Google Scholar] [CrossRef]
- Helmi, K.; Skraber, S.; Gantzer, C.; Willame, R.; Hoffmann, L.; Cauchie, H.M. Interactions of Cryptosporidium parvum, Giardia lamblia, vaccinal poliovirus type 1, and bacteriophages phiX174 and MS2 with a drinking water biofilm and a wastewater biofilm. Appl. Environ. Microbiol. 2008, 74, 2079–2088. [Google Scholar] [CrossRef]
- Kauppinen, A.; Ikonen, J.; Pursiainen, A.; Pitkänen, T.; Miettinen, I.T. Decontamination of a drinking water pipeline system contaminated with adenovirus and Escherichia coli utilizing peracetic acid and chlorine. J. Water Health 2012, 10, 406–418. [Google Scholar] [CrossRef]
- Allen, M.; Clark, R.; Cotruvo, J.A.; Grigg, N. Drinking water and public health in an era of aging distribution infrastructure. Public Work. Manag. Policy 2018, 23, 301–309. [Google Scholar] [CrossRef]
- Barratt, J.L.; Harkness, J.; Marriott, D.; Ellis, J.T.; Stark, D. A review of Dientamoeba fragilis carriage in humans: Several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes 2011, 2, 3–12. [Google Scholar] [CrossRef]
- Wong, Z.W.; Faulder, K.; Robinson, J.L. Does Dientamoeba fragilis cause diarrhea? A systematic review. Parasitol. Res. 2018, 117, 971–980. [Google Scholar] [CrossRef] [PubMed]
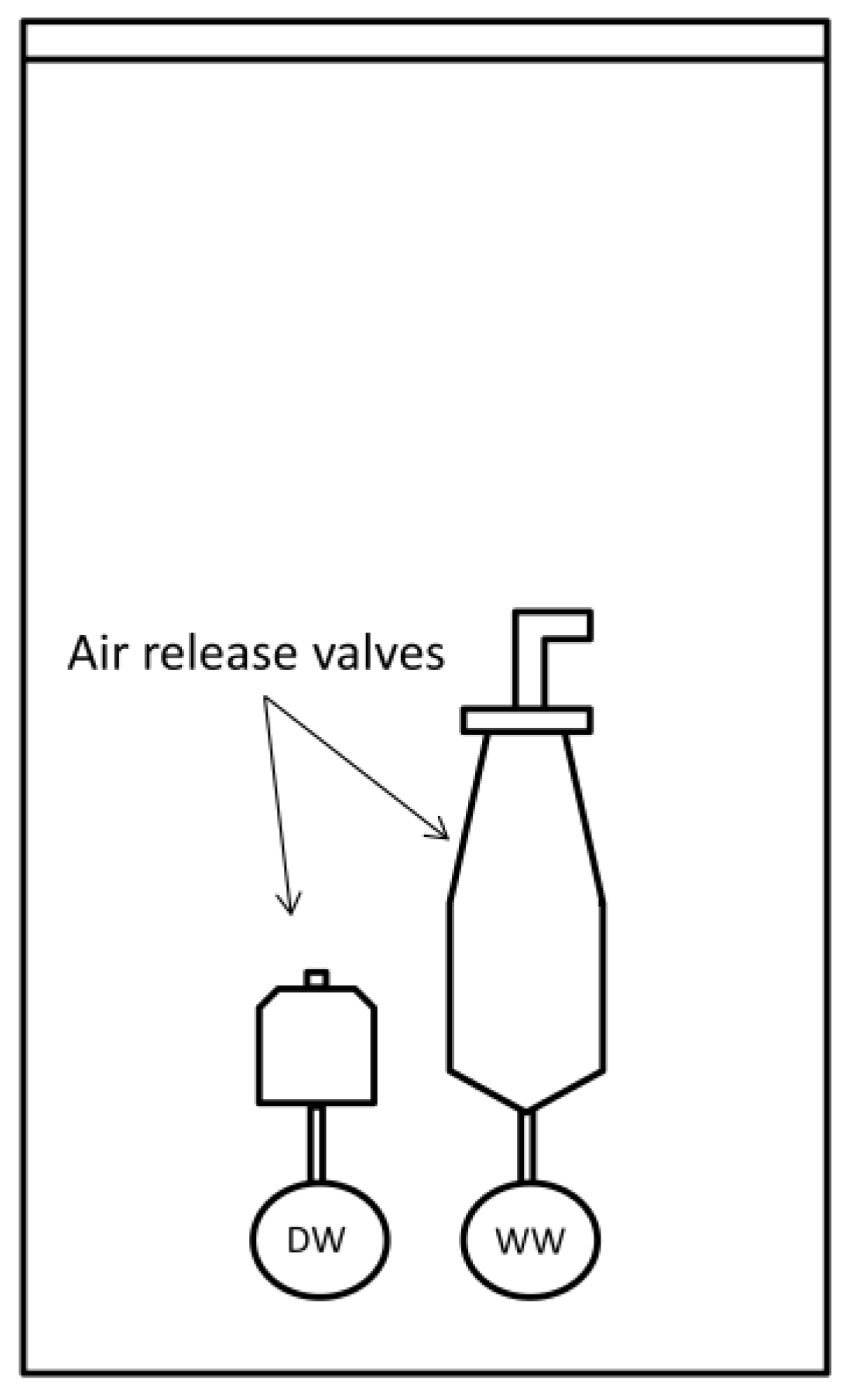
| Microbe | Outbreak I | Outbreak II |
|---|---|---|
| Sapovirus 1 | 16/31 | 11/33 |
| Norovirus 1 | 0/31 | 16/33 |
| Adenovirus 1 | 3/31 | 0/33 |
| Astrovirus 1 | 0/31 | 7/33 |
| Rotavirus 1 | 0/31 | 0/33 |
| ETEC 1 | Nd 3 | 2/34 |
| EHEC 1 | Nd | 1/34 |
| Plesiomonas shigelloides2 | 0/37 | 1/34 |
| Yersinia spp., Salmonella spp., Campylobacter spp. 2 | 0/37 | 0/34 |
| Cryptosporidium spp., Giardia lamblia, Entamoeba histolytica 1 | 0/5 | 0/38 |
| Dientamoeba fragilis1 | 2/5 | Nd |
| Microbe | Raw Water | Water Before Chlorination | Water After Chlorination | Biofilms | Sewage | Contamination Site |
