Environmental Pollution as a Risk Factor in Testicular Tumour Development: Focus on the Interaction between Bisphenol A and the Associated Immune Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Preparation of BPA
2.4. Perinatal BPA Exposure in Mice
2.5. Quantification of BPA Serum Levels
2.6. Cell Culture
2.7. Tumour Induction Model
2.8. Flow Cytometry Analysis
2.9. Haematoxylin-Eosin Staining
2.10. RT-PCR Assays
2.11. Statistical Analysis
3. Results
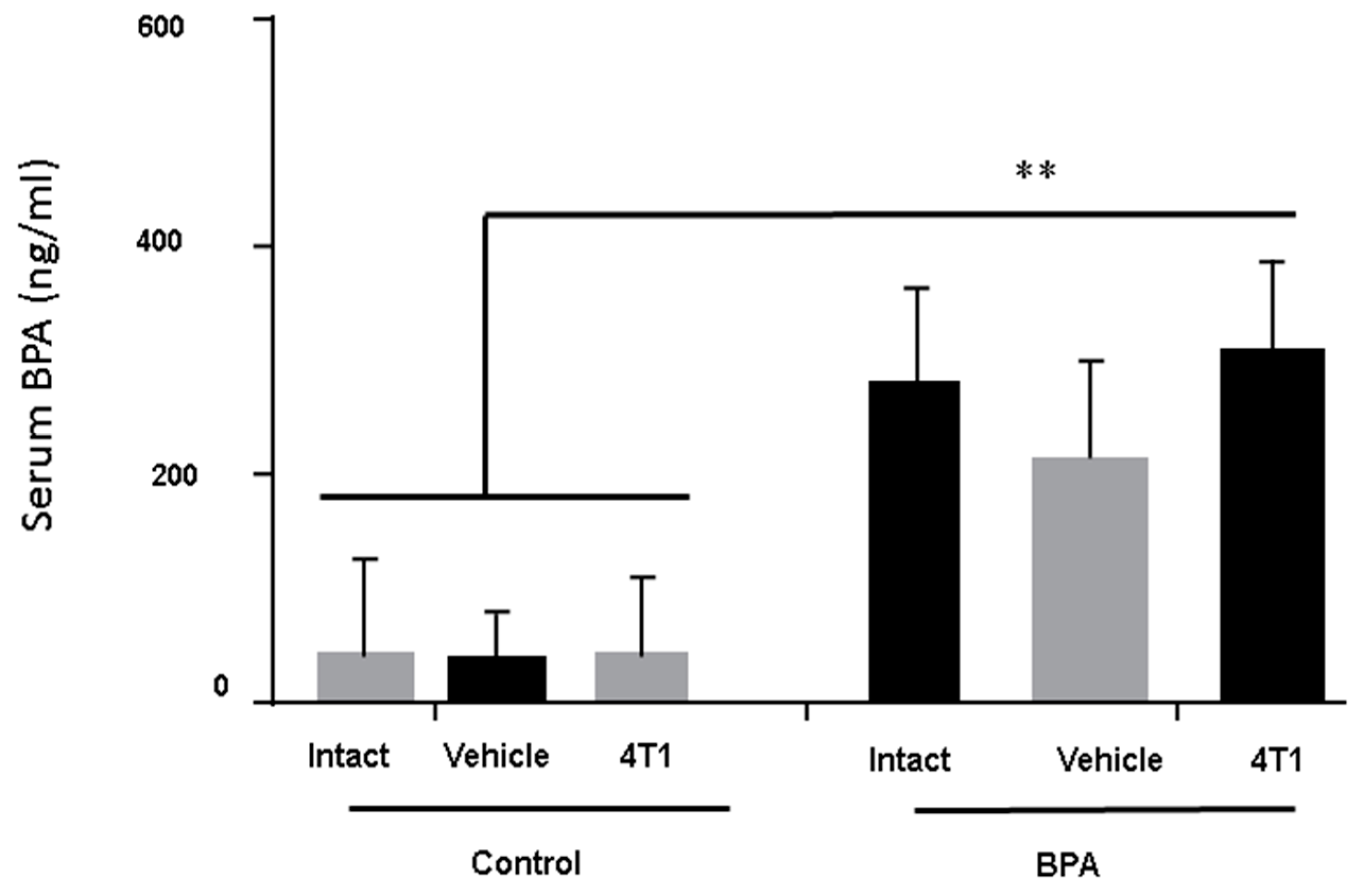
3.1. BPA-Serum Levels
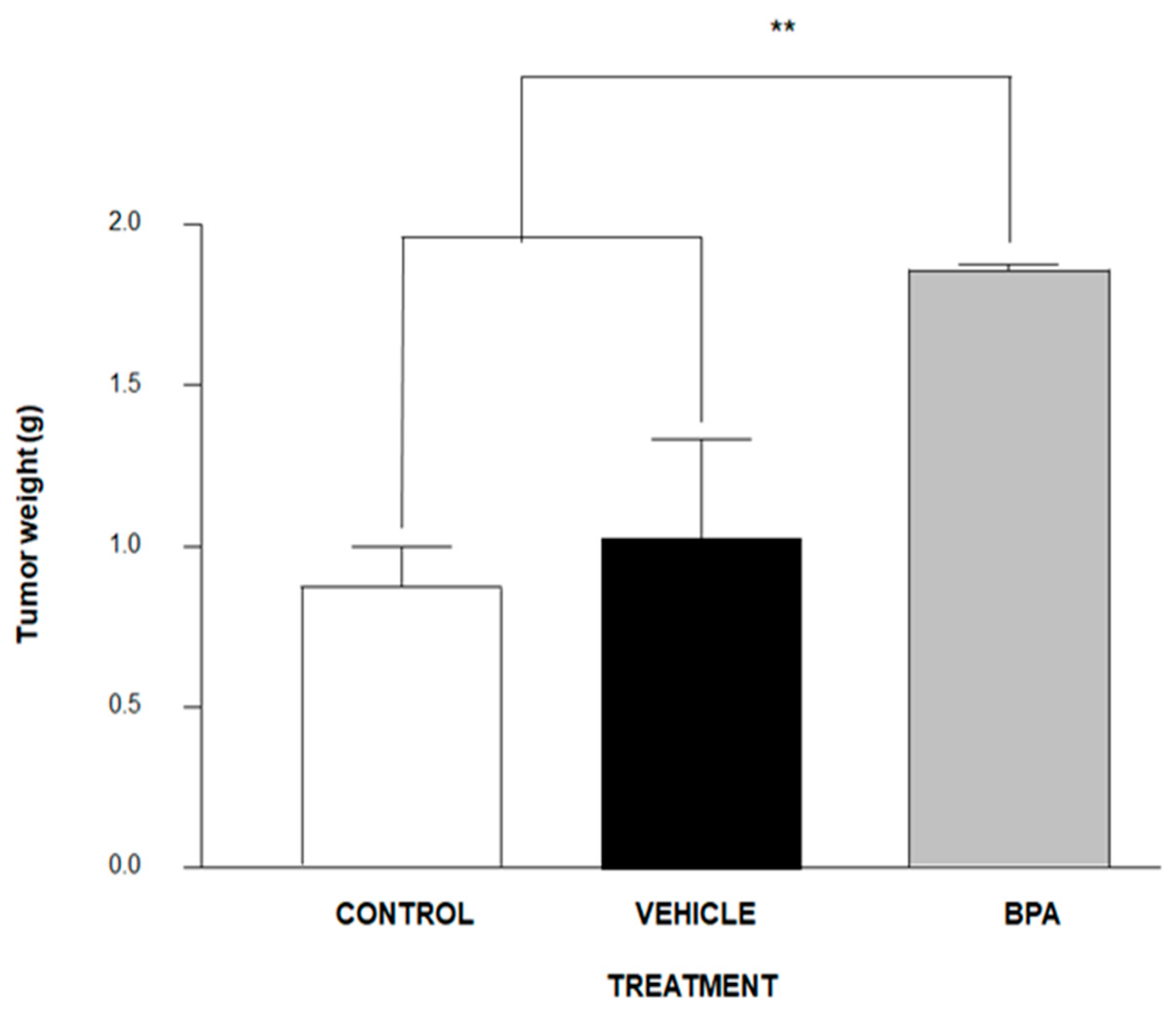
3.2. Tumour Induction
3.3. Haematoxilyn–Eosin (H&E) Staining
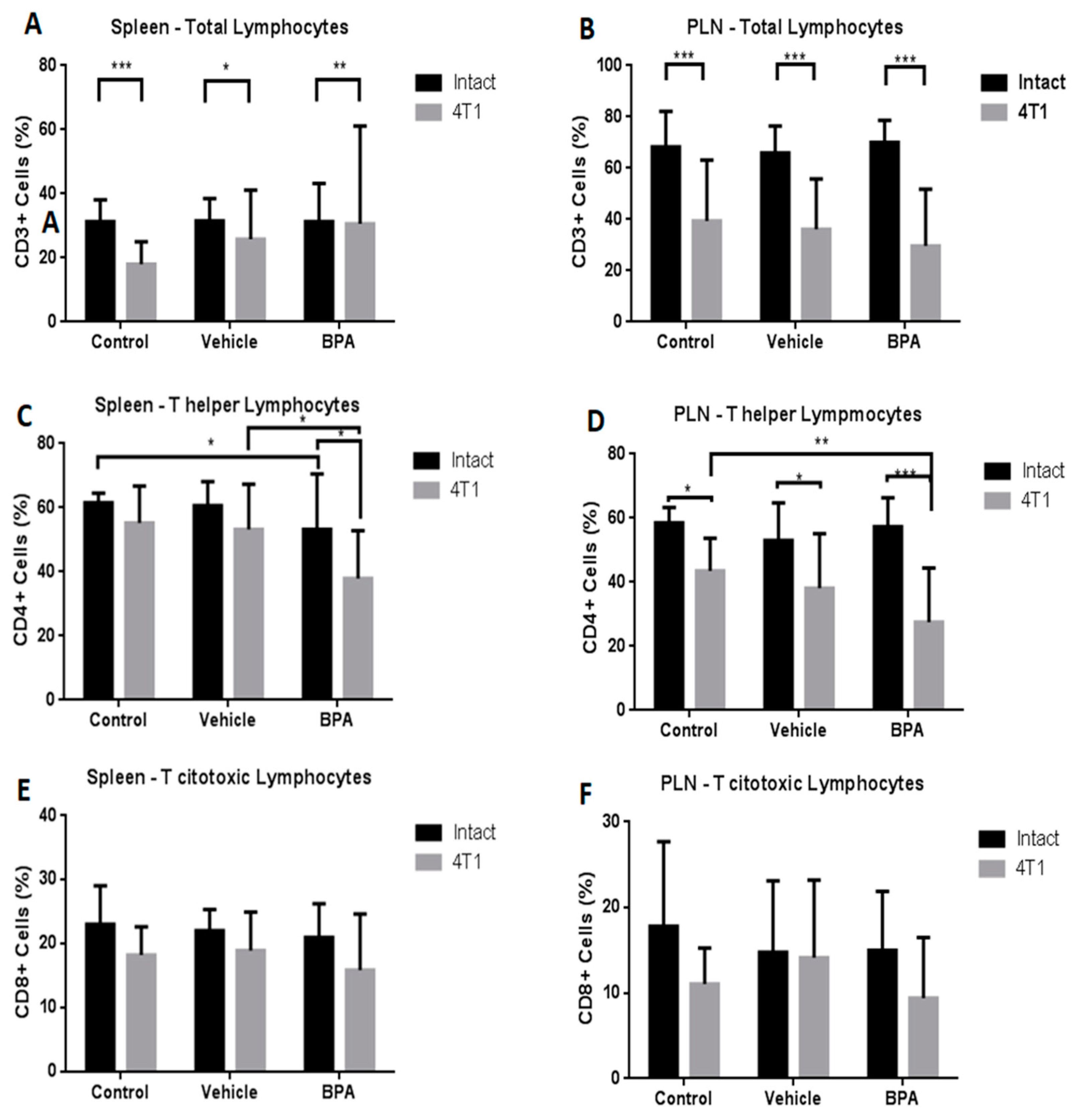
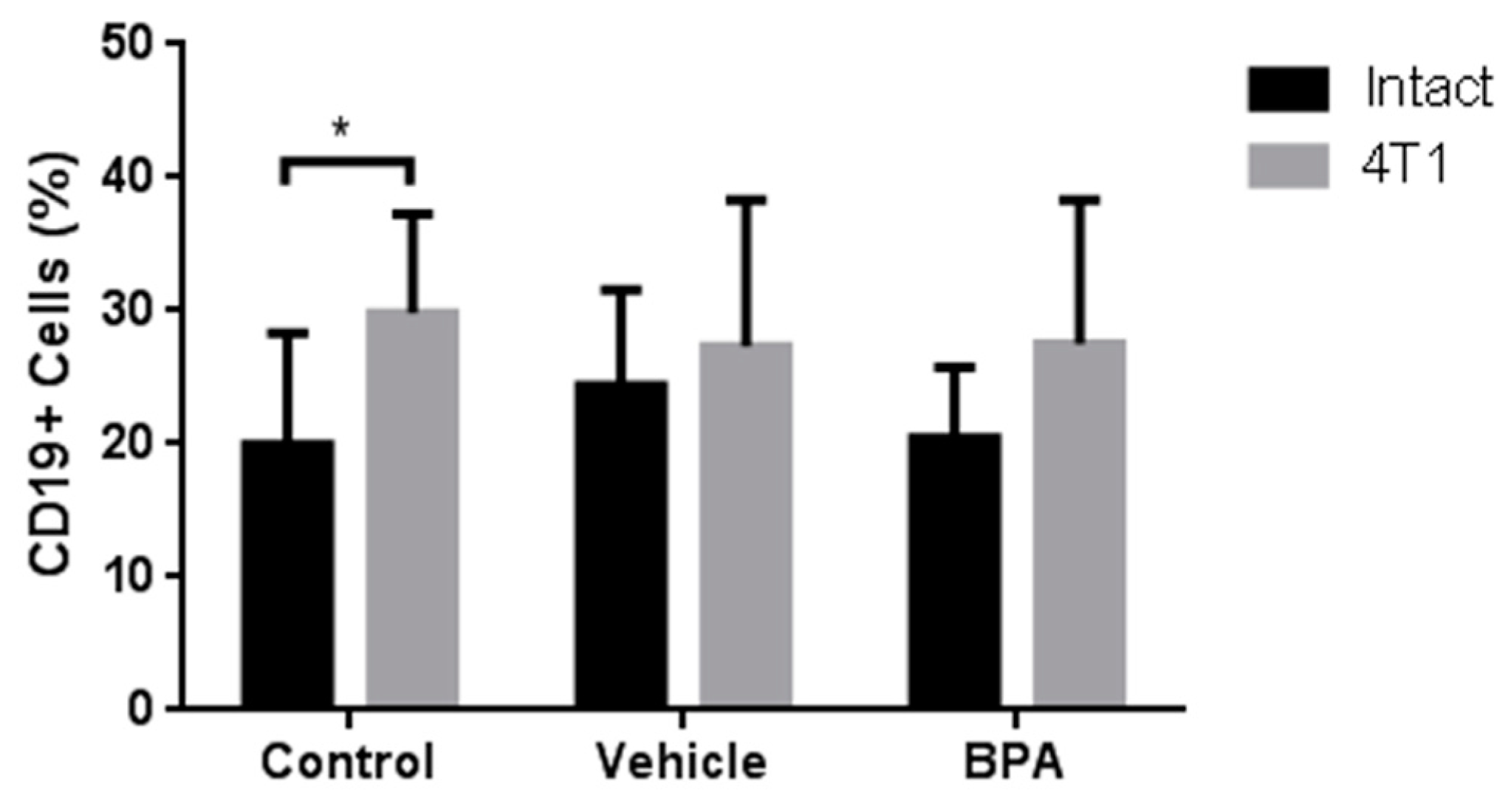
3.4. Percentage of Immune Cell Populations
3.5. Relative Expression of Intra-Tumoural Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kavlock, R.J.; Ankley, G.T. A perspective on the risk assessment process for endocrine-disruptive effects on wildlife and human health. Risk Anal. 1996, 16, 731–739. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. Ann. Endocrinol. 2013, 74, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, M.H.; Shin, K.T.; Niu, Y.J.; Ahn, Y.D.; Kim, N.H.; Cui, X.S. The possible molecular mechanisms of bisphenol A action on porcine early embryonic development. Sci. Rep. 2017, 7, 8632. [Google Scholar] [CrossRef] [PubMed]
