Stable H-O Isotopic Composition and Water Quality Assessment of Surface Water and Groundwater: A Case Study in the Dabie Mountains, Central China
Abstract
1. Introduction
2. Materials and Methods
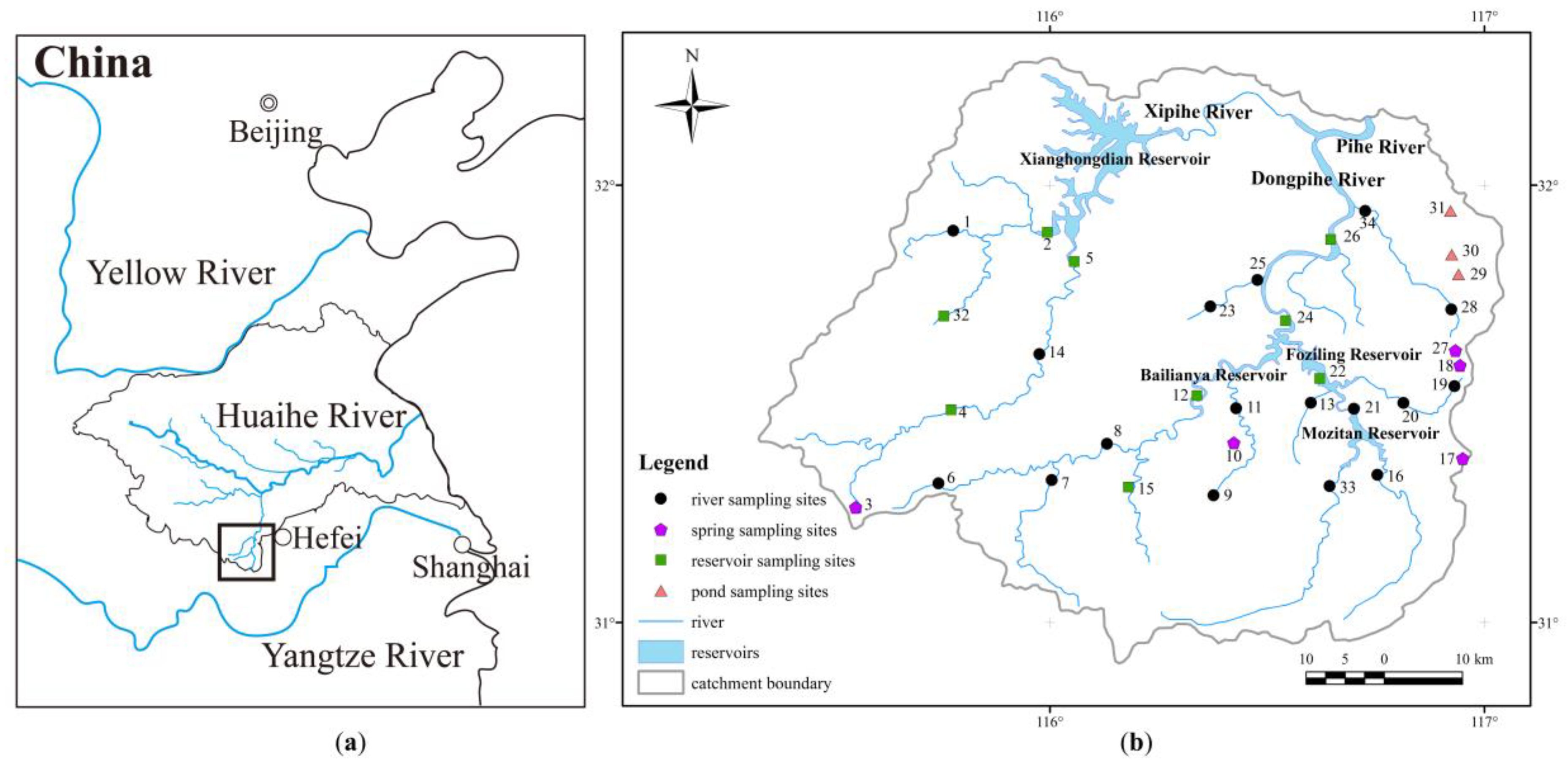
2.1. Background of the Study Area
2.1.1. Location
2.1.2. Climate
2.1.3. Reservoirs
2.2. Sampling Processes
2.3. Major Cations and Anions
2.4. Isotope Analysis
3. Results and Discussion
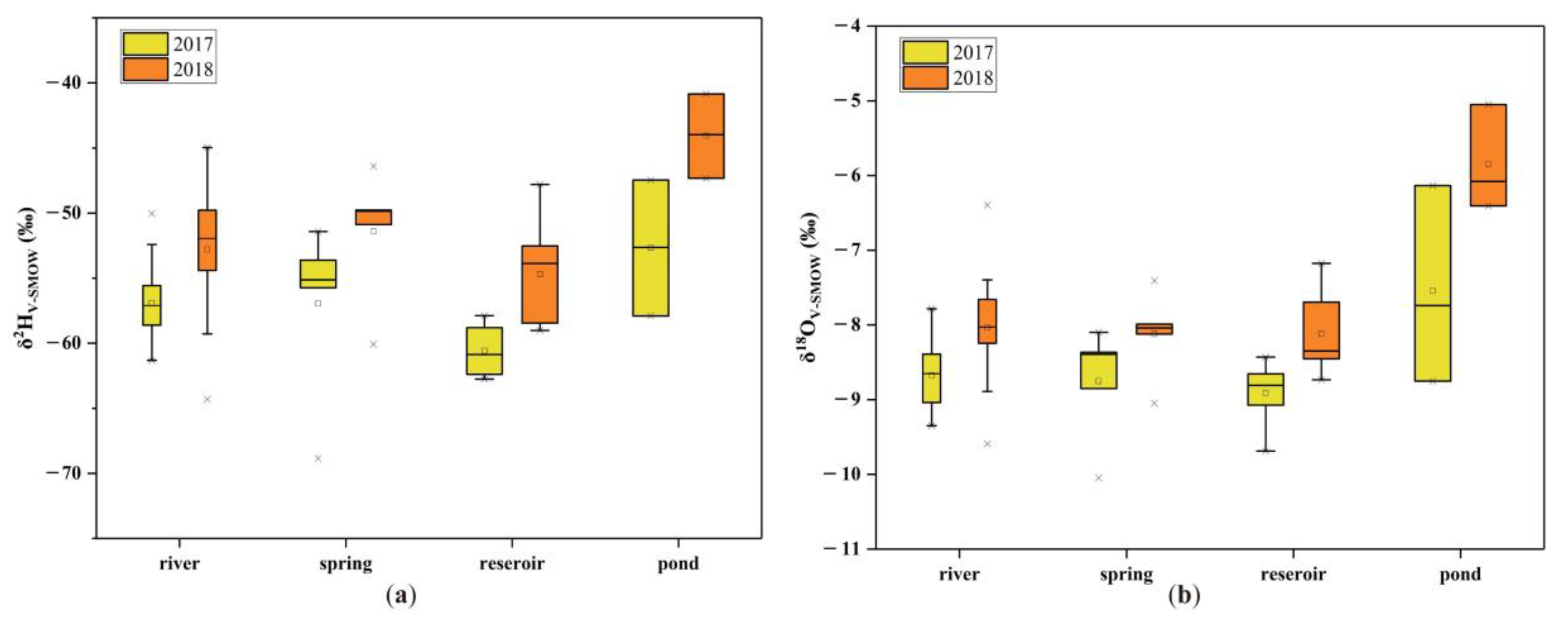
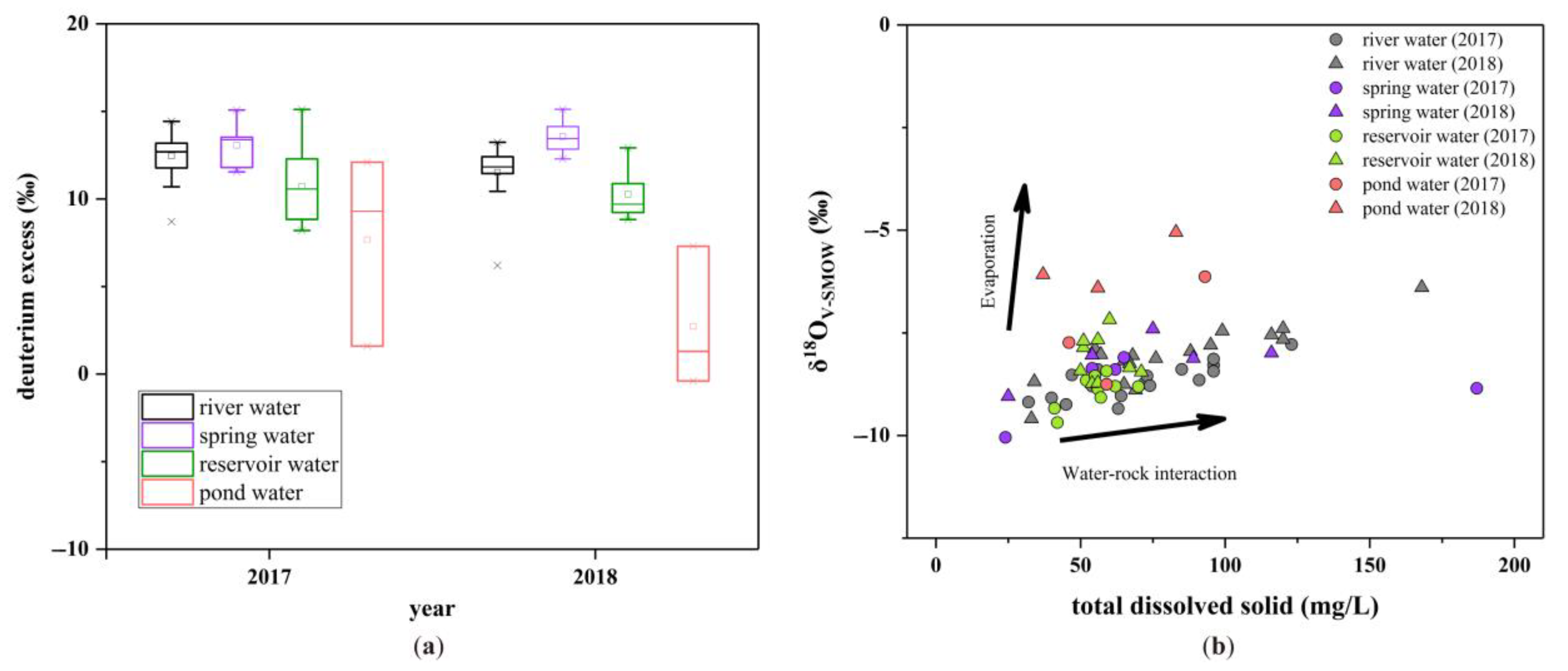
3.1. Stable H-O Isotopic Geochemistry
3.1.1. Hydrogen and Oxygen Isotopic Composition
3.1.2. Impact of Water–Rock Interaction and Evaporation
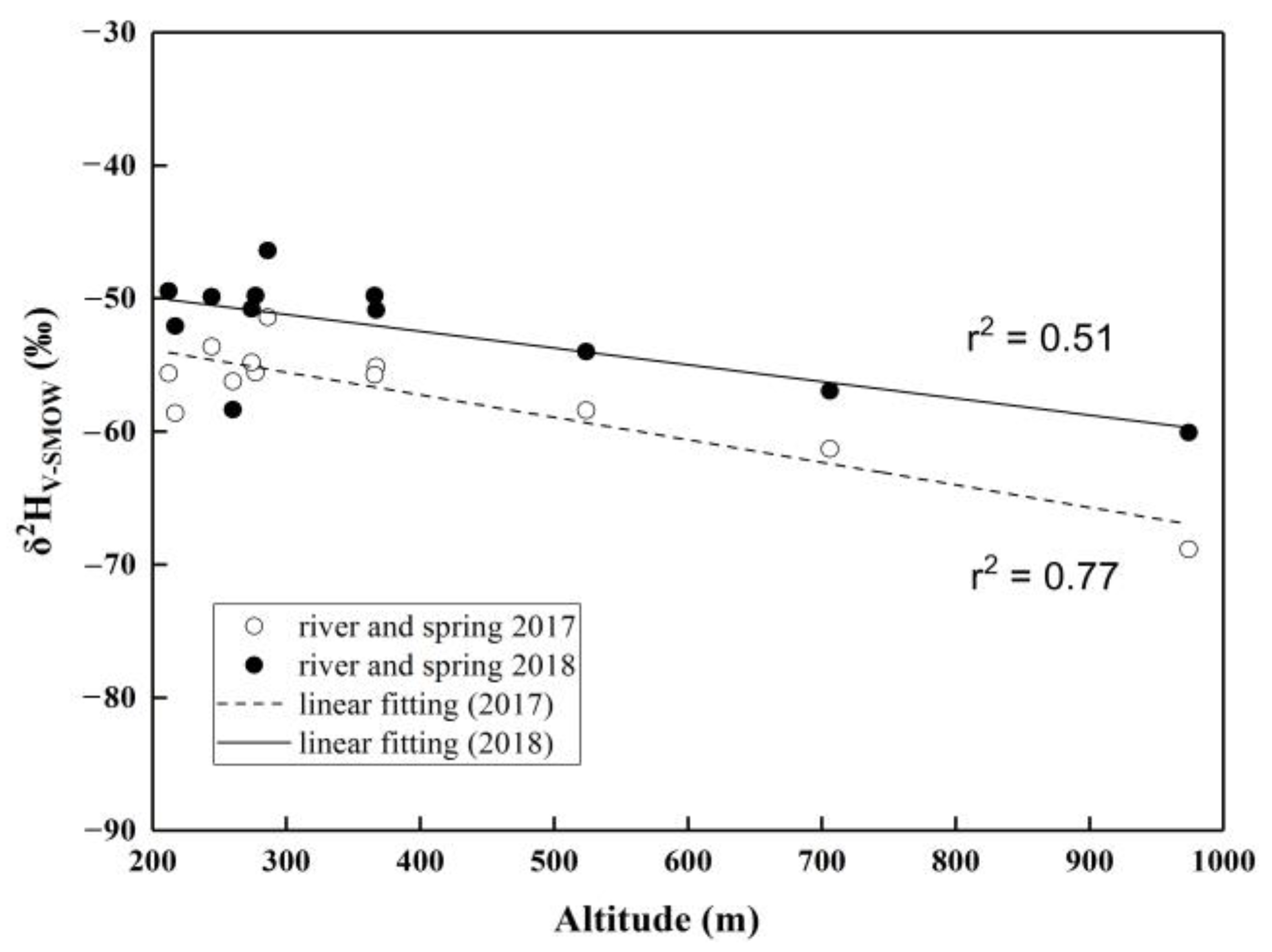
3.1.3. Isotopic Distribution in the Upper Pi River Basin
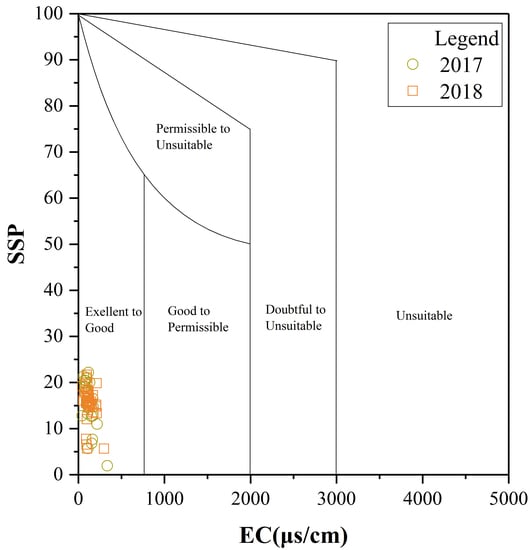
3.2. Assessment of Water Quality for Irrigation Purposes
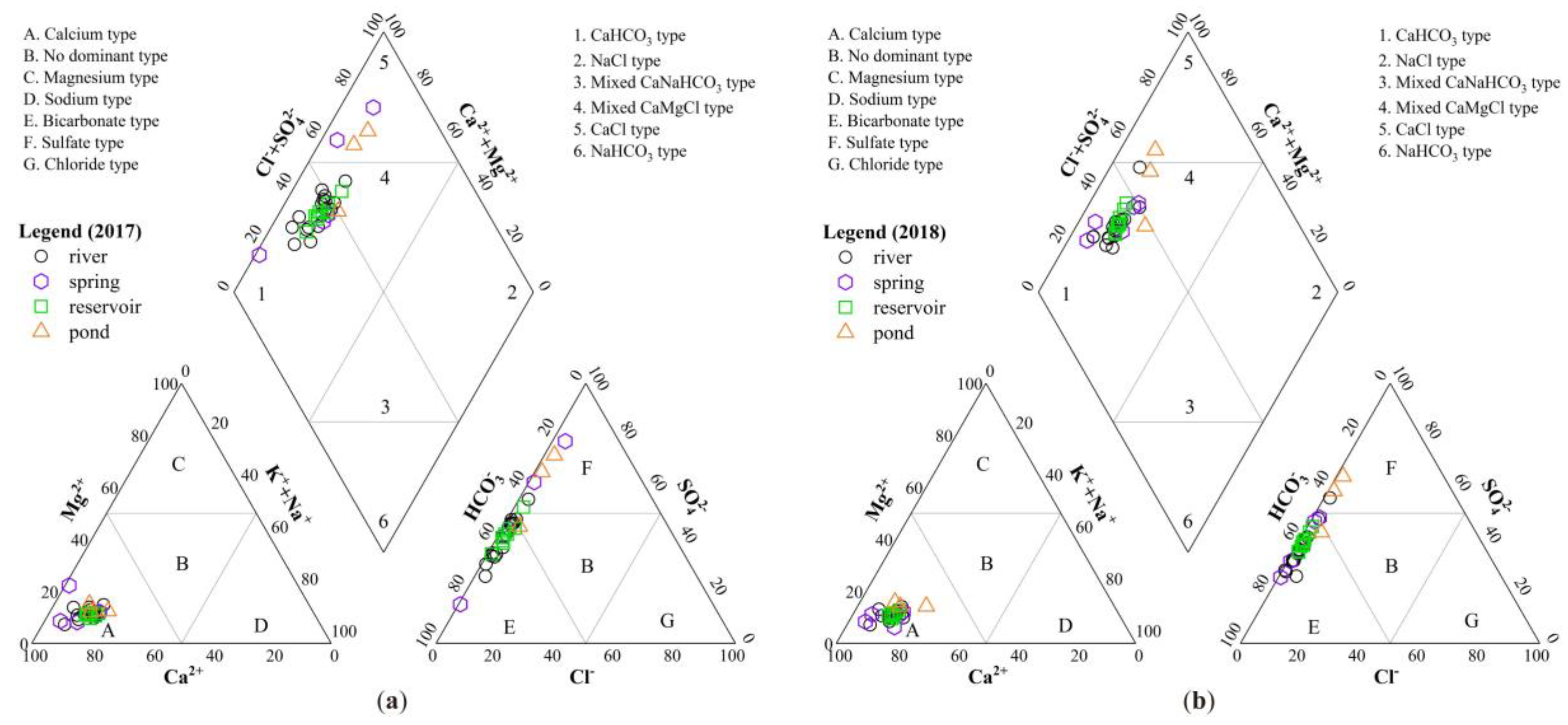
3.2.1. Hydro-Chemical Classification
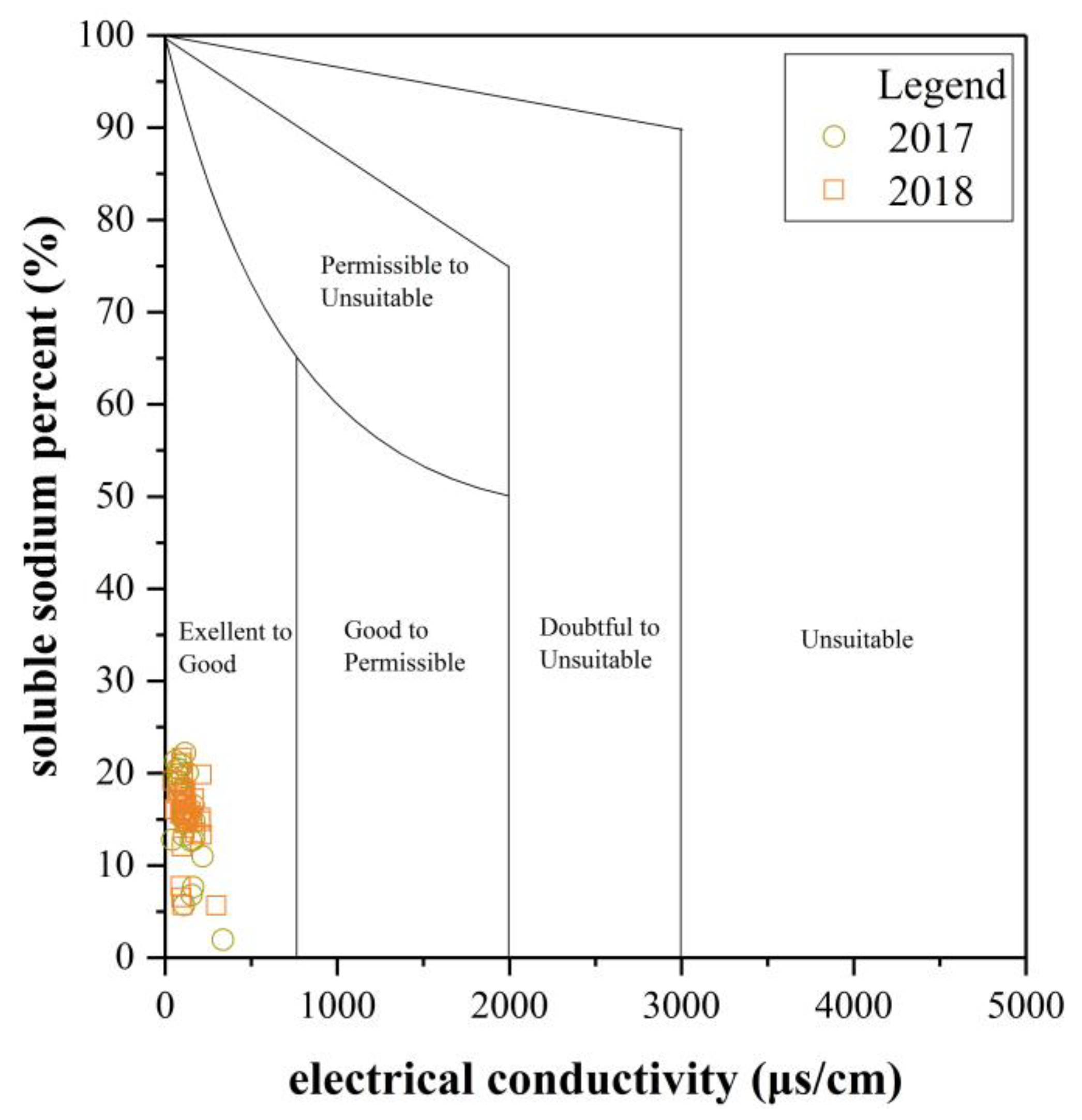
3.2.2. Water Quality Assessment
3.3. Instructions for Local Water Management
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buytaert, W.; Celleri, R.; De Bievre, B.; Cisneros, F.; Wyseure, G.; Deckers, J.; Hofstede, R. Human impact on the hydrology of the Andean paramos. Earth Sci. Rev. 2006, 79, 53–72. [Google Scholar] [CrossRef]
- Zeng, J.; Yue, F.-J.; Wang, Z.-J.; Wu, Q.; Qin, C.-Q.; Li, S.-L. Quantifying depression trapping effect on rainwater chemical composition during the rainy season in karst agricultural area, southwestern China. Atmos. Environ. 2019, 218, 116998. [Google Scholar] [CrossRef]
