A Detoxification Intervention for Gulf War Illness: A Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
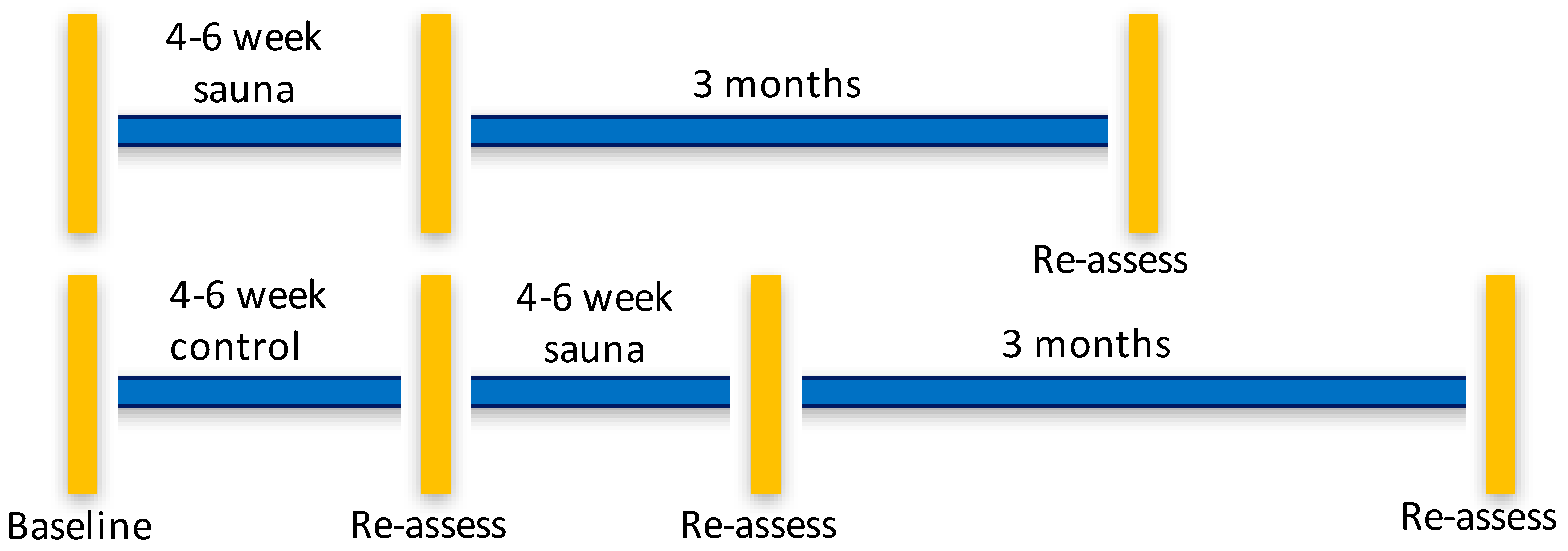
2.1. Study Design
2.2. Participants
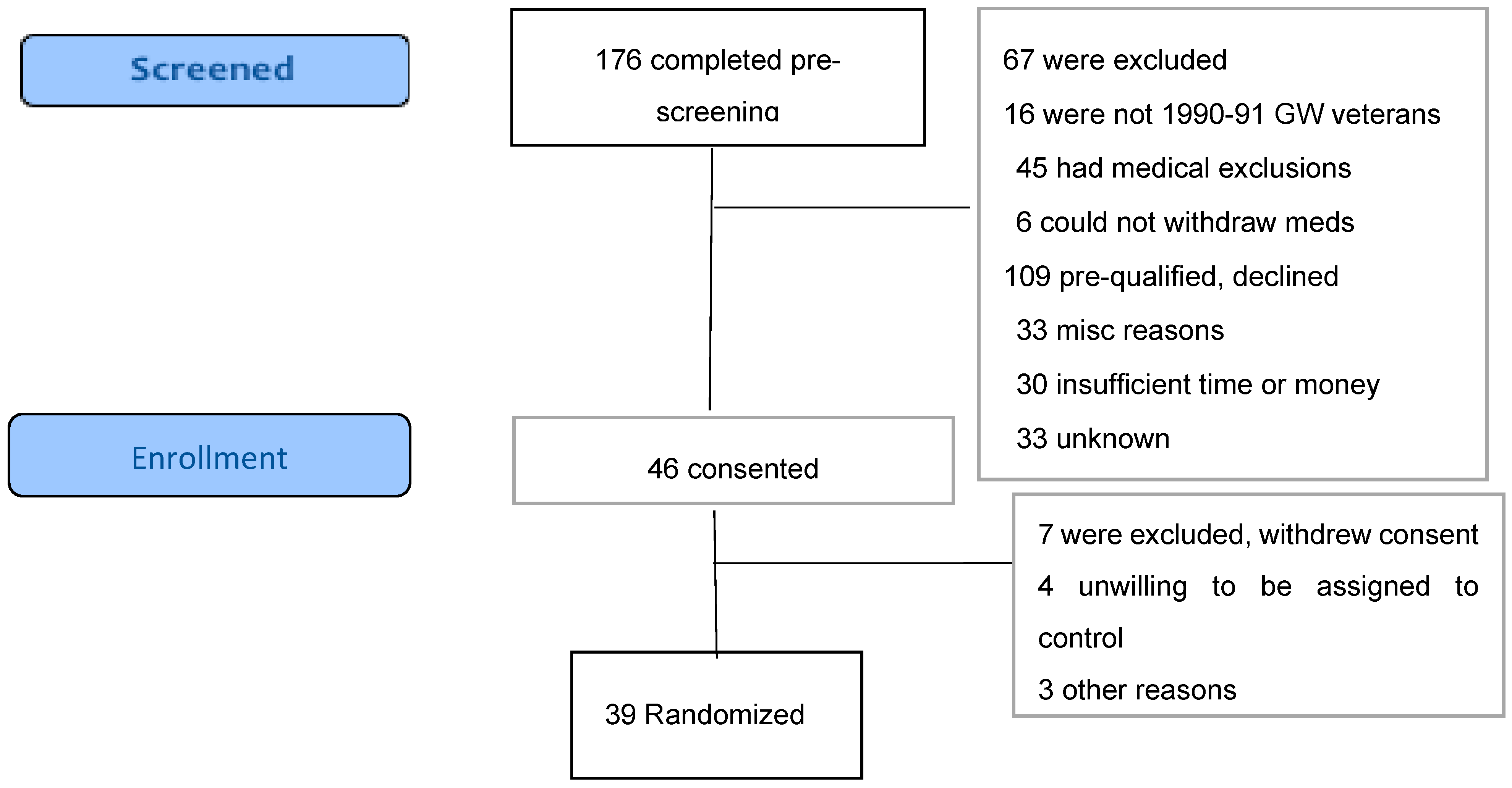
2.3. Recruitment and Enrollment
2.4. Randomization and Blinding
2.5. Intervention
2.6. Waitlist Control
2.7. Safety Aspects
2.8. Outcome Measures
2.8.1. Feasibility
2.8.2. Safety
2.8.3. Heath Related Quality of Life and Function
2.8.4. Symptoms and Case Status
2.9. Statistical Methods
3. Results
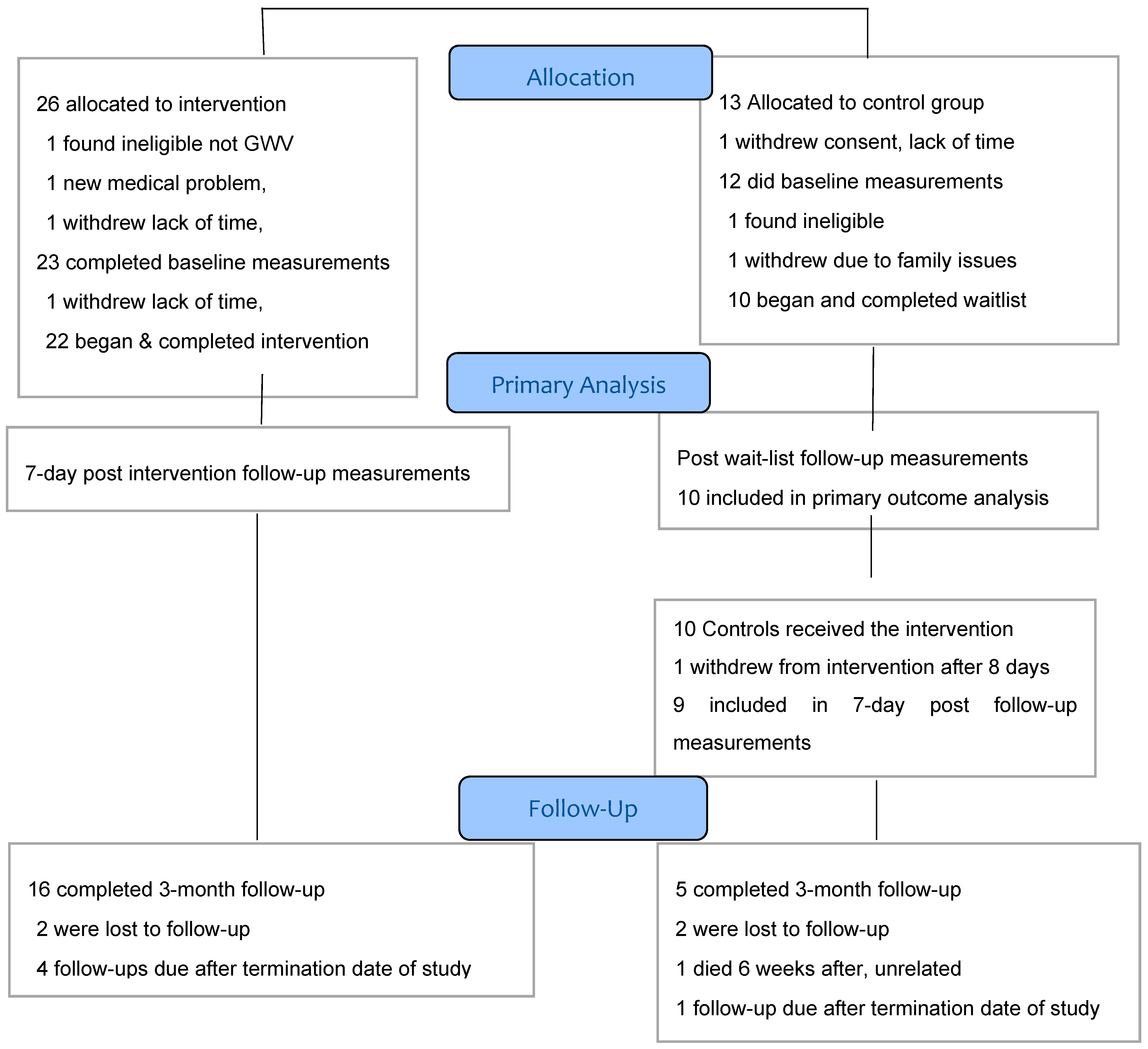
3.1. Recruitment. and Participant Flow
3.2. Baseline Characteristics
3.3. Quality of Life and Symptom Outcomes
3.4. Three-Month Follow-Up Outcomes
3.4.1. Between-Group Difference’s at 3 Months
3.4.2. Stability at 3 Months
3.5. Laboratory Results
3.6. Adverse Events
4. Discussion
4.1. Feasibility of the Intervention and Study Methods
4.2. Changes in Health Measures Outcomes
4.3. Adverse Events
4.4. Potential Mechanisms Underlying the Effects of the Regimen on Outcomes
4.5. Exposure Essessment
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Research Advisory Committee on Gulf War Veterans’ Illnesses. Gulf War Illness and the Health of Gulf War Veterans. Scientific Findings and Recommendations; Government Printing Office: Washington, DC, USA, 2008.
- Kerr, K.J. Gulf War illness: An overview of events, most prevalent health outcomes, exposures, and clues as to pathogenesis. Rev. Environ. Health 2015, 30, 273–286. [Google Scholar] [CrossRef] [PubMed]
- White, R.F.; Steele, L.; O’Callaghan, J.P.; Sullivan, K.; Binns, J.H.; Golomb, B.A.; Bloom, F.E.; Bunker, J.A.; Crawford, F.; Graves, J.C.; et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 2016, 74, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Donta, S.T.; Clauw, D.J.; Engel, C.C.; Guarino, P.; Peduzzi, P.; Williams, D.A.; Skinner, J.S.; Barkhuizen, A.; Taylor, T.; Kazis L., E.; et al. Cognitive behavioral therapy and aerobic exercise for Gulf War veterans’ illnesses - A randomized controlled trial. Jama-J. Am. Med. Assoc. 2003, 289, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, M.S.; Eisen, S.A.; Alpern, R.; Karlinsky, J.; Toomey, R.; Reda, D.J.; Pepper, L.; Clapp, R.; Sutker, P.B.; Vasterling, J.J.; et al. Chronic multisymptom illness complex in Gulf War I veterans 10 years later. Am. J. Epidemiol. 2006, 163, 66–75. [Google Scholar] [CrossRef]
- Golomb, B.A.; Allison, M.; Koperski, S.; Koslik, H.J.; Devaraj, S.; Ritchie, J.B. Coenzyme Q10 Benefits Symptoms in Gulf War Veterans: Results of a Randomized Double-Blind Study. Neural Comput. 2014, 26, 2594–2651. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; El-Amin, S.; Corey, R.; Rayhan, R.; Timbol, C. Carnosine treatment for gulf war illness: A randomized controlled trial. Glob. J. Health Sci. 2013, 5, 69–81. [Google Scholar] [CrossRef]