- Swedenborg, E.; Ruegg, J.; Makela, S.; Pongratz, I. Endocrine disruptive chemicals: Mechanisms of action and involvement in metabolic disorders. J. Mol. Endocrinol. 2009, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Calemine, J.; Zalenka, J.; Karpuzoglu-Sahin, E.; Ward, D.L.; Lengi, A.; Ahmed, S.A. The immune system of geriatric mice is modulated by estrogenic endocrine disruptors (diethylstilbestrol, alpha-zearalanol, and genistein): Effects on interferon-gamma. Toxicology 2003, 194, 115–128. [Google Scholar] [CrossRef]
- Csaba, G. Hormones in the immune system and their possible role. A critical review. Acta Microbiol. Immunol. Hung. 2014, 61, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.Y.; Park, H.Y.; Lee, J.W.; Jung, I.O.; Choi, K.H.; Kim, K.; Cho, K.H. Evaluation of the immune response following exposure of mice to bisphenol A: Induction of Th1 cytokine and prolactin by BPA exposure in the mouse spleen cells. Arch Pharm. Res. 2002, 25, 946–953. [Google Scholar] [CrossRef]
- Yoshino, S.; Yamaki, K.; Li, X.; Sai, T.; Yanagisawa, R.; Takano, H.; Mori, Y. Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology 2004, 112, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, T.; Uemura, Y.; Jiao, S.; Wang, D.; Lin, Z.; Ishihara, O. Bisphenol A in combination with TNF-alpha selectively induces Th2 cell-promoting dendritic cells in vitro with an estrogen-like activity. Cell. Mol. Immunol. 2010, 7, 227–234. [Google Scholar] [PubMed]
- Palacios-Arreola, M.I.; Nava-Castro, K.E.; Rio-Araiza, V.H.D.; Perez-Sanchez, N.Y.; Morales-Montor, J. A single neonatal administration of Bisphenol A induces higher tumour weight associated to changes in tumour microenvironment in the adulthood. Sci. Rep. 2017, 7, 10573. [Google Scholar] [CrossRef] [PubMed]