- Han, G.; Song, Z.; Tang, Y.; Wu, Q.; Wang, Z. Ca and Sr isotope compositions of rainwater from Guiyang city, Southwest China: Implication for the sources of atmospheric aerosols and their seasonal variations. Atmos. Environ. 2019, 214. [Google Scholar] [CrossRef]
- Kalbus, E.; Reinstorf, F.; Schirmer, M. Measuring methods for groundwater-surface water interactions: A review. Hydrol. Earth Syst. Sci. 2006, 10, 873–887. [Google Scholar] [CrossRef]
- Correa, A.; Breuer, L.; Crespo, P.; Celleri, R.; Feyen, J.; Birkel, C.; Silva, C.; Windhorst, D. Spatially distributed hydro-chemical data with temporally high-resolution is needed to adequately assess the hydrological functioning of headwater catchments. Sci. Total Environ. 2019, 651, 1613–1626. [Google Scholar] [CrossRef]
- Han, G.; Tang, Y.; Wu, Q.; Liu, M.; Wang, Z. Assessing Contamination Sources by Using Sulfur and Oxygen Isotopes of Sulfate Ions in Xijiang River Basin, Southwest China. J. Environ. Qual. 2019, 48, 1507–1516. [Google Scholar] [CrossRef]
- Xia, J.; She, D.; Zhang, Y.; Du, H. Spatio-temporal trend and statistical distribution of extreme precipitation events in Huaihe River Basin during 1960–2009. J. Geogr. Sci. 2012, 22, 195–208. [Google Scholar] [CrossRef]
- Ogrinc, N.; Kanduc, T.; Stichler, W.; Vreca, P. Spatial and seasonal variations in delta O-18 and delta D values in the River Sava in Slovenia. J. Hydrol. 2008, 359, 303–312. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Huang, R. The health risk level of water quality in the centralized drinking water sources of the River Pihe irrigation district. Acta Sci. Circumstantiae 2014, 34, 781–787. [Google Scholar]