- Kearney, D.J.; Simpson, T.L.; Malte, C.A.; Felleman, B.; Martinez, M.E.; Hunt, S.C. Mindfulness-based Stress Reduction in Addition to Usual Care Is Associated with Improvements in Pain, Fatigue, and Cognitive Failures Among Veterans with Gulf War Illness. Am. J. Med. 2016, 129, 204–214. [Google Scholar] [CrossRef]
- Conboy, L.; Gerke, T.; Hsu, K.-Y.; John, M.S.; Goldstein, M.; Schnyer, R. The Effectiveness of Individualized Acupuncture Protocols in the Treatment of Gulf War Illness: A Pragmatic Randomized Clinical Trial. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Institute of Medicine. Gulf War and Health: Treatment for Chronic Multisymptom Illness; The National Academies Press: Washington, DC, USA, 2013. [Google Scholar]
- CDC. A Research Planning Conference. In The Health Impact of Chemical Exposures During the Gulf War; Centers for Disease Control and Prevention: Atlanta, GA, USA, 1999. [Google Scholar]
- Proctor, S.P.; Heeren, T.; White, R.F.; Wolfe, J.; Borgos, M.S.; Davis, J.D.; Pepper, L.; Clapp, R.; Sutker, P.B.; Vasterling, J.J.; et al. Health status of Persian Gulf War veterans: Self-reported symptoms, environmental exposures and the effect of stress. Int. J. Epidemiol. 1998, 27, 1000–1010. [Google Scholar] [CrossRef]
- Tuite, J.J.; Haley, R.W. Meteorological and Intelligence Evidence of Long-Distance Transit of Chemical Weapons Fallout from Bombing Early in the 1991 Persian Gulf War. Neuroepidemiology 2013, 40, 160–177. [Google Scholar] [CrossRef]
- Gackstetter, G.D.; Hooper, T.I.; al Qahtani, M.S.; Smith, T.C.; Memish, Z.A.; Schlangen, K.M.; Cruess, D.F.; Barrett, D.H.; Ryan, M.A.K.; Gray, G.C.; et al. Assessing the potential health impact of the 1991 Gulf War on Saudi Arabian National Guard Soldiers. Int. J. Epidemiol. 2005, 34, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A. A Review of the Scientific Literature as It Pertains to Gulf War Illnesses: Volume 2: Pyridostigmine Bromide; RAND Corporation: Santa Monica, CA, USA, 1999. [Google Scholar]
- Lorber, M.; Gibb, H.; Grant, L.; Pinto, J.; Pleil, J.; Cleverly, D. Assessment of inhalation exposures and potential health risks to the general population that resulted from the collapse of the World Trade Center towers. Risk Anal. 2007, 27, 1203–1221. [Google Scholar] [CrossRef] [PubMed]
- Steele, L.; Sastre, A.; Gerkovich, M.M.; Cook, M.R. Complex Factors in the Etiology of Gulf War Illness: Wartime Exposures and Risk Factors in Veteran Subgroups. Environ. Health Perspect. 2012, 120, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Research Advisory Committee on Gulf War Veterans’ Illnesses. Gulf War Illness and the Health of Gulf War Veterans: Research Update and Recommendations, 2009–2013; Government Printing Office: Boston, MA, USA, 2014.