- Shanmugalingam, T.; Soultati, A.; Chowdhury, S.; Rudman, S.; Van Hemelrijck, M. Global incidence and outcome of testicular cancer. Clin. Epidemiol. 2013, 5, 417–427. [Google Scholar]
- Milose, J.C.; Filson, C.P.; Weizer, A.Z.; Hafez, K.S.; Montgomery, J.S. Role of biochemical markers in testicular cancer: Diagnosis, staging, and surveillance. Open Access J. Urol. 2011, 4, 1–8. [Google Scholar]
- Chevalier, N.; Bouskine, A.; Fenichel, P. Bisphenol A promotes testicular seminoma cell proliferation through GPER/GPR30. Int. J. Cancer 2012, 130, 241–242. [Google Scholar] [CrossRef]
- Prins, G.S.; Tang, W.Y.; Belmonte, J.; Ho, S.M. Developmental exposure to bisphenol A increases prostate cancer susceptibility in adult rats: Epigenetic mode of action is implicated. Fertil. Steril. 2008, 89 (Suppl. 2), e41. [Google Scholar]
- Jorgensen, A.; Blomberg Jensen, M.; Nielsen, J.E.; Juul, A.; Rajpert-De Meyts, E. Influence of vitamin D on cisplatin sensitivity in testicular germ cell cancer-derived cell lines and in a NTera2 xenograft model. J. Steroid Biochem. Mol. Biol. 2013, 136, 238–246. [Google Scholar]
- Heaney, J.D.; Nadeau, J.H. Testicular germ cell tumors in mice: New ways to study a genetically complex trait. Methods Mol. Biol. 2008, 450, 211–231. [Google Scholar]