- Han, G.; Lv, P.; Tang, Y.; Song, Z. Spatial and temporal variation of H and O isotopic compositions of the Xijiang River system, Southwest China. Isot. Environ. Health Stud. 2018, 54, 137–146. [Google Scholar] [CrossRef]
- Yang, K.; Han, G.; Liu, M.; Li, X.; Liu, J.; Zhang, Q. Spatial and Seasonal Variation of O and H Isotopes in the Jiulong River, Southeast China. Water 2018, 10, 1677. [Google Scholar] [CrossRef]
- Cao, X.; Wu, P.; Zhou, S.; Han, Z.; Tu, H.; Zhang, S. Seasonal variability of oxygen and hydrogen isotopes in a wetland system of the Yunnan-Guizhou Plateau, southwest China: A quantitative assessment of groundwater inflow fluxes. Hydrol. J. 2018, 26, 215–231. [Google Scholar] [CrossRef]
- Halder, J.; Terzer, S.; Wassenaar, L.I.; Araguas-Araguas, L.J.; Aggarwal, P.K. The Global Network of Isotopes in Rivers (GNIR): Integration of water isotopes in watershed observation and riverine research. Hydrol. Earth Syst. Sci. 2015, 19, 3419–3431. [Google Scholar] [CrossRef]
- Vystavna, Y.; Diadin, D.; Huneau, F. Defining a stable water isotope framework for isotope hydrology application in a large trans-boundary watershed (Russian Federation/Ukraine). Isot. Environ. Health Stud. 2018, 54, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Li, W. Temporal and spatial variation of water stable isotopes (O-18 and H-2) in the Kaidu River basin, Northwestern China. Hydrol. Process. 2014, 28, 653–661. [Google Scholar] [CrossRef]
- Kumar, A.; Sanyal, P.; Agrawal, S. Spatial distribution of delta O-18 values of water in the Ganga river basin: Insight into the hydrological processes. J. Hydrol. 2019, 571, 225–234. [Google Scholar] [CrossRef]
- Duc Anh, T.; Minh Thi Nguyet, L.; Quynh Thi Phuong, L. Use of stable isotopes to understand run-off generation processes in the Red River Delta. Hydrol. Process. 2017, 31, 3827–3843. [Google Scholar] [CrossRef]