|---|---|---|---|---|---|---|
| Sapovirus | 0/1 | Nd 1 | 0/7 | 0/9 | 1/1 | 1/1 (1.0 × 107) |
| Adenovirus | 0/1 | Nd | 0/3 | 0/9 | Nd | 1/1 (5.0 × 106) |
| Giardia lamblia, Entamoeba histolytica, Cryptosporidium spp. and Dientamoeba fragilis | 0/1 | Nd | 0/7 | 0/9 | Dientamoeba fragilis | Dientamoeba fragilis |
| GenBac3 (rDNA) | 0/1 | Nd | 0/3 | 0/9 | Nd | 1/1 (4.5 × 107) |
| GenBac3 (rRNA) | 1/1 (8.0 × 100) | Nd | 1/3 (7.0×100) | 0/9 | Nd | 1/1 (7.5 × 108) |
| HF183 (rDNA) | 0/1 | Nd | 0/3 | 0/9 | Nd | 1/1 (6.3 × 106) |
| HF183 (rRNA) | 0/1 | Nd | 0/3 | 0/9 | Nd | 1/1 (2.7 × 107) |
| E. coli | 0/1 | 1/2 | 0/91 | 0/9 | Nd | 1/1 (2.8 × 105) |
| Coliform bacteria | 1/1 (3.0 × 10−2) | 1/2 | 3/91 (1.0 – 4.0×100) | 2/9 (1.0 – 2.0 × 101) | Nd | 1/1 (1.2 × 106) |
| Intestinal enterococci | 0/1 | Nd | 0/29 | 0/9 | Nd | Nd |
| C. perfringens | 0/1 | Nd | 0/12 | 0/9 | Nd | 1/1 (2.0 × 104) |
| Microbe | Water before Chlorination | Water after Chlorination | Water after Intensive Chlorination | Biofilms | Contamination Site |
|---|---|---|---|---|---|
| Sapovirus | 0/1 | 1/2 (<LOQ 2) | 0/3 | 0/1 | 1/1 (1.9 × 105) |
| Norovirus GI | 0/1 | 0/2 | Nd | 0/1 | 1/1 (2.3 × 105) |
| Norovirus GII | 0/1 | 0/2 | Nd | 0/1 | 1/1 (2.8 × 103) |
| Adenovirus | 0/1 | 0/1 | Nd | Nd | 0/1 |
| ETEC, EPEC, EHEC and EAEC 3 | 0/1 | 1/2 (EPEC) | 0/3 | 0/1 | 1/1 (EHEC, ETEC, EAEC) |
| Campylobacter spp. | 0/1 | 0/2 | Nd | Nd | 0/1 |
| Giardia spp. and Cryptosporidium spp. | 0/1 | 0/2 | 0/3 | 0/1 | 0/1 |
| GenBac3 (rDNA) | 1/1 (5.9 × 103) | 1/2 (6.6 × 103) | 3/3 (<LOQ) | 1/1 (3.1 × 102) | 1/1 (6.3 × 1010) |
| GenBac3 (rRNA) | 1/1 (4.4 × 105) | 1/2 (1.9 × 105) | 3/3 (<LOQ) | 1/1 (1.7 × 104) | 1/1 (1.1 × 1013) |
| HF183 (rDNA) | 1/1 (7.7 × 102) | 1/2 (9.0 × 102) | 0/3 | 0/1 | 1/1 (6.1 × 109) |