- Li, W.B.; Gerstmann, U.C.; Hoellriegl, V.; Szymczak, W.; Roth, P.; Hoeschen, C.; Oeh, U. Radiation dose assessment of exposure to depleted uranium. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 502–514. [Google Scholar] [CrossRef][Green Version]
- Glass, D.C.; Sim, M.R. The challenges of exposure assessment in health studies of Gulf War veterans. Philos. Trans. R. Soc. B-Biol. Sci. 2006, 361, 627–637. [Google Scholar] [CrossRef]
- Haley, R.W.; Tuite, J.J. Epidemiologic Evidence of Health Effects from Long-Distance Transit of Chemical Weapons Fallout from Bombing Early in the 1991 Persian Gulf War. Neuroepidemiology 2013, 40, 178–189. [Google Scholar] [CrossRef]
- Heller, J.M.; Legge, W.E. Final Report Kuwait oil Fire Health Risk Assessment; U.S. Army Environmental Hygiene Agency, Aberdeen Proving Ground: Aberdeen, MD, USA, 1991. [Google Scholar]
- Gevao, B.; Aba, A.A.; Al-Ghadban, A.N.; Uddin, S. Depositional History of Polychlorinated Biphenyls in a Dated Sediment Core from the Northwestern Arabian Gulf. Arch. Environ. Contam. Toxicol. 2012, 62, 549–556. [Google Scholar] [CrossRef]
- Alawi, M.A.; Al-Tameemi, F.T. Levels of polychlorinated biphenyls (PCBs) in human adipose tissue from Baghdad/Iraq. Toxin Rev. 2016, 35, 83–89. [Google Scholar] [CrossRef]
- Kehe, K.; Balszuweit, F.; Emmler, J.; Kreppel, H.; Jochum, M.; Thiermann, H. Sulfur mustard research--strategies for the development of improved medical therapy. Eplasty 2008, 8, e32. [Google Scholar]
- Valciukas, J.A.; Lilis, R.; Anderson, H.A.; Wolff, M.S.; Petrocci, M. The neurotoxicity of polybrominated biphenyls: Results of a medical field survey. Ann. N. Y. Acad. Sci. 1979, 320, 337–367. [Google Scholar] [CrossRef]
- Heaton, K.J.; Palumbo, C.L.; Proctor, S.P.; Killiany, R.J.; Yurgelun-Todd, D.A.; White, R.F. Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin. Neurotoxicology 2007, 28, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.E.; Richards, P.G. The potential for toxic effects of chronic, low-dose exposure to organophosphates. Toxicol. Lett. 2001, 120, 343–351. [Google Scholar] [CrossRef]
- Jokanovic, M.; Kosanovic, M. Neurotoxic effects in patients poisoned with organophosphorus pesticides. Environ. Toxicol. Pharmacol. 2010, 29, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Araki, S.; Murata, K.; Nishikitani, M.; Okumura, T.; Ishimatsu, S.; Takasu, N. Chronic neurobehavioral and central and autonomic nervous system effects of Tokyo subway sarin poisoning. J. Physiol. Paris 1998, 92, 317–323. [Google Scholar] [CrossRef]
- Steele, L.; Lockridge, O.; Gerkovich, M.M.; Cook, M.R.; Sastre, A. Butyrylcholinesterase genotype and enzyme activity in relation to Gulf War illness: Preliminary evidence of gene-exposure interaction from a case-control study of 1991 Gulf War veterans. Environ. Health 2015, 14, 4. [Google Scholar] [CrossRef]
- Koslik, H.J.; Hamilton, G.; Golomb, B.A. Mitochondrial Dysfunction in Gulf War Illness Revealed by 31Phosphorus Magnetic Resonance Spectroscopy: A Case-Control Study. PLoS ONE 2014, 9, e92887. [Google Scholar] [CrossRef]
- Hodgson, E.; Rose, R.L. Human metabolism and metabolic interactions of deployment-related chemicals. Drug Metab. Rev. 2005, 37, 1–39. [Google Scholar] [CrossRef]
- Chen, Y.; Meyer, J.N.; Hill, H.Z.; Lange, G.; Condon, M.R.; Klein, J.C.; Ndirangu, D.; Falvo, M.J. Role of mitochondrial DNA damage and dysfunction in veterans with Gulf War Illness. PLoS ONE 2017, 12, e0184832. [Google Scholar]
- Chao, L.L.; Abadjian, L.; Hlavin, J.; Meyerhoff, D.J.; Weiner, M.W. Effects of low-level sarin and cyclosarin exposure and Gulf War Illness on brain structure and function: A study at 4T. Neurotoxicology 2011, 32, 814–822. [Google Scholar] [CrossRef]
- Engdahl, B.E.; James, L.M.; Miller, R.D.; Leuthold, A.C.; Lewis, S.M.; Carpenter, A.F.; Georgopoulos, A.P. A Magnetoencephalographic (MEG) Study of Gulf War Illness (GWI). Ebiomedicine 2016, 12, 127–132. [Google Scholar] [CrossRef]
- Christova, P.; James, L.M.; Engdahl, B.E.; Lewis, S.M.; Carpenter, A.F.; Georgopoulos, A.P. Subcortical brain atrophy in Gulf War Illness. Exp. Brain Res. 2017, 235, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Abou-Donia, M.B.; Conboy, L.A.; Kokkotou, E.; Jacobson, E.; Elmasry, E.M.; Elkafrawy, P.; Neely, M.; Bass, C.R.; Sullivan, K. Screening for novel central nervous system biomarkers in veterans with Gulf War Illness. Neurotoxicol. Teratol. 2017, 61, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, A.P.; James, L.M.; Carpenter, A.F.; Engdahl, B.E.; Leuthold, A.C.; Lewis, S.M. Gulf War illness (GWI) as a neuroimmune disease. Exp. Brain Res. 2017, 235, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the human health effects of chemical mixtures. Environ. Health Perspect. 2002, 110, 25–42. [Google Scholar] [CrossRef]
- Sexton, K. Cumulative risk assessment: An overview of methodological approaches for evaluating combined health effects from exposure to multiple environmental stressors. Int. J. Environ. Res. Public Health 2012, 9, 370–390. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. The exposome: From concept to utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef]
- Rappaport, S.M.; Smith, M.T. Epidemiology. Environment and disease risks. Science 2010, 330, 460–461. [Google Scholar] [CrossRef]
- Hubbard, L.R. The Technical Bulletins; Bridge Publications, Inc.: Los Angeles, CA, USA, 1978; Volume 12. [Google Scholar]
- Hubbard, L.R. Clear Body Clear Mind; Bridge Publications, Inc.: Los Angeles, CA, USA, 1990. [Google Scholar]