- Kendall, C.; Coplen, T.B. Distribution of oxygen-18 and deuterium in river waters across the United States. Hydrol. Process. 2001, 15, 1363–1393. [Google Scholar] [CrossRef]
- Rank, D.; Wyhlidal, S.; Schott, K.; Weigand, S.; Oblin, A. Temporal and spatial distribution of isotopes in river water in Central Europe: 50 years experience with the Austrian network of isotopes in rivers. Isot. Environ. Health Stud. 2018, 54, 115–136. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, R.; Song, X.; Xia, J. Spatial variation of stable isotopic composition in surface waters of the Huai River basin, China and the regional hydrological implication. Hydrol. Res. 2018, 49, 1452–1466. [Google Scholar] [CrossRef]
- IAEA/WMO. Global Network of Isotopes in Precipitation. Available online: http://www-naweb.iaea.org/napc/ih/IHS_resources_gnip.html (accessed on 22 May 2019).
- Brooks, J.R.; Wigington, P.J., Jr.; Phillips, D.L.; Comeleo, R.; Coulombe, R. Willamette River Basin surface water isoscape (delta O-18 and delta H-2): Temporal changes of source water within the river. Ecosphere 2012, 3. [Google Scholar] [CrossRef]
- Zhao, X.; Li, F. Isotope evidence for quantifying river evaporation and recharge processes in the lower reaches of the Yellow River. Environ. Earth Sci. 2017, 76. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Z.; Zhao, M.; Zeng, Q.; Zeng, C.; Chen, B.; Chen, C.; He, H.; Cai, X.; Ou, Y.; et al. Using deuterium excess, precipitation and runoff data to determine evaporation and transpiration: A case study from the Shawan Test Site, Puding, Guizhou, China. Geochim. Cosmochim. Acta 2018, 242, 21–33. [Google Scholar] [CrossRef]