| HF183 (rRNA) | 1/1 (5.1 × 103) | 1/2 (3.8 × 102) | 0/3 | 0/1 | 1/1 (8.0 × 1012) |
| E. coli | 1/5 (2.0 × 100) | 0/19 | 0/48 | 0/1 | 1/1 (5.5 × 105) |
| Coliform bacteria | 1/5 (1.2 × 101) | 1/19 (3.0 × 100) | 0/48 | 0/1 | 1/1 (1.7 × 106) |
| Intestinal enterococci | 2/5 (1.0 – 2.0 × 100) | 2/19 (1.0–2.0 × 100) | 2/48 (4.0 × 10−1 – 1.0 × 100) | 0/1 | 1/1 (9.0 × 104) |
| C. perfringens | Nd 1 | 1/9 (1.0 × 10−1) | 0/22 | 0/1 | 1/1 (5.9 × 103) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauppinen, A.; Pitkänen, T.; Al-Hello, H.; Maunula, L.; Hokajärvi, A.-M.; Rimhanen-Finne, R.; Miettinen, I.T. Two Drinking Water Outbreaks Caused by Wastewater Intrusion Including Sapovirus in Finland. Int. J. Environ. Res. Public Health 2019, 16, 4376. https://doi.org/10.3390/ijerph16224376
Kauppinen A, Pitkänen T, Al-Hello H, Maunula L, Hokajärvi A-M, Rimhanen-Finne R, Miettinen IT. Two Drinking Water Outbreaks Caused by Wastewater Intrusion Including Sapovirus in Finland. International Journal of Environmental Research and Public Health. 2019; 16(22):4376. https://doi.org/10.3390/ijerph16224376
Chicago/Turabian StyleKauppinen, Ari, Tarja Pitkänen, Haider Al-Hello, Leena Maunula, Anna-Maria Hokajärvi, Ruska Rimhanen-Finne, and Ilkka T. Miettinen. 2019. "Two Drinking Water Outbreaks Caused by Wastewater Intrusion Including Sapovirus in Finland" International Journal of Environmental Research and Public Health 16, no. 22: 4376. https://doi.org/10.3390/ijerph16224376
APA StyleKauppinen, A., Pitkänen, T., Al-Hello, H., Maunula, L., Hokajärvi, A.-M., Rimhanen-Finne, R., & Miettinen, I. T. (2019). Two Drinking Water Outbreaks Caused by Wastewater Intrusion Including Sapovirus in Finland. International Journal of Environmental Research and Public Health, 16(22), 4376. https://doi.org/10.3390/ijerph16224376