- Klaassen, C. Casarett & Doull’s Toxicology: The Basic Science of Poisons, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Ghannoum, M.; Gosselin, S. Enhanced Poison Elimination in Critical Care. Adv. Chronic Kidney Dis. 2013, 20, 94–101. [Google Scholar] [CrossRef]
- Cohn, W.J.; Boylan, J.J.; Blanke, R.V.; Fariss, M.W.; Howell, J.R.; Guzelian, P.S. Treatment of chlordecone (kepone) toxicity with cholestyramine-results of a controlled clinical-trial. N. Engl. J. Med. 1978, 298, 243–248. [Google Scholar] [CrossRef]
- Jandacek, R.J.; Heubi, J.E.; Buckley, D.D.; Khoury, J.C.; Turner, W.E.; Sjodin, A.; Olson, J.R.; Shelton, C.; Helms, K.; Bailey, T.D.; et al. Reduction of the body burden of PCBs and DDE by dietary intervention in a randomized trial. J. Nutr. Biochem. 2014, 25, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Tretjak, Z.; Shields, M.; Beckmann, S.L. PCB reduction and clinical improvement by detoxification: An unexploited approach? Hum. Exp. Toxicol. 1990, 9, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Schnare, D.W.; Ben, M.; Shields, M.G. Body burden reductions of polychlorinated biphenyls, polybrominated biphenyls and chlorinated pesticides in human subjects. Ambio 1984, 13, 378–380. [Google Scholar]
- Schnare, D.W.; Denk, G.; Shields, M.; Brunton, S. Evaluation of a detoxification regimen for fat stored xenobiotics. Med. Hypotheses 1982, 9, 265–282. [Google Scholar] [CrossRef]
- Kilburn, K.H.; Warsaw, R.H.; Shields, M.G. Neurobehavioral dysfunction in firemen exposed to polychlorinated biphenyls (PCBs): Possible improvement after detoxification. Arch. Environ. Health 1989, 44, 345–350. [Google Scholar] [CrossRef]
- Tsyb, A.S.; Parshkov, E.M.; Barnes, J.; Yarzutkin, V.V.; Vorontsov, N.V.; Dedov, V.I. Rehabilitation of a Chernobyl Affected Population Using a Detoxification Method. In Proceedings of the US EPA International Radiological Post-Emergency Response Issues Conference, Washington, DC, USA, 9–11 September 1998; pp. 162–166. [Google Scholar]
- Ross, G.H.; Sternquist, M.C. Methamphetamine exposure and chronic illness in police officers: Significant improvement with sauna-based detoxification therapy. Toxicol. Ind. Health 2012, 28, 758–768. [Google Scholar] [CrossRef]
- Cecchini, M.; Root, D.; Rachunow, J.; Gelb, P. Chemical Exposures at the World Trade Center Use of the Hubbard Sauna Detoxification Regimen to Improve the Health Status of New York City Rescue Workers Exposed to Toxicants. Townsend Lett. 2006, 263, 58–65. [Google Scholar]
- Weiler, B.A.; Colby, T.V.; Floreth, T.J.; Hines, S.E. Small airways disease in an Operation Desert Storm Deployer: Case report and review of the literature on respiratory health and inhalational exposures from Gulf War I. Am. J. Ind. Med. 2018, 61, 793–801. [Google Scholar] [CrossRef]
- Elliott, S.A.; Brown, J.S.L. What are we doing to waiting list controls? Behav. Res. Ther. 2002, 40, 1047–1052. [Google Scholar] [CrossRef]
- Ozakinci, G.; Hallman, W.K.; Kipen, H.M. Persistence of symptoms in veterans of the First Gulf War: 5-year follow-up. Environ. Health Perspect. 2006, 114, 1553–1557. [Google Scholar] [CrossRef]
- Dursa, E.K.; Barth, S.K.; Schneiderman, A.I.; Bossarte, R.M. Physical and Mental Health Status of Gulf War and Gulf Era Veterans Results From a Large Population-Based Epidemiological Study. J. Occup. Environ. Med. 2016, 58, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Steele, L. Prevalence and patterns of Gulf War illness in Kansas veterans: Association of symptoms with characteristics of person, place, and time of military service. Am. J. Epidemiol. 2000, 152, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined; The National Academies Press: Washington, DC, USA, 2014. [Google Scholar]
- Fukuda, K.; Nisenbaum, R.; Stewart, G.; Thompson, W.W.; Robin, L.; Washko, R.M.; Noah, D.L.; Barrett, D.H.; Randall, B.; Herwaldt, B.L.; et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA 1998, 280, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Use of Dietary Supplements by Military Personnel; Greenwood, M.R.C., Oria, M., Eds.; The National Academies Press: Washington, DC, USA, 2008. [Google Scholar]
- Laukkanen, J.A.; Laukkanen, T.; Kunutsor, S.K. Cardiovascular and Other Health Benefits of Sauna Bathing: A Review of the Evidence. Mayo Clin. Proc. 2018, 93, 1111–1121. [Google Scholar] [CrossRef]
- Elsawy, B.; Higgins, K.E. Physical Activity Guidelines for Older Adults. Am. Fam. Physician 2010, 81, 55–59. [Google Scholar]
- al-Zaki, T.; Jolly, B.T. Severe hyponatremia after “purification”. Ann. Emerg. Med. 1997, 29, 194–195. [Google Scholar]
- Berende, A.; ter Hofstede, H.J.; Vos, F.J.; van Middendorp, H.; Vogelaar, M.L.; Tromp, M.; van den Hoogen, F.H.; Donders, A.R.T.; Evers, A.W.M.; Kullberg, B.J. Randomized Trial of Longer-Term Therapy for Symptoms Attributed to Lyme Disease. N. Engl. J. Med. 2016, 374, 1209–1220. [Google Scholar] [CrossRef]
- Golier, J.A.; Caramanica, K.; Michaelides, A.C.; Makotkine, I.; Schmeidler, J.; Harvey, P.D.; Yehuda, R. A randomized, double-blind, placebo-controlled, crossover trial of mifepristone in Gulf War veterans with chronic multisymptom illness. Psychoneuroendocrinology 2016, 64, 22–30. [Google Scholar] [CrossRef]
- Frayne, S.M.; Parker, V.A.; Christiansen, C.L.; Loveland, S.; Seaver, M.R.; Kazis, L.E.; Skinner, K.M. Health status among 28,000 women veterans-The VA women’s health program evaluation project. J. Gen. Intern. Med. 2006, 21, S40–S46. [Google Scholar] [CrossRef]
- Diaz-Arribas, M.J.; Fernandez-Serrano, M.; Royuela, A.; Kovacs, F.M.; Gallego-Izquierdo, T.; Ramos-Sanchez, M.; Llorca-Palomera, R.; Pardo-Hervas, P.; Martin-Pariente, O.S. Minimal Clinically Important Difference in Quality of Life for Patients With Low Back Pain. Spine 2017, 42, 1908–1916. [Google Scholar] [CrossRef]