- Li, C.; Yang, S.; Lian, E.; Yang, C.; Deng, K.; Liu, Z. Damming effect on the Changjiang (Yangtze River) river water cycle based on stable hydrogen and oxygen isotopic records. J. Geochem. Explor. 2016, 165, 125–133. [Google Scholar] [CrossRef]
- Bowen, G.J.; Kennedy, C.D.; Liu, Z.; Stalker, J. Water balance model for mean annual hydrogen and oxygen isotope distributions in surface waters of the contiguous United States. J. Geophys. Res.-Biogeosci. 2011, 116. [Google Scholar] [CrossRef]
- Su, J.; Lv, S.; Li, M.; Ma, X. Research on the Impacts of LUCC on Runoff of Basin above Hengpaitou. China Rural Water Hydropower 2014, 10, 43–48. [Google Scholar]
- Ye, J.; Li, Z.; Liu, J.; Yang, Z. Identification of early meteorological risk warning indicators for flash flood disasters. Torrential Rain Disasters 2016, 35, 25–30. [Google Scholar]
- NMIC. China Meteorological Data Service Centre. Available online: http://data.cma.cn (accessed on 20 May 2019).
- Wang, S.; Wu, R.; Xie, W.; Lu, Y. Rainstorm-Induced Mountain Flood Disaster Risk Zoning Based on FloodArea Inundation Model Taking Pihe River Valley as A Case. Progress. Inquisitiones De Mutat. Clim. 2016, 12, 432–441. [Google Scholar]
- Jager, H.I.; Smith, B.T. Sustainable reservoir operation: Can we generate hydropower and preserve ecosystem values? River Res. Appl. 2008, 24, 340–352. [Google Scholar] [CrossRef]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- IAEA. Global Network of Isotopes in Rivers. Available online: http://www-naweb.iaea.org/napc/ih/IHS_resources_gnir.html (accessed on 22 May 2019).