- Weber Lozada, K.; Keri, R.A. Bisphenol A increases mammary cancer risk in two distinct mouse models of breast cancer. Biol. Reprod. 2011, 85, 490–497. [Google Scholar] [CrossRef]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ. Health Perspect. 2009, 117, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Stanta, G.; Bonin, S. Overview on Clinical Relevance of Intra-Tumor Heterogeneity. Front. Med. 2018, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Elbakry, R.H.; Bayomy, N.A. Effect of bisphenol A on morphology, apoptosis and proliferation in the resting mammary gland of the adult albino rat. Int. J. Exp. Pathol. 2016, 97, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Perez-Diez, A.; Joncker, N.T.; Choi, K.; Chan, W.F.; Anderson, C.C.; Lantz, O.; Matzinger, P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007, 109, 5346–5354. [Google Scholar] [CrossRef] [PubMed]
- Deng, G. Tumor-infiltrating regulatory T cells: Origins and features. Am. J. Clin. Exp. Immunol. 2018, 7, 81–87. [Google Scholar] [PubMed]
- Huber, S.; Hoffmann, R.; Muskens, F.; Voehringer, D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood 2010, 116, 3311–3320. [Google Scholar] [CrossRef]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- O’Brien, E.; Dolinoy, D.C.; Mancuso, P. Perinatal bisphenol A exposures increase production of pro-inflammatory mediators in bone marrow-derived mast cells of adult mice. J. Immunotoxicol. 2014, 11, 205–212. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jeong, H.G. Down-regulation of inducible nitric oxide synthase and tumor necrosis factor-alpha expression by bisphenol A via nuclear factor-kappaB inactivation in macrophages. Cancer Lett. 2003, 196, 69–76. [Google Scholar]