- Nordin, A.; Taft, C.; Lundgren-Nilsson, A.; Dencker, A. Minimal important differences for fatigue patient reported outcome measures-a systematic review. BMC Med. Res. Methodol. 2016, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.; Brown, M.; Evans, M.; Anderson, V.; Lerch, A.; Brown, A.; Hunnell, J.; Porter, N. Measuring substantial reductions in functioning in patients with chronic fatigue syndrome. Disabil. Rehabil. 2010, 33, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Turk, D.C.; Revicki, D.A.; Harding, G.; Coyne, K.S.; Peirce-Sandner, S.; Bhagwat, D.; Everton, D.; Burke, L.B.; Cowan, P.; et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 2009, 144, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, T.I.; Turk, D.C.; Morasco, B.J. Evaluation of the Psychometric Properties of the Revised Short-Form McGill Pain Questionnaire. J. Pain 2012, 13, 1250–1257. [Google Scholar] [CrossRef]
- Gauthier, L.R.; Young, A.; Dworkin, R.H.; Rodin, G.; Zimmermann, C.; Warr, D.; Librach, S.L.; Moore, M.; Shepherd, F.A.; Riddell, R.P.; et al. Validation of the Short-Form McGill Pain Questionnaire-2 in Younger and Older People With Cancer Pain. J. Pain 2014, 15, 756–770. [Google Scholar] [CrossRef]
- Kachooei, A.R.; Ebrahimzadeh, M.H.; Erfani-Sayyar, R.; Salehi, M.; Salimi, E.; Razi, S. Short Form-McGill Pain Questionnaire-2 (SF-MPQ-2): A Cross-Cultural Adaptation and Validation Study of the Persian Version in Patients with Knee Osteoarthritis. Arch. Bone Jt. Surg.-Abjs 2015, 3, 45–50. [Google Scholar]
- Norman, G.R.; Sloan, J.A.; Wyrwich, K.W. Interpretation of changes in health-related quality of life-The remarkable universality of half a standard deviation. Med. Care 2003, 41, 582–592. [Google Scholar] [CrossRef]
- Smets, E.M.; Garssen, B.; Bonke, B.; de Haes, J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Elbers, R.G.; van Wegen, E.E.H.; Verhoef, J.; Kwakkel, G. Reliability and structural validity of the Multidimensional Fatigue Inventory (MFI) in patients with idiopathic Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, 532–536. [Google Scholar] [CrossRef]
- Murdock, K.W.; Wang, X.S.; Shi, Q.; Cleeland, C.S.; Fagundes, C.P.; Vernon, S.D. The utility of patient-reported outcome measures among patients with myalgic encephalomyelitis/chronic fatigue syndrome. Qual. Life Res. 2017, 26, 913–921. [Google Scholar] [CrossRef]
- Jason, L.A.; Evans, M.; Brown, M.; Porter, N.; Brown, A.; Hunnell, J.; Anderson, V.; Lerch, A. Fatigue Scales and Chronic Fatigue Syndrome: Issues of Sensitivity and Specificity. Disabil. Stud. Q. 2011, 31, 1375. [Google Scholar] [CrossRef] [PubMed]
- Donta, S.T.; Engel, C.C.; Collins, J.F.; Baseman, J.B.; Dever, L.L.; Taylor, T.; Boardman, K.D.; Kazis, L.E.; Martin, S.E.; Horney, R.A.; et al. Benefits and harms of doxycycline treatment for Gulf War veterans’ illnesses. Ann. Intern. Med. 2004, 141, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hayer, S.D.; Rabago, D.P.; Amaza, I.P.; Kille, T.; Coe, C.L.; Zgierska, A. Effectiveness of nasal irrigation for chronic rhinosinusitis and fatigue in patients with Gulf War illness: Protocol for a randomized controlled trial. Contemp. Clin. Trials 2015, 41, 219–226. [Google Scholar] [CrossRef][Green Version]
- Proctor, S.P.; Heaton, K.J.; Heeren, T.; White, R.F. Effects of sarin and cyclosarin exposure during the 1991 Gulf War on neurobehavioral functioning in US army veterans. Neurotoxicology 2006, 27, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.; Fleming, J.; Bennett, S.; Burmeister, B.; Haines, T. Determining the minimal clinically important difference criteria for the Multidimensional Fatigue Inventory in a radiotherapy population. Support. Care Cancer 2010, 18, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Grp, P.C. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ-Br. Med. J. 2016, 355, i5239. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Statistics notes-How to randomise. Br. Med. J. 1999, 319, 703–704. [Google Scholar] [CrossRef] [PubMed]
- Lachin, J.M. Properties of simple randomization in clinical-trials. Control. Clin. Trials 1988, 9, 312–326. [Google Scholar] [CrossRef]
- Haley, R.W.; Maddrey, A.M.; Gershenfeld, H.K. Severely reduced functional status in veterans fitting a case definition of Gulf War syndrome. Am. J. Public Health 2002, 92, 46–47. [Google Scholar] [CrossRef]
- Lee, D.H.; Jacobs, D.R. Serum gamma-glutamyltransferase: New insights about an old enzyme. J. Epidemiol. Community Health 2009, 63, 884–886. [Google Scholar] [CrossRef]
- Lacher, J.W.; Schrier, R.W. Sweating treatment for chronic renal-failure. Nephron 1978, 21, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Rayhan, R.U.; Stevens, B.W.; Timbol, C.R.; Adewuyi, O.; Walitt, B.; VanMeter, J.W.; Baraniuk, J.N. Increased Brain White Matter Axial Diffusivity Associated with Fatigue, Pain and Hyperalgesia in Gulf War Illness. PLoS ONE 2013, 8, e58493. [Google Scholar] [CrossRef] [PubMed]
- Erickson, L.C.; Ritchie, J.B.; Javors, J.M.; Golomb, B.A. Recruiting a special sample with sparse resources: Lessons from a study of Gulf War veterans. Clin. Trials 2013, 10, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Doebbeling, B.N.; Merchant, J.A.; Barrett, D.H.; Black, D.W.; Burmeister, L.F.; Clarke, W.R.; Falter, K.H.; Hall, D.B.; Jones, M.F.; et al. Self-reported illness and health status among gulf war veterans-A population-based study. JAMA-J. Am. Med. Assoc. 1997, 277, 238–245. [Google Scholar]
- Nakamura, Y.; Lipschitz, D.L.; Donaldson, G.W.; Kida, Y.; Williams, S.L.; Landward, R.; Glover, D.W.; West, G.; Tuteja, A.K. Investigating Clinical Benefits of a Novel Sleep-Focused Mind-Body Program on Gulf War Illness Symptoms: A Randomized Controlled Trial. Psychosom. Med. 2017, 79, 706–718. [Google Scholar] [CrossRef]