- Su, X.; Lin, X.; Liao, Z.; Wang, J. Variation of isotopes in the Yellow River along the flow path and its affecting factors. Geochimica 2003, 32, 349–357. [Google Scholar]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Meredith, K.T.; Hollins, S.E.; Hughes, C.E.; Cendon, D.I.; Hankin, S.; Stone, D.J.M. Temporal variation in stable isotopes (O-18 and H-2) and major ion concentrations within the Darling River between Bourke and Wilcannia due to variable flows, saline groundwater influx and evaporation. J. Hydrol. 2009, 378, 313–324. [Google Scholar] [CrossRef]
- Araguas-Araguas, L.; Froehlich, K.; Rozanski, K. Stable isotope composition of precipitation over southeast Asia. J. Geophys. Res.-Atmos. 1998, 103, 28721–28742. [Google Scholar] [CrossRef]
- Laonamsai, J.; Putthividhya, A. Preliminary Assessment of Groundwater and Surface Water Characteristics in the Upper Chao Phraya River Basin Land Using a Stable Isotope Fingerprinting Technique; ASCE: Reston, VA, USA, 2016. [Google Scholar]
- Nguyen Le, D.; Heidbuechel, I.; Meyer, H.; Merz, B.; Apel, H. What controls the stable isotope composition of precipitation in the Mekong Delta? A model-based statistical approach. Hydrol. Earth Syst. Sci. 2018, 22, 1239–1262. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.W.; Letolle, R.; Jusserand, C. Major-Element Chemistry of the Huanghe (Yellow-River), China-Weathering Processes and Chemical Fluxes. J. Hydrol. 1995, 168, 173–203. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Liang, X.; Li, X.; Wang, F. Cumulative effects of cascade dams on river water cycle: Evidence from hydrogen and oxygen isotopes. J. Hydrol. 2019, 568, 604–610. [Google Scholar] [CrossRef]
- Jodar, J.; Custodio, E.; Javier Lamban, L.; Martos-Rosillo, S.; Herrera-Lameli, C.; Sapriza-Azuri, G. Vertical variation in the amplitude of the seasonal isotopic content of rainfall as a tool to jointly estimate the groundwater recharge zone and transit times in the Ordesa and Monte Perdido National Park aquifer system, north-eastern Spain. Sci. Total Environ. 2016, 573, 505–517. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhao, X.; Teng, Y.; Li, X.; Zhang, J.; Wu, J.; Zuo, R. Groundwater nitrate pollution and human health risk assessment by using HHRA model in an agricultural area, NE China. Ecotoxicol. Environ. Saf. 2017, 137, 130–142. [Google Scholar] [CrossRef]
- Zhang, B.; Song, X.; Zhang, Y.; Han, D.; Tang, C.; Yang, L.; Wang, Z.-L. The relationship between and evolution of surface water and groundwater in Songnen Plain, Northeast China. Environ. Earth Sci. 2015, 73, 8333–8343. [Google Scholar] [CrossRef]
- Piper, M. A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Dinka, M.O. Hydrochemical composition and origin of surface water and groundwater in the Matahara area, Ethiopia. Inland Waters 2017, 7, 297–304. [Google Scholar] [CrossRef]
- Alavi, N.; Nozari, V.; Mazloumzadeh, S.M.; Nezamabadi-pour, H. Irrigation water quality evaluation using adaptive network-based fuzzy inference system. Paddy Water Environ. 2010, 8, 259–266. [Google Scholar] [CrossRef]
- Talib, M.A.; Tang, Z.; Shahab, A.; Siddique, J.; Faheem, M.; Fatima, M. Hydrogeochemical Characterization and Suitability Assessment of Groundwater: A Case Study in Central Sindh, Pakistan. Int. J. Environ. Res. Public Health 2019, 16, 886. [Google Scholar] [CrossRef]
- Vincy, M.V.; Brilliant, R.; Pradeepkumar, A.P. Hydrochemical characterization and quality assessment of groundwater for drinking and irrigation purposes: A case study of Meenachil River Basin, Western Ghats, Kerala, India. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef]
- Wilcox, L.V. Classification and Use of Irrigation Waters; Department of Agriculture: Washington, DC, USA, 1955.
- Amiri, V.; Sohrabi, N.; Dadgar, M.A. Evaluation of groundwater chemistry and its suitability for drinking and agricultural uses in the Lenjanat plain, central Iran. Environ. Earth Sci. 2015, 74, 6163–6176. [Google Scholar] [CrossRef]
- Todd, D.K. Groundwater Hydrology, 2nd ed.; Wiley: New York, NY, USA, 1980. [Google Scholar]
- U.S.S.L. Diagnosis and Improvement of Saline and Alkali Soils; Agriculture Department, U.S.: Washington, DC, USA, 1954; p. 160.