- De Leon-Nava, M.A.; Nava, K.; Soldevila, G.; Lopez-Griego, L.; Chavez-Rios, J.R.; Vargas-Villavicencio, J.A.; Morales-Montor, J. Immune sexual dimorphism: Effect of gonadal steroids on the expression of cytokines, sex steroid receptors, and lymphocyte proliferation. J. Steroid Biochem. Mol. Biol. 2009, 113, 57–64. [Google Scholar] [CrossRef] [PubMed]



| Oligonucleotide | Sequence | MT (°C) | Product (bp) |
|---|---|---|---|
| IL-4 Sense | TCATGGGATGATGATGATAACCTGCT | 62 | 502 |
| IL-4 Antisense | CCCATACTTTAGGAAGACACGGATT | ||
| IL-10 Sense | AACTGGTAGAAGTGATGCCCCAGGCA | 63 | 237 |
| IL-10 Antisense | CTATGCAGTTGATGAAGATGTCAAA | ||
| TNF-α Sense | GGCAGGTCTACTTTGGAGTCATTGC | 63 | 300 |
| TNF-α Antisense | ACATTCGAGGCTCCAGTGAATTCGG | ||
| IFN-γ Sense | AGCGGCTGACTGAACTCAGATTGTAG | 60 | 247 |
| IFN-γ Antisense | GTCACAGTTTTCAGCTGTATAGGG | ||
| TGF-β Sense | CTTCAGCTCCACAGAGAAGAACTGA | 61 | 298 |
| TGF-β Antisense | CACAATCATGTTGGACAACTGCTCC | ||
| 18S Sense | CGCGGTTCTATTTTGTTGGT | 60 | 219 |
| 18S Antisense | AGTCGGCATCGTTTATGGTC |
| Cytokine | Control | Vehicle | BPA |
|---|---|---|---|
| IL-4 | 20.5 ± 3.8 | 22.9 ± 6.3 | ** 83.2 ± 4.1 |
| IL-10 | 10.1 ± 2.2 | 13.3 ± 1.2 | ** 57.0 ± 2.2 |
| TNF-α | 80.5 ± 8.3 | 78.9 ± 5.9 | * 41.1 ± 8.2 |
| IFN-γ | 72.3 ± 3.8 | 69.4 ± 9.1 | * 33.2 ± 9.1 |
| TGF-β | 39.5 ± 7.8 | 42.9 ± 8.4 | * 89.6 ± 10.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Castro, K.E.; Ramírez-Nieto, R.; Méndez-García, L.A.; Girón-Pérez, M.I.; Segovia-Mendoza, M.; Navidad-Murrieta, M.S.; Morales Montor, J. Environmental Pollution as a Risk Factor in Testicular Tumour Development: Focus on the Interaction between Bisphenol A and the Associated Immune Response. Int. J. Environ. Res. Public Health 2019, 16, 4113. https://doi.org/10.3390/ijerph16214113