- Magni, G.; Amici, A.; Emanuelli, M.; Orsomando, G.; Raffaelli, N.; Ruggieri, S. Enzymology of NAD+ homeostasis in man. Cell. Mol. Life Sci. 2004, 61, 19–34. [Google Scholar] [CrossRef]
- Monteiro, J.P.; da Cunha, D.F.; Filho, D.C.; Silva-Vergara, M.L.; Santos, V.M.D.; da Costa, J.C.J.; Etchebehere, R.M.; Goncalves, J.; de Carvalho da Cunha, S.F.; Jordao, A.A. Niacin metabolite excretion in alcoholic pellagra and AIDS patients with and without diarrhea. Nutrition 2004, 20, 778–782. [Google Scholar] [CrossRef]
- Creider, J.C.; Hegele, R.A.; Joy, T.R. Niacin: Another look at an underutilized lipid-lowering medication. Nat. Rev. Endocrinol. 2012, 8, 517–528. [Google Scholar] [CrossRef]
- Ganji, S.H.; Kashyap, M.L.; Kamanna, V.S. Niacin inhibits fat accumulation, oxidative stress, and inflammatory cytokine IL-8 in cultured hepatocytes: Impact on non-alcoholic fatty liver disease. Metab.-Clin. Exp. 2015, 64, 982–990. [Google Scholar] [CrossRef]
- de Paula, E.S.; Carneiro, M.F.H.; Grotto, D.; Hernandes, L.C.; Antunes, L.M.G.; Barbosa, F. Protective effects of niacin against methylmercury-induced genotoxicity and alterations in antioxidant status in rats. J. Toxicol. Environ. Health-Part A-Curr. Issues 2016, 79, 174–183. [Google Scholar] [CrossRef]
- Altschul, R.; Hoffer, A.; Stephen, J.D. Influence of nicotinic acid on serum cholesterol in man. Arch. Biochem. 1955, 54, 558–559. [Google Scholar] [CrossRef]
- Cooper, D.L.; Murrell, D.E.; Roane, D.S.; Harirforoosh, S. Effects of formulation design on niacin therapeutics: Mechanism of action, metabolism, and drug delivery. Int. J. Pharm. 2015, 490, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.N. Plasma free fatty acid rebound induced by nicotinic acid. J. Lipid Res. 1967, 8, 239–244. [Google Scholar] [PubMed]
- Wang, W.; Basinger, A.; Neese, R.A.; Christiansen, M.; Hellerstein, M.K. Effects of nicotinic acid on fatty acid kinetics, fuel selection, and pathways of glucose production in women. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E50–E59. [Google Scholar] [CrossRef]
- Carlson, L.A.; Oro, L. Effect of nicotinic acid on plasma free fatty acids-demonstration of a metabolic type of sympathicolysis. Acta Med. Scand. 1962, 172, 641–645. [Google Scholar] [CrossRef]
- Mitjavila, S.; Carrera, G.; Fernandez, Y. Evaluation of the toxic risk of accumulated DDT in the rat: During fat mobilization. Arch. Environ. Contam. Toxicol. 1981, 10, 471–481. [Google Scholar] [CrossRef]
- Imbeault, P.; Chevrier, J.; Dewailly, E.; Ayotte, P.; Despres, J.P.; Mauriege, P.; Tremblay, A. Increase in plasma pollutant levels in response to weight loss is associated with the reduction of fasting insulin levels in men but not in women. Metab.-Clin. Exp. 2002, 51, 482–486. [Google Scholar] [CrossRef]
- al Mulla, N.; Simonsen, L.; Bulow, J. Post-exercise adipose tissue and skeletal muscle lipid metabolism in humans: The effects of exercise intensity. J. Physiol.-Lond. 2000, 524, 919–928. [Google Scholar] [CrossRef]
- Taylor, J.K.; Plaisance, E.P.; Mahurin, A.J.; Mestek, M.L.; Moncada-Jimenez, J.; Grandjean, P.W. Paraoxonase responses to exercise and niacin therapy in men with metabolic syndrome. Redox Rep. 2015, 20, 42–48. [Google Scholar] [CrossRef]
- Kimbrough, R.D.; Korver, M.P.; Burse, V.W.; Groce, D.F. Effect of different diets or mineral-oil on liver pathology and polybrominated biphenyl concentration in tissues. Toxicol. Appl. Pharmacol. 1980, 52, 442–453. [Google Scholar] [CrossRef]
- Hussain, J.N.; Mantri, N.; Cohen, M.M. Working Up a Good Sweat-The Challenges of Standardising Sweat Collection for Metabolomics Analysis. Clin. Biochem. Rev. 2017, 38, 13–34. [Google Scholar] [PubMed]
- Mosher, H.H. Simultaneous study of constituents of urine and perspiration. J. Biol. Chem. 1933, 99, 781–790. [Google Scholar]
- Hanafusa, N.; Lodebo, B.T.; Shah, A.; Kopple, J.D. Is There a Role for Diaphoresis Therapy for Advanced Chronic Kidney Disease Patients? J. Ren. Nutr. 2017, 27, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ishizaka, T.; Suzuki, T.; Takeda, M.; Uchiyama, M. Organochlorine chemicals in skin lipids as an index of their accumulation in the human body. Arch. Environ. Contam. Toxicol. 1991, 21, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, T.; Watanabe, M.; Tanaka, K.; Yoshida, H.; Nonaka, S.; Tsukazaki, N.; Rikioka, Y. Polychlorinated biphenyls (PCBs) and polychlorinated quaterphenyls (PCQs) concentrations in skin surface lipids and blood of patients with yusho. Fukuoka Igaku Zasshi 1993, 84, 212–216. [Google Scholar]
- Levisky, J.A.; Bowerman, D.L.; Jenkins, W.W.; Karch, S.B. Drug deposition in adipose tissue and skin: Evidence for an alternative source of positive sweat patch tests. Forensic Sci. Int. 2000, 110, 35–46. [Google Scholar] [CrossRef]
- Huestis, M.A.; Oyler, J.M.; Cone, E.J.; Wstadik, A.T.; Schoendorfer, D.; Joseph, R.E.J. Sweat testing for cocaine, codeine and metabolites by gas chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1999, 733, 247–264. [Google Scholar] [CrossRef]
- Vree, T.B.; Jm, V.; Muskens, A.T.J. Excretion of amphetamines in human sweat. Arch. Int. Pharmacodyn. Ther. 1972, 199, 311–317. [Google Scholar]
- de Giovanni, N.; Fucci, N. The Current Status of Sweat Testing For Drugs of Abuse: A Review. Curr. Med. Chem. 2013, 20, 545–561. [Google Scholar]
- Hopf, N.B.; Ruder, A.M.; Waters, M.A.; Succop, P. Concentration-dependent half-lives of polychlorinated biphenyl in sera from an occupational cohort. Chemosphere 2013, 91, 172–178. [Google Scholar] [CrossRef]