- Paliwal, K.V.; Heine, R.W. Irrigation with Saline Water. Q. Rev. Biol. 1972, 198. [Google Scholar]
- Szabolcs, I. The influence of irrigation water of high sodium-carbonate content on soils. Agrokémia Talajt. 1964, 13, 237–246. [Google Scholar]
- Kelley, P.W. USE of Saline Irrigation Water. Soil Sci. 1963, 95, 385–391. [Google Scholar] [CrossRef]
- Bouderbala, A. Assessment of Groundwater Quality and its Suitability for Agricultural Uses in the Nador Plain, North of Algeria. Water Qual. Expo. Health 2015, 7, 445–457. [Google Scholar] [CrossRef]



| Water Types | No. | Stream | Log. °E | Lat. °N | Alt. m | δ2H | δ18O | d-Excess | δ2H | δ18O | d-Excess |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ‰ | ‰ | ‰ | ‰ | ‰ | ‰ | ||||||
| 2017 | 2018 | ||||||||||
| River water | 1 | Xipi | 115.89 | 31.45 | 146 | −58.4 | −8.73 | 11.5 | −59.3 | −8.89 | 11.8 |
| 6 | Manshuim | 115.87 | 31.16 | 524 | −58.4 | −8.39 | 8.7 | −54.0 | −8.18 | 11.5 | |
| 7 | Wugui | 116.00 | 31.16 | 277 | −55.6 | −8.55 | 12.8 | −49.8 | −7.66 | 11.5 | |
| 8 | Manshui | 116.07 | 31.20 | 212 | −55.6 | −8.29 | 10.7 | −49.4 | −7.55 | 10.9 | |
| 9 | Shiyangm | 116.19 | 31.15 | 706 | −61.3 | −9.19 | 12.2 | −56.9 | −8.69 | 12.6 | |
| 11 | Shiyang | 116.21 | 31.24 | 217 | −58.6 | −8.80 | 11.8 | −52.1 | −8.05 | 12.3 | |
| 13 | 116.30 | 31.25 | 154 | −55.8 | −8.53 | 12.4 | −49.8 | −7.88 | 13.2 | ||
| 14 | Maotan | 115.99 | 31.31 | 260 | −56.2 | −8.44 | 11.3 | −58.3 | −8.75 | 11.7 | |
| 16 | Qingtan | 116.38 | 31.17 | 192 | −59.6 | −9.09 | 13.1 | −53.3 | −8.24 | 12.6 | |
| 19 | Saozhou | 116.47 | 31.27 | 274 | −54.8 | −8.65 | 14.4 | −50.8 | −7.80 | 11.6 | |
| 21 | 116.35 | 31.24 | 123 | −61.0 | −9.24 | 13.0 | −64.3 | −9.59 | 12.4 | ||
| 20 | Saozhou | 116.41 | 31.25 | 174 | −53.7 | −8.39 | 13.4 | −49.2 | −7.45 | 10.4 | |
| 23 | Kongjia | 116.18 | 31.36 | 125 | −57.9 | −9.04 | 14.4 | −51.9 | −8.12 | 13.1 | |
| 25 | Kongjia | 116.24 | 31.39 | 94 | −57.1 | −8.79 | 13.2 | −51.6 | −7.95 | 12.1 | |
| 28 | Danjiamiao | 116.46 | 31.36 | 132 | −52.4 | −8.14 | 12.7 | −47.1 | −7.40 | 12.0 | |
| 33 | Huangwei | 116.32 | 31.16 | 198 | −60.7 | −9.35 | 14.1 | −54.4 | −8.03 | 9.8 | |
| 34 | Xiongjia | 116.36 | 31.47 | 48 | −50.0 | −7.79 | 12.2 | −45.0 | −6.40 | 6.2 | |
| Spring water | 3 | 115.78 | 31.13 | 974 | −68.8 | −10.05 | 11.5 | −60.1 | −9.05 | 12.3 | |
| 10 | 116.21 | 31.21 | 367 | −55.1 | −8.36 | 11.8 | −50.9 | −8.04 | 13.4 | ||
| 17 | 116.48 | 31.19 | 286 | −51.4 | −8.10 | 13.4 | −46.4 | −7.41 | 12.8 | ||
| 18 | 116.47 | 31.29 | 366 | −55.7 | −8.85 | 15.1 | −49.8 | −7.99 | 14.1 | ||
| 27 | 116.47 | 31.31 | 244 | −53.6 | −8.39 | 13.5 | −49.9 | −8.12 | 15.1 | ||
| Reservoir water | 2 | Xianghongdian | 116.00 | 31.45 | 122 | −58.6 | −8.43 | 8.8 | −47.8 | −7.18 | 9.6 |
| 4 | 115.89 | 31.24 | 391 | −60.9 | −8.66 | 8.4 | −53.9 | −8.35 | 12.9 | ||
| 5 | 116.03 | 31.41 | 190 | −62.8 | −8.87 | 8.2 | −59.0 | −8.73 | 10.8 | ||
| 12 | Baiyunya | 116.17 | 31.26 | 202 | −62.0 | −9.07 | 10.6 | −52.5 | −7.67 | 8.8 | |
| 15 | 116.09 | 31.15 | 376 | −62.4 | −9.34 | 12.3 | −56.1 | −8.42 | 11.3 | ||
| 22 | Foziling | 116.31 | 31.28 | 127 | −58.8 | −8.55 | 9.6 | −53.6 | −7.86 | 9.2 | |
| 24 | Foziling | 116.27 | 31.34 | 129 | −59.5 | −8.80 | 10.9 | −51.9 | −7.70 | 9.7 | |
| 26 | Banjiezhuizi | 116.32 | 31.44 | 60 | −57.9 | −8.81 | 12.6 | −58.5 | −8.45 | 9.2 | |
| 32 | 115.88 | 31.35 | 263 | −62.4 | −9.69 | 15.1 | −58.9 | −8.72 | 10.9 | ||
| Pond water | 29 | 116.47 | 31.40 | 133 | −47.5 | −6.13 | 1.6 | −40.8 | −5.05 | −0.4 | |
| 30 | 116.46 | 31.42 | 147 | −57.9 | −8.75 | 12.1 | −44.0 | −6.41 | 7.3 | ||
| 31 | 116.46 | 31.47 | 113 | −52.6 | −7.74 | 9.3 | −47.3 | −6.08 | 1.3 | ||
| Max. T | Min. T | Avg. T | Avg. H | Monthly Rainfall | Rainfall Days | |
|---|---|---|---|---|---|---|
| °C | °C | °C | % | mm | Days | |
| October 2017 | 20.0 | 12.4 | 15.5 | 88.8 | 180.2 | 16 |
| September 2018 | 28.1 | 19.5 | 23.0 | 83.3 | 128.2 | 14 |
| Water Types | δ2H | δ18O | δ2H | δ18O | |
|---|---|---|---|---|---|
| ‰ | ‰ | ‰ | ‰ | ||
| 2017 | 2018 | ||||
| River water | Min. | −61.3 | −9.35 | −64.3 | −9.59 |
| Max. | −50.0 | −7.79 | −45.0 | −6.40 | |
| Avg. | −56.9 | −8.67 | −52.8 | −8.04 | |
| Spring water | Min. | −68.8 | −10.05 | −60.1 | −9.05 |
| Max. | −51.4 | −8.10 | −46.4 | −7.41 | |
| Avg. | −56.9 | −8.75 | −51.4 | −8.12 | |
| Reservoir water | Min. | −62.8 | −9.69 | −59.0 | −8.73 |