Nava-Castro KE, Ramírez-Nieto R, Méndez-García LA, Girón-Pérez MI, Segovia-Mendoza M, Navidad-Murrieta MS, Morales Montor J. Environmental Pollution as a Risk Factor in Testicular Tumour Development: Focus on the Interaction between Bisphenol A and the Associated Immune Response. International Journal of Environmental Research and Public Health. 2019; 16(21):4113. https://doi.org/10.3390/ijerph16214113
Chicago/Turabian StyleNava-Castro, Karen Elizabeth, Ricardo Ramírez-Nieto, Lucía Angélica Méndez-García, Manuel Iván Girón-Pérez, Mariana Segovia-Mendoza, Migdalia Sarahy Navidad-Murrieta, and Jorge Morales Montor. 2019. "Environmental Pollution as a Risk Factor in Testicular Tumour Development: Focus on the Interaction between Bisphenol A and the Associated Immune Response" International Journal of Environmental Research and Public Health 16, no. 21: 4113. https://doi.org/10.3390/ijerph16214113
APA StyleNava-Castro, K. E., Ramírez-Nieto, R., Méndez-García, L. A., Girón-Pérez, M. I., Segovia-Mendoza, M., Navidad-Murrieta, M. S., & Morales Montor, J. (2019). Environmental Pollution as a Risk Factor in Testicular Tumour Development: Focus on the Interaction between Bisphenol A and the Associated Immune Response. International Journal of Environmental Research and Public Health, 16(21), 4113. https://doi.org/10.3390/ijerph16214113