- Quinn, C.L.; Wania, F. Understanding Differences in the Body Burden-Age Relationships of Bioaccumulating Contaminants Based on Population Cross Sections versus Individuals. Environ. Health Perspect. 2012, 120, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Dennis, K.K.; Marder, E.; Balshaw, D.M.; Cui, Y.X.; Lynes, M.A.; Patti, G.J.; Rappaport, S.M.; Shaughnessy, D.T.; Vrijheid, M.; Barr, D.B. Biomonitoring in the Era of the Exposome. Environ. Health Perspect. 2017, 125, 502–510. [Google Scholar] [CrossRef] [PubMed]
- White, R.F.; Proctor, S.P.; Heeren, T.; Wolfe, J.; Krengel, M.; Vasterling, J.; Lindem, K.; Heaton, K.J.; Sutker, P.; Ozonoff, D.M. Neuropsychological function in Gulf War Veterans: Relationships to self-reported toxicant exposures. Am. J. Ind. Med. 2001, 40, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Cecil, P.E.; Guilmartin, J.F. Assessing possible exposures of ground troops to agent orange during the Vietnam war: The use of contemporary military records. Environ. Sci. Pollut. Res. 2004, 11, 349–358. [Google Scholar] [CrossRef]
- McNeil, R.B.; Thomas, C.M.; Coughlin, S.S.; Hauser, E.; Huang, G.D.; Goldstein, K.M.; Johnson, M.R.; Dunn-Thomas, T.; Provenzale, D.T. An assessment of survey measures used across key epidemiologic studies of United States Gulf War I Era Veterans. Environ. Health 2013, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, M.A. Considerations in determining sample size for pilot studies. Res. Nurs. Health 2008, 31, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Teasell, R.; Mamdani, M.; Hall, J.; McIlroy, W.; Cheung, D.; Thorpe, K.E.; Cohen, L.G.; Bayley, M. Effectiveness of Virtual Reality Using Wii Gaming Technology in Stroke Rehabilitation A Pilot Randomized Clinical Trial and Proof of Principle. Stroke 2010, 41, 1477–1484. [Google Scholar] [CrossRef]
- King, M.T. A point of minimal important difference (MID): A critique of terminology and methods. Expert Rev. Pharm. Outcomes Res. 2011, 11, 171–184. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Burke, J.F.; Sussman, J.B.; Prescott, H.C.; Hayward, R.A.; Angus, D.C. Implications of Heterogeneity of Treatment Effect for Reporting and Analysis of Randomized Trials in Critical Care. Am. J. Respir. Crit. Care Med. 2015, 192, 1045–1051. [Google Scholar] [CrossRef]
- Chao, L.D.L.; Zhang, Y. Effects of low-level sarin and cyclosarin exposure on hippocampal microstructure in Gulf War Veterans. Neurotoxicol. Teratol. 2018, 68, 36–46. [Google Scholar] [CrossRef]
- Schnare, D.W.; Robinson, P.C. Reduction of the human body burdens of hexachlorobenzene and polychlorinated biphenyls. IARC Sci. Publ. 1986, 77, 597–603. [Google Scholar]
- Tretjak, Z.; Root, D.E.; Tretjak, A.; Slivnik, R.; Edmondson, E.; Graves, R.; Beckmann, S.L. Xenobiotic Reduction and Clinical Improvements in Capacitor Workers: A Feasible Method. J. Environ. Sci. Health 1990, 25, 731–751. [Google Scholar] [CrossRef]
- Dahlgren, J.; Cecchini, M.; Takhar, H.; Paepke, O. Persistent organic pollutants in 9/11 world trade center rescue workers: Reduction following detoxification. Chemosphere 2007, 69. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.J.; Slater, B.C.S.; Leis, L.A.; Rector, T.S.; Bach, R.R. Blood Biomarkers of Chronic Inflammation in Gulf War Illness. PLoS ONE 2016, 11, e0153157. [Google Scholar] [CrossRef] [PubMed]
- Roumelioti, M.E.; Nolin, T.; Unruh, M.L.; Argyropoulos, C. Revisiting the Middle Molecule Hypothesis of Uremic Toxicity: A Systematic Review of Beta 2 Microglobulin Population Kinetics and Large Scale Modeling of Hemodialysis Trials In Silico. PLoS ONE 2016, 11, e0153157. [Google Scholar] [CrossRef] [PubMed]
- Hooton, K.; Li, L. Nonocclusive Sweat Collection Combined with Chemical Isotope Labeling LC-MS for Human Sweat Metabolomics and Mapping the Sweat Metabolomes at Different Skin Locations. Anal. Chem. 2017, 89, 7847–7851. [Google Scholar] [CrossRef]
- Mallon, T.M.; Rohrbeck, P.; Haines, K.M.; Jones, D.P.; Utell, M.; Hopke, P.K.; Phipps, R.P.; Walker, D.I.; Thatcher, T.; Woeller, C.F.; et al. Introduction to Department of Defense Research on Burn Pits, Biomarkers, and Health Outcomes Related to Deployment in Iraq and Afghanistan. J. Occup. Environ. Med. 2016, 58, S3–S11. [Google Scholar] [CrossRef]
| Characteristic | Intervention n = 22 | Control n = 10 |
|---|---|---|
| Age (years) mean ± SD | 51.7 ± 7.9 | 50.2 ± 5 |
| BMI (kg/m2) mean ± SD | 32.3 ± 6.2 | 32.0 ± 6.3 |
| Sex [n (%)] | ||
| Male | 14 (64) | 7 (70) |
| Female | 8 (36) | 3 (30) |
| Race [n (%)] | ||
| White | 20 (91) | 5 (50) |
| Black | 1 (1) | 5 (50) |
| Other | 1 (1) | 0 (0) |
| Married [n (%)] | 15 (65) | 4 (36) |
| Employment [n (%)] | ||
| Fulltime | 9 (41) | 10 (100) |
| Part time, unemployed, retired | 3 (13) | 0 (0) |
| Disabled | 10 (41) | 0 (0) |
| Current smoker [n (%)] | 4 (18) | 0 (0) |
| Smoked during Gulf War [n (%)] | 3 (14) | 3 (30) |
| Comorbidities [n (%)] | ||
| # diabetes (DM) | 3 (13) | 2 (15) |
| # who remained on medications (DM, blood pressure, Other) | 11 (48) | 5 (45) |
| # who recently stopped antidepressants | 11 (48) | 1 (8) |
| # who recently stopped analgesics | 8 (35) | 2 (6) |
| Symptom and function assessments- mean ± SD | ||
| VR-36 physical component summary | 30.3 ± 8.9 | 36.7 ± 10.5 |
| VR-36 mental component summary | 38.4 ± 12.1 | 40.5 ± 14.1 |
| physical functioning | 43.8 ± 20 | 59.4 ± 28.8 |
| role-physical | 35.6 ± 29.3 | 54.6 ± 37.2 |
| bodily pain | 32 ± 26.2 | 44.0 ± 29.1 |
| general health | 27.3 ± 18.5 | 40.9 ± 27.3 |
| vitality | 23.3 ± 23.1 | 32.0 ± 25.3 |
| social functioning | 36.9 ± 27.7 | 46.6 ± 35.0 |
| role-emotional | 53 ± 31.3 | 61.7 ± 34.5 |
| mental health | 53 ± 22.2 | 58.0 ± 24.2 |