| Max. | −57.9 | −8.43 | −47.8 | −7.18 | |
| Avg. | −60.6 | −8.91 | −54.7 | −8.12 | |
| Pond water | Min. | −57.9 | −8.75 | −47.3 | −6.41 |
| Max. | −47.5 | −6.13 | −40.8 | −5.05 | |
| Avg. | −52.7 | −7.54 | −44.0 | −5.85 | |
| Water Types | No. | EC (μs/cm) | Na% | SAR | MH | KR | EC (μs/cm) | Na% | SAR | MH | KR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | ||||||||||
| River water | 1 | 128 | 15.55 | 6.47 | 12.82 | 0.20 | 124 | 14.72 | 6.23 | 13.61 | 0.19 |
| 6 | 99 | 19.58 | 7.36 | 13.14 | 0.26 | 117 | 16.08 | 6.75 | 13.24 | 0.22 | |
| 7 | 131 | 20.03 | 8.74 | 13.94 | 0.27 | 213 | 19.84 | 7.33 | 16.52 | 0.27 | |
| 8 | 166 | 16.52 | 7.93 | 13.08 | 0.21 | 208 | 15.14 | 6.25 | 11.42 | 0.19 | |
| 9 | 60 | 21.34 | 5.93 | 11.60 | 0.29 | 62 | 16.06 | 6.64 | 12.82 | 0.21 | |
| 11 | 96 | 18.11 | 6.59 | 13.63 | 0.24 | 112 | 17.75 | 5.11 | 13.78 | 0.25 | |
| 13 | 84 | 20.38 | 6.98 | 17.73 | 0.28 | 98 | 15.71 | 6.79 | 14.36 | 0.21 | |
| 14 | 171 | 14.75 | 7.14 | 15.86 | 0.19 | 116 | 14.55 | 6.73 | 12.66 | 0.19 | |
| 16 | 72 | 20.50 | 6.62 | 12.33 | 0.29 | 120 | 18.18 | 6.75 | 11.13 | 0.24 | |
| 19 | 154 | 6.80 | 2.94 | 14.86 | 0.08 | 170 | 17.24 | 7.12 | 10.91 | 0.23 | |
| 21 | 80 | 19.04 | 6.35 | 13.12 | 0.26 | 58 | 16.09 | 6.18 | 12.57 | 0.21 | |
| 20 | 151 | 12.68 | 5.65 | 11.99 | 0.15 | 176 | 13.59 | 5.17 | 13.70 | 0.18 | |
| 23 | 113 | 16.06 | 6.09 | 14.76 | 0.21 | 136 | 15.47 | 5.42 | 11.85 | 0.20 | |
| 25 | 136 | 15.55 | 6.53 | 13.37 | 0.20 | 158 | 15.35 | 6.40 | 11.98 | 0.20 | |
| 28 | 163 | 7.64 | 3.56 | 7.83 | 0.09 | 214 | 13.33 | 4.22 | 17.91 | 0.17 | |
| 33 | 111 | 15.83 | 6.03 | 12.70 | 0.20 | 101 | 5.67 | 2.98 | 11.90 | 0.06 | |
| 34 | 218 | 10.97 | 6.17 | 10.36 | 0.14 | 298 | 5.65 | 2.58 | 8.82 | 0.06 | |
| Spring water | 3 | 42 | 12.78 | 2.94 | 9.15 | 0.16 | 46 | 19.19 | 10.83 | 12.41 | 0.26 |
| 10 | 96 | 20.90 | 7.83 | 14.22 | 0.29 | 96 | 6.52 | 3.11 | 14.18 | 0.08 | |
| 17 | 116 | 22.18 | 8.79 | 14.50 | 0.31 | 136 | 15.06 | 10.13 | 9.71 | 0.20 | |
| 18 | 335 | 1.95 | 1.18 | 22.57 | 0.02 | 207 | 14.78 | 5.69 | 12.70 | 0.19 | |
| 27 | 111 | 5.70 | 2.08 | 9.09 | 0.06 | 158 | 15.28 | 7.18 | 18.40 | 0.24 | |
| Reservoir water | 2 | 107 | 14.92 | 5.69 | 12.39 | 0.19 | 107 | 17.75 | 7.41 | 11.98 | 0.24 |
| 4 | 93 | 18.18 | 6.59 | 11.24 | 0.24 | 119 | 16.88 | 9.29 | 12.99 | 0.22 | |
| 5 | 100 | 15.66 | 5.81 | 13.67 | 0.21 | 96 | 21.08 | 6.67 | 11.98 | 0.30 | |
| 12 | 102 | 16.63 | 6.33 | 13.16 | 0.22 | 99 | 12.02 | 5.97 | 11.76 | 0.15 | |
| 15 | 73 | 19.89 | 6.43 | 12.62 | 0.27 | 90 | 7.75 | 4.03 | 7.73 | 0.09 | |
| 22 | 99 | 15.66 | 5.71 | 12.75 | 0.20 | 92 | 18.49 | 6.65 | 12.11 | 0.25 | |
| 24 | 112 | 15.18 | 5.85 | 12.46 | 0.20 | 89 | 15.59 | 5.26 | 12.97 | 0.20 | |
| 26 | 124 | 15.07 | 6.17 | 12.69 | 0.19 | 126 | 16.65 | 6.16 | 14.86 | 0.22 | |
| 32 | 74 | 19.09 | 6.02 | 13.76 | 0.27 | 100 | 21.69 | 9.62 | 14.54 | 0.30 | |
| Pond water | 29 | 167 | 12.79 | 6.61 | 15.40 | 0.19 | 148 | 15.84 | 4.73 | 16.52 | 0.21 |
| 30 | 107 | 13.23 | 4.55 | 17.04 | 0.17 | 101 | 17.13 | 4.76 | 7.40 | 0.24 | |
| 31 | 84 | 17.81 | 5.49 | 14.06 | 0.24 | 72 | 17.82 | 6.62 | 12.82 | 0.24 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Han, G.; Song, C.; Zhang, P. Stable H-O Isotopic Composition and Water Quality Assessment of Surface Water and Groundwater: A Case Study in the Dabie Mountains, Central China. Int. J. Environ. Res. Public Health 2019, 16, 4076. https://doi.org/10.3390/ijerph16214076
Yang K, Han G, Song C, Zhang P. Stable H-O Isotopic Composition and Water Quality Assessment of Surface Water and Groundwater: A Case Study in the Dabie Mountains, Central China. International Journal of Environmental Research and Public Health. 2019; 16(21):4076. https://doi.org/10.3390/ijerph16214076
Chicago/Turabian StyleYang, Kunhua, Guilin Han, Chao Song, and Peng Zhang. 2019. "Stable H-O Isotopic Composition and Water Quality Assessment of Surface Water and Groundwater: A Case Study in the Dabie Mountains, Central China" International Journal of Environmental Research and Public Health 16, no. 21: 4076. https://doi.org/10.3390/ijerph16214076
APA StyleYang, K., Han, G., Song, C., & Zhang, P. (2019). Stable H-O Isotopic Composition and Water Quality Assessment of Surface Water and Groundwater: A Case Study in the Dabie Mountains, Central China. International Journal of Environmental Research and Public Health, 16(21), 4076. https://doi.org/10.3390/ijerph16214076