| Multidimensional fatigue inventory | ||
| general fatigue | 16.9 ± 3.2 | 15.3 ± 5.3 |
| physical fatigue | 16.1 ± 4.0 | 12.6 ± 5.7 |
| reduced activity | 15 ± 4.3 | 12.4 ± 4.7 |
| reduced motivation | 13.7 ± 4.0 | 11.8 ± 3.8 |
| mental fatigue | 14.6 ± 2.8 | 15.4 ± 4.6 |
| SF McGill Pain Q-2 total pain | 4.2 ± 1.8 | 3.9 ± 2.3 |
| Kansas GWI case criteria (number positive of 6) | 4.5 ± 1.2 | 4.6 ± 0.7 |
| Health Measures | Waitlist (WL) Control (Usual Care) n = 10 | Intervention n = 22 | Adjusted between Group Differences1 Comparing Scores between WL and Intervention at Week 6 | p-Value | ||
|---|---|---|---|---|---|---|
| VR−36 quality of life | Baseline | 6-wk follow-up | Baseline | 6-wk follow-up | (95% confidence interval) | |
| VR-36 physical component summary | 36.7 (10.4) | 35.7(12.10) | 30.3 (8.9) | 38.2 (10.3) | 6.9 (−0.3, 14.2) | 0.06 |
| VR-36 mental component summary | 40.5 (14.1) | 41.1 (12.8) | 38.4 (12.1) | 49.2 (9.3) | 9.5 (3.1, 15.8) | 0.003 |
| physical functioning | 59.4 (28.8) | 63.5 (30.4) | 43.8 (20.0) | 59.5 (26.7) | 2.7 (−18.1, 23.5) | 0.8 |
| role-physical | 54.6 (37.2) | 45.0 (36.9) | 35.6 (29.3) | 61.9 (29.9)] | 27.6 (6.9, 48.3) | 0.009 |
| bodily pain | 44.0 (29.1) | 36.0 (28.0) | 32.1 (26.2) | 56.2 (27.0) | 26.4 (8.5, 44.4) | 0.004 |
| general health | 40.9 (27.3) | 38.5 (31.1) | 27.3 (18.5) | 45.7 (24.2) | 20.7 (9.2, 32.3) | < 0.001 |
| vitality | 32.0 (25.3) | 30.0 (28.6) | 23.3 (23.1) | 53.9 (25.2) | 31.2 (15.6, 46.9) | < 0.001 |
| social functioning | 46.6 (35.0) | 52.5 (32.2)′ | 36.9 (27.7) | 64.2 (26.5) | 15.9 (−3.9, 35.7) | 0.1 |
| role-emotional | 61.7 (34.5) | 59.2 (41.1) | 53.0 (31.3) | 68.9 (26.1) | 15.2 (−4.9, 35.2) | 0.1 |
| mental health | 58.0 (24.2) | 59.6 (25.6) | 53.0 (22.2) | 73.8 (18.4) | 17.7 (5.3, 30.0) | 0.005 |
| Multidimensional Fatigue Inventory | ||||||
| general fatigue | 15.3 (5.3) | 15.7 (3.6) | 17.4 (2.6) | 12.8 (4.8) | −4.3 (−7.4, −1.3) | 0.006 |
| physical fatigue | 12.5 (5.7) | 14.6 (4.8) | 16.2 (4.0) | 13.1 (4.7) | −3.5 (−6.9, −0.2) | 0.04 |
| reduced activity | 12.4 (4.7) | 15.4 (4.9) | 15.2 (4.3) | 12.8 (4.4) | −4.0 (−7.3, −0.7) | 0.02 |
| reduced motivation | 11.8 (3.8) | 12.2 (3.2) | 13.7 (4.0) | 10.2 (3.9) | −3.1 (−5.6, −0.5) | 0.02 |
| mental fatigue | 15.4 (4.6) | 16.2 (4.5) | 14.6 (2.8) | 10.4 (4.0) | −5.7 (−8.7, −2.7) | < 0.001 |
| SF McGill Pain Questionnaire-2 | ||||||
| Total pain score | 3.9 (2.5) | 3.0 (2.0) | 4.2 (1.8) | 2.1 (1.5) | −1.1 (−2.0, −0.2) | 0.02 |
| Kansas GWI case criteria | ||||||
| Total of six domains score | 4.7 (0.7) | 3.3 (1.8) | 4.5 (5.3) | 2.7 (3.7) | −0.5 (−1.9, 0.9) | 0.5 |
| Proportion positive | 10/10 (100%) | 8/10 (80%) | 22/22 (100%) | 11/22 (50%) | ||
| Health Measures | Waitlisted | Waitlisted | Intervention | Intervention |
|---|---|---|---|---|
| Parameter | Week 6 § vs. Baseline * | Month 3 vs. Week 6 | Week 6 vs. Baseline | Month 3 vs. Week 6 |
| VR-36 quality of life | ||||
| VR-36 PCS | 5.8 (0.45, 11) | −1.3 (−8.2, 5.7) | 7.8 (4.2, 11.5) | −1.3 (−5.3, 2.7) |
| VR-36 MCS | 2.2 (−3.9, 8.4) | 7.3 (−0.7, 15.3) | 11.1 (6.7, 15.3) | −0.1 (−4.8, 4.5) |
| VR-36 Sub scores | ||||
| Physical functioning | 11 (−3.9, 25.9) | −4.9 (−24.1, 14.4) | 15.6 (5.5, 25.7) | −0.2 (−11.5, 11) |
| Role−physical | 15 (−1.1, 31.1) | 2.1 (−18.7, 23.0) | 26.7 (15.7, 37.6) | −6.5 (−18.6, 5.7) |
| Bodily pain | 7.5 (−8.1, 23.8) | 2.4 (−18.7, 23.6) | 22.9 (11.8, 34.1) | −7.9 (−20.3, 4.4) |
| General health | 16.6 (5.9, 27.4) | 1.4 (−12.7, 15.5) | 18.2 (10.8, 25.5) | −2.7 (−10.9, 5.5) |
| Vitality | 11.5 (−3.1, 26.1) | 29.6 (10.7, 48.5) | 31.4 (21.5, 41.4) | 13.3 (2.3, 24.3) |
| Social functioning | 6.2 (−10.5, 23) | 3 (−18.7, 24.7) | 27.6 (16.2, 39) | −6.5 (−19.1, 6.2) |
| Role−emotional | 9.2 (−8.4, 26.7) | 4.1 (−18.7, 26.9) | 15.8 (3.9, 27.8) | 2.6 (−10.7, 16) |
| Mental health | 4 (−7.2, 15.2) | 10.4 (−4.1, 25) | 21.3 (13.7, 28.9) | −6.6 (−15, 1.9) |
| Multidimensional fatigue inventory | ||||
| General fatigue | −2.8 (−5.5, −0.1) | −1.6 (−4.7, 1.5) | −4.1 (−5.9, −2.4) | 1.2 (−0.8, 3.1) |
| Physical fatigue | −3 (5.9, − 0.1) | −1.1 (−4.5, 2.2) | −3 (−4.9, −1.2) | 0.5 (−1.6, 2.6) |
| Reduced activity | −3.6 (−6.9, −0.2) | −3.6 (−7.5, 0.3) | −2.3 (−4.5, −0.2) | −0.4 (−2.8, 2) |
| Reduced motivation | −2.7 (−5.2, −0.2) | −1.3 (−4.2, 1.6) | −3.2 (−4.8, −1.6) | 0.6 (−1.2, 2.4) |
| Mental fatigue | −2.3 (−5.2, 0.5) | −3.9 (−7.2, −0.7) | −4.1 (−5.9, −2.4) | 1.7 (−0.3, 3.7) |
| SF McGill Pain Questionnaire-2 | ||||
| Total pain score | −1 (−1.9, −0.1) | 0.6 (−0.5, 1.7) | −2.2 (−2.7, −1.6) | 0.3 (−0.3, 1.0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerr, K.; Morse, G.; Graves, D.; Zuo, F.; Lipowicz, A.; Carpenter, D.O. A Detoxification Intervention for Gulf War Illness: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16, 4143. https://doi.org/10.3390/ijerph16214143
Kerr K, Morse G, Graves D, Zuo F, Lipowicz A, Carpenter DO. A Detoxification Intervention for Gulf War Illness: A Pilot Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2019; 16(21):4143. https://doi.org/10.3390/ijerph16214143
Chicago/Turabian StyleKerr, Kathleen, Gayle Morse, Donald Graves, Fei Zuo, Alain Lipowicz, and David O. Carpenter. 2019. "A Detoxification Intervention for Gulf War Illness: A Pilot Randomized Controlled Trial" International Journal of Environmental Research and Public Health 16, no. 21: 4143. https://doi.org/10.3390/ijerph16214143
APA StyleKerr, K., Morse, G., Graves, D., Zuo, F., Lipowicz, A., & Carpenter, D. O. (2019). A Detoxification Intervention for Gulf War Illness: A Pilot Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 16(21), 4143. https://doi.org/10.3390/ijerph16214143